Abstract
Streptococcus pneumoniae (pneumococcus) is a leading cause of bacterial meningitis and neurological sequelae in children worldwide. Acute bacterial meningitis is widely considered to result from bacteremia that leads to blood-brain barrier breakdown and bacterial dissemination throughout the central nervous system (CNS). Previously, we showed that pneumococci can gain access to the CNS through a nonhematogenous route without peripheral blood infection. This access is thought to occur when the pneumococci in the upper sinus follow the olfactory nerves and enter the CNS through the olfactory bulbs. In this study, we determined whether the addition of exogenous sialic acid postcolonization promotes nonhematogenous invasion of the CNS. Previously, others showed that treatment with exogenous sialic acid post-pneumococcal infection increased the numbers of CFU recovered from an intranasal mouse model of infection. Using a pneumococcal colonization model, an in vivo imaging system, and a multiplex assay for cytokine expression, we demonstrated that sialic acid can increase the number of pneumococci recovered from the olfactory bulbs and brains of infected animals. We also show that pneumococci primarily localize to the olfactory bulb, leading to increased expression levels of proinflammatory cytokines and chemokines. These findings provide evidence that sialic acid can enhance the ability of pneumococci to disseminate into the CNS and provide details about the environment needed to establish nonhematogenous pneumococcal meningitis.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is a common asymptomatic colonizer of the nasopharynx of healthy individuals. Colonization can occur at any point during a person's life but occurs most frequently in the first few years of life, with colonization rates of 50% to 70% in hosts ≤3 years of age (1). Colonization in the young and elderly can lead to bacterial pneumonia, otitis media, meningitis, and sepsis, with approximately 4 million new cases of illness and 22,000 deaths annually in the United States (2). Pneumococcal meningitis is traditionally thought to be established when bacteria disseminate into the lower respiratory tract and cause a focal pneumonia, which is proceeded by septicemia and subsequent crossing of the blood-brain barrier (3–5). We and others have shown that pneumococci and other pathogens have the ability to invade the central nervous system (CNS) through a nonhematogenous route after nasopharynx colonization. However, further research is needed to understand conditions that may contribute to CNS dissemination (6–11).
Clinically, there have been cases of bacterial meningitis reported in the absence of a positive blood culture. A study of child cerebral malaria in Kenyan children found that of 29 cases of acute bacterial meningitis, 10 cases had negative blood cultures but positive cerebrospinal fluid (CSF) samples, and half of these infections were caused by Streptococcus pneumoniae (12). A 2005 study of neonatal meningitis found that 38% of cases of confirmed bacterial meningitis had negative blood cultures. The authors of that study concluded that meningitis can often occur in the absence of bacteremia (13). This idea was further corroborated by a study of the efficacy of the use of blood culture to identify the causative agent of bacterial meningitis. While the results of that study support the use of blood cultures to help identify the possible bacterial etiology of meningitis, those authors also found that 9% of the cases of meningitis in patients without antibiotic treatment had negative blood cultures. They also showed that S. pneumoniae and Neisseria meningitidis, two colonizers of the nasopharynx, have a lower rate of recovery from the blood in cases of meningitis caused by these agents than in cases of meningitis caused by Haemophilus influenzae (14). The sensitivity of detection of the causative agent of bacterial meningitis via blood culture ranges from 40% to 90%. Patients with pneumococcal meningitis have been estimated to have the causative agent recoverable in the blood in 75% of cases (4, 14, 15). These observations provide further support for the hypothesis that some of the meningitis cases in young children are the result of direct invasion of the CNS from colonization. The clinical data reinforce the need for a model of nonhematogenous meningitis in order to fully investigate factors that may contribute to its establishment.
Previously, we demonstrated that in a mouse colonization model, we could consistently infect the CNS in the absence of bacteremia. This route of infection was attributed to the ability of pneumococci to bind to gangliosides, which allow the bacteria to travel to the CNS via the olfactory nerve (6). Another study showed that a pneumococcal mutant deficient in capsule, which cannot survive in the peripheral blood, given intranasally (i.n.) could still cause experimental meningitis (7). Other pathogens that colonize the nasopharynx have also demonstrated the ability to directly invade the CNS. Sjolinder and Jonsson established that N. meningitidis can invade the CNS in a transgenic mouse line expressing human CD46 via the olfactory nerve in the absence of bacteremia (8). Herbert et al. also showed that when the olfactory epithelium was damaged, Staphylococcus aureus administered intranasally was localized along the olfactory nerve bundles and in the olfactory bulbs 6 h after inoculation (9). These studies support the idea that pathogens can invade the CNS directly from the nasopharynx, but given the high rate of bacterial colonization in human populations compared to the low reported incidences of nonhematogenous meningitis cases, colonization must not be the only determinant of CNS invasion. There is a need to identify a signal that may promote nonhematogenous dissemination.
Carbohydrate availability and pneumococcal pathogenesis have been studied extensively (16–19). Pneumococci possess up to 3 neuraminidases (neuraminidase A [NanA], NanB, and sometimes NanC), which are capable of cleaving α-2,6 and α-2,3 linkages of N-acetylneuraminic acid (Neu5Ac) (sialic acid) to galactose and N-acetylgalactosamine (20). Sialic acid is a general term used to describe a family over of 40 derivatives of neuraminic acid, which are 9-carbon monosaccharides that occupy the terminal end of many glycoconjugates. The most common sialic acid found in humans is Neu5Ac, typically found in its bound form and ubiquitously expressed on most cell surfaces in mammals. N-Glycolylneuraminic acid (Neu5Gc) is also found in the human body but is typically associated with disease (21). Sialic acid availability has been shown to be important in pneumococcal pathogenesis. It was demonstrated previously that exogenous sialic acid promotes nasal colonization and lung invasion when given to mice postinfection, and the addition of sialic acid in vitro leads to the upregulation of NanA and NanB in pneumococci (22). Furthermore, it was shown that pneumococci have the ability to metabolize sialic acid, and when the locus responsible for sialic acid transport was deleted, colonization was inhibited in mice (23, 24). This finding and evidence that bound sialic acid cleaved by influenza virus may exacerbate pneumococcal infection and contribute to invasive disease underscore the importance of sialic acid availability (25–27).
The purpose of this study was to investigate whether sialic acid promotes nonhematogenous invasion of the CNS by pneumococci. To this end, we developed a model of CNS invasion that is a modification of our previously reported intranasal colonization model (28). Using this model, we showed that sialic acid treatment can increase the numbers of recoverable pneumococcal CFU from the sinus tissue, olfactory bulbs, and brain of mice infected intranasally and that this increase happens without any detectable bacteremia. We verified invasion using an in vivo imaging system (IVIS) to show the localization of pneumococci directly on the olfactory bulbs and brains of infected animals. We also correlate the presence of the bacteria with increased levels of proinflammatory cytokines and chemokines. These findings indicate that administration of sialic acid soon after intranasal inoculation with pneumococci can promote pneumococcal invasion of the CNS in the absence of bacteremia, providing evidence that free sialic acid can enhance nonhematogenous pneumococcal meningitis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae strains used in our studies were EF3030 (serotype 19F); TIGR4 (serotype 4); D39.1, a mouse-passaged D39 strain (serotype 2); a TIGR4 ΔnanA mutant; and EF3030 Xen 11, which possesses a stable copy of the lux operon on the bacterial chromosome (Caliper LifeSciences). The EF3030 strain was used because it can easily colonize the nasopharynx and is incapable of establishing bacteremia when administered intravenously (29). TIGR4 is more virulent than EF3030, but when given intranasally in a moderate inoculum, it can colonize with no apparent bacteremia (6). D39.1 was used because it is more virulent in intravenous infection than TIGR4 and readily disseminates into the blood.
The pneumococcal EF3030, TIGR4, D39.1, and TIGR4 ΔnanA strains were grown in Todd-Hewitt medium containing 0.5% yeast extract at 37°C to an optical density at 600 nm (OD600) of 0.5 and then stored frozen in aliquots at −80°C in the same broth supplemented with 10% sterile glycerol. EF3030 Xen 11 was grown in a manner similar to that mentioned above but modified to include brain heart infusion medium instead of Todd-Hewitt medium.
Construction of ΔnanA mutants.
Strains SAM001 and JCP001 of parental background TIGR4 are ΔnanA mutants derived through insertion-duplication mutagenesis techniques (30–32). To obtain the mutants, TIGR4 was used as the recipient for the transformation of donor chromosomal DNA prepared from the ΔnanA D39 strain (33). Examination of the supernatants or lysed pellets of our ΔnanA mutant strains revealed no neuraminidase activity (data not shown) using the fluorigenic substrate 2′-(4-methylumbelliferyl)-alpha-d-N-acetylneuraminic acid (Sigma Chemical Company, St. Louis, MO) (34). Examination by Western blotting using an antibody to NanA revealed whole NanA in the D39 and TIGR4 parental strains but fragmented NanA in the mutant strain (data not shown).
Mice.
Eight- to 12-week-old C57BL/6J (female) and 6- to 8-week-old BALB/cByJ (female and male) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/cByJ mice were used for the whole-body imaging experiments because their white fur does not block light to the same degree as the black skin or fur of C57BL/6J mice. Mice were maintained under pathogen-free conditions, which includes being separated into groups and housed in microisolator cages. Cubicles of infected mice were physically separated and housed in different rooms from noninfected mice. Additionally, laboratory personnel and University of Alabama at Birmingham (UAB) veterinary staff work from “noninfected to infected” by entering noninfected areas first and never backtracking. Animals were under the supervision of the UAB veterinary staff, and all animal protocols used for these studies were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Mouse intranasal and intravenous infection models.
For our intranasal colonization model and intravenous infection model, mice were infected as previously described (28, 35). A frozen inoculum containing known concentrations of viable bacteria was diluted in lactated Ringer's solution. Mice were then inoculated i.n. with the indicated pneumococcal strain in a volume of 20 μl. At 24 h postinfection, mice were intranasally administered lactated Ringer's solution or the indicated concentrations of N-acetylneuraminic acid (catalog number A0812; Sigma), N-glycolylneuraminic acid (catalog number G9793; Sigma), or glucose (catalog number 15023021; Gibco) reconstituted in lactated Ringer's solution (20-μl volume/mouse). For intravenous infection, mice received the indicated amounts of either EF3030 or D39.1 in a 100-μl volume via tail vein injection. Mice were monitored daily and sacrificed at the indicated times postinfection. Numbers of CFU in the inoculum were confirmed by plating as described previously (6, 28).
Whole-body imaging.
Mice challenged intranasally were imaged after 4 days by using the IVIS-100 system (Caliper Life Sciences) equipped with a charge-coupled-device (CCD) camera (36, 37). Mice were anesthetized until they reached a surgical plane of anesthesia with inhaled isoflurane and O2 (flow rate, 2.5 liters/min). The mice were then positioned inside the Xenogen imaging cabinet, where they received further anesthesia and were placed onto a warm plate (37°C) for the duration of imaging. Acquisition properties and signal quantification were performed by using Living Image 4.2 software (Caliper). The total counts were taken from a region of interest (ROI) (minus background). Mice were first perfused with 20 ml of lactated Ringer's solution via intracardial puncture with a 27-gauge butterfly needle before individual tissues were collected and imaged.
Tissue collection and enumeration.
Tissue collection was done as previously described, with few a modifications (6, 28). Mice were placed under light anesthesia (inhaled isoflurane), and ∼75 μl of blood was collected into heparinized capillary tubes via retroorbital bleeding. Eight 3-fold serial dilutions of blood were made, each dilution was plated onto blood agar plates, and CFU were enumerated after 24 h. The anesthetized mice were then euthanized via CO2-induced hypoxia. A nasal wash specimen was collected via an incision made in the trachea and a Tygon tube (0.075-cm diameter) inserted into the trachea. A syringe was attached to the tube, and the nasopharynx was then washed with 1 ml of lactated Ringer's solution. The wash specimen was collected as it was expelled from the nares of the animal. Mice were then perfused with 20 ml of lactated Ringer's solution via intracardinal puncture with a 27-gauge butterfly needle. The sinus tissue, olfactory bulbs, and brain were collected as previously described (6, 28). Eight 3-fold serial dilutions were made for the sinus tissue and nasal wash specimens, while the olfactory bulbs and brains were homogenized in 1 ml of lactated Ringer's solution, and 100 μl of the sample was plated. Each sample was plated onto blood agar plates, and CFU were counted after 24 h. The limit of detection was back-calculated based on the dilution factors used to process each sample. The limits of detection were 1.49 log CFU for blood, 1.0 log CFU for brains/olfactory bulbs, 1.6 log CFU for sinus tissue, and 0.3 log CFU for nasal wash specimens.
Cytokine expression assay.
Nasal wash specimen and olfactory bulb homogenate supernatants were assayed by using the Millipore (Billerica, MA) mouse cytokine/chemokine magnetic bead panel (Mcytomag-70K) as described previously (38). Tumor necrosis factor alpha (TNF-α), interleukin-1α (IL-1α), and IL-1β are all proinflammatory cytokines that have been shown in patients and animal models to be present at increased levels in the CSF in cases of bacterial meningitis. The level of IL-1α is increased systemically during sepsis and fever, and IL-1α has been identified as being very sensitive in sensing bacterial meningitis. Macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and keratinocyte chemoattractant (KC) are chemokines that are produced by activated macrophages and attract granulocytes, and in this case, they were used to indicate immune cell infiltration. IL-10 is an important anti-inflammatory cytokine and has been shown to downregulate TNF-α and KC during pneumococcal meningitis (4, 39–41). Expression levels of IL-1α, IL-1β, KC, MIP-1α, MIP-1β, and TNF-α were adjusted by the total protein levels in each sample and are reported in picograms per milliliter.
Statistics.
Statistical comparisons were done by using an unpaired two-tailed Mann-Whitney U test and one-way analysis of variance (ANOVA). Samples with no detectable CFU were set to the limit of detection according to their respective sample types. Significance is indicated in the figure legends.
RESULTS
Increased colonization and dissemination of pneumococcal species into the CNS after treatment with sialic acid.
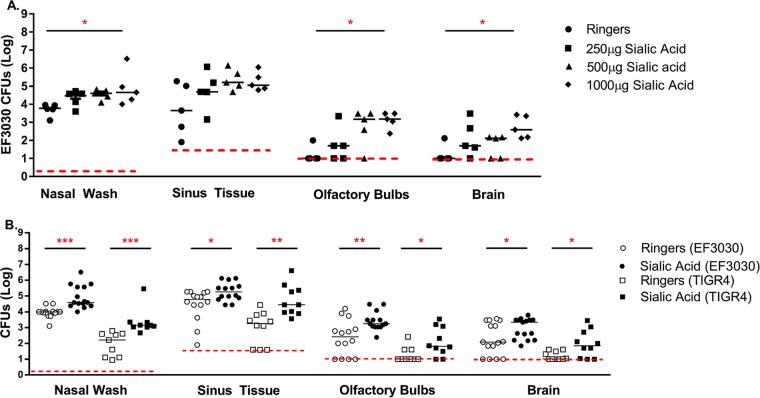
Previously, it was reported that intranasal inoculations of free sialic acid could increase the colonization of the nasopharynx in infected mice (22, 23). To determine if sialic acid availability plays a role in the direct dissemination of pneumococci from the nasopharynx to the CNS, we incorporated N-acetylneuraminic acid (Neu5Ac) in our colonization model. C57BL/6J mice were infected with EF3030, a capsule type 19F pneumococcal strain. All of the mice treated with sialic acid had increased numbers of CFU recovered compared to Ringer's solution-treated animals. However, mice that were treated with 1 mg of sialic acid had significantly more CFU recovered from nasal wash specimens, olfactory bulbs, and brain than control mice (Fig. 1A).
FIG 1.
Increased colonization and dissemination of pneumococcal species into the CNS after treatment with sialic acid. Eight- to 12-week-old C57BL/6J mice were intranasally challenged with 107 CFU of EF3030 (A and B) or 3 × 106 CFU of pneumococcal strain TIGR4 (B) without anesthesia. After 24 h, mice were treated with the indicated amounts of N-acetylneuraminic acid (A) or 1,000 μg of N-acetylneuraminic acid (B). Mice were then sacrificed at 4 days postinfection, and pneumococcal CFU were enumerated from nasal wash specimens and tissue homogenates. Each point on the graph represents data for an individual mouse. The red dashed line indicates the minimum level of detection, and the solid bar represents the median. Statistical significance was determined by using one-way ANOVA (A) or a Mann-Whitney rank sum test (B) (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [indicating significant differences between populations]) (sialic acid is N-acetylneuraminic acid). Before euthanasia, all mice were bled, and the blood was plated to determine CFU. In all cases, no bacteremia was observed (data not shown).
In this study, it was important to determine whether sialic acid-dependent increases in colonization and dissemination were likely to be independent of the capsular serotype. C57BL/6J mice were infected with EF3030 and TIGR4, capsule type 19F and 4 strains, respectively, and then treated with 1 mg of sialic acid. TIGR4 displayed the same enhanced ability to colonize and invade animals treated with sialic acid as EF3030 (Fig. 1B). There was also an increased number of sialic acid-treated mice with recoverable CFU in the brain compared to Ringer's solution-treated animals. This indicated that sialic acid not only increased colonization but also affected the frequency with which the pneumococci could enter normally sterile sites. Blood samples were taken from every mouse before sacrifice, and at no time was there any detectable CFU recovered from the blood (data not shown). We also wanted to determine if bacteremia was detectable at earlier time points during infection. We compared intravenous infection with D39.1, a strain with a pneumococcal serotype associated with bacteremia in animal models, to intranasal infection with EF3030. We took blood samples at 1, 4, 8, 12, 24, 48, 72, and 96 h postinfection. Our results indicated that intranasal infection with EF3030 does not produce significant bacteremia, even when blood is sampled at earlier time points during infection. Additionally, we found that even though D39.1 can establish robust bacteremia, it did not lead to significant CNS invasion. This gives credence to the idea that the blood-brain barrier is still intact early during infection and that another route must be available to bacteria (see Fig. S1 in the supplemental material).
Sialic acid treatment increases pneumococcal colonization without dissemination into the blood.
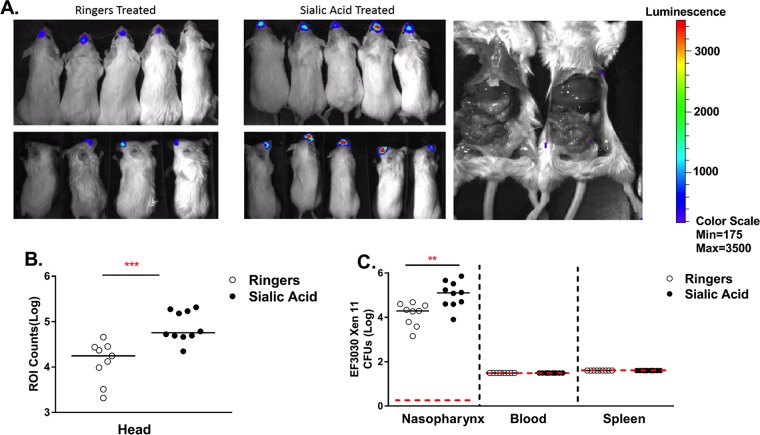
In our model of intranasal colonization without anesthesia, pneumococci are found in the lungs only at low levels (28, 42). However, it was not known if the addition of sialic acid would cause systemic distribution of pneumococci in animals. To determine if sialic acid can cause the spread of bacteria from the nasopharynx to other organs, we employed EF3030 Xen 11, which stably expresses the lux operon on the bacterial chromosome. EF3030 Xen 11 allowed us to use an in vivo imaging system (IVIS) to track bacterial dissemination. The IVIS revealed that mice treated with sialic acid had increased levels of nasopharynx colonization but no detectable bacteremia or organ invasion (Fig. 2A and B). Opening of the body cavity of infected animals still revealed no additional bacterial luciferase expression from the liver, spleen, or intestines (Fig. 2A). CFU data for the different tissues complemented the IVIS images showing that the level of recovery from the nasopharynx was highest in sialic acid-treated mice. Moreover, pneumococci were not detectable by IVIS imaging of the whole mouse or by determination of CFU in blood or spleen (Fig. 2C). The lack of systemic spread of EF3030 was further substantiated when we infected mice intravenously with EF3030 and found that EF3030 failed to establish bacteremia or invade the CNS. However, we were able to recover low levels of EF3030 in the spleen and liver of intravenously infected mice (see Fig. S2 in the supplemental material). The IVIS is not sensitive enough to detect small amounts of systemic pneumococci, but IVIS imaging along with our intravenous challenge with EF3030 make us confident that pneumococci are not present in the blood in large enough numbers to invade the CNS.
FIG 2.
Sialic acid treatment increases pneumococcal colonization without dissemination into the blood. Six- to eight-week-old BALB/cByJ mice were intranasally infected with 107 CFU of EF3030 Xen 11 without anesthesia. (A) After 24 h, mice were treated with either lactated Ringer's solution or 1 mg of Neu5Ac and imaged at 4 days postinfection. (B) Light emission was determined from the region of interest (ROI), the head of each mouse. Each point represents the total light detected on one animal. The horizontal red dashed line represents the lower limit of detection in each assay, which is where CFU-negative samples were plotted. The solid horizontal lines represent the medians for each data set. (C) CFU from nasal wash specimens, blood, and spleens from infected animals were enumerated. Statistical significance was determined by a Mann-Whitney rank sum test (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [indicating significant differences between populations]).
Treatment with N-acetylneuraminic acid but not N-glycolylneuraminic acid leads to increased invasion into the brain.
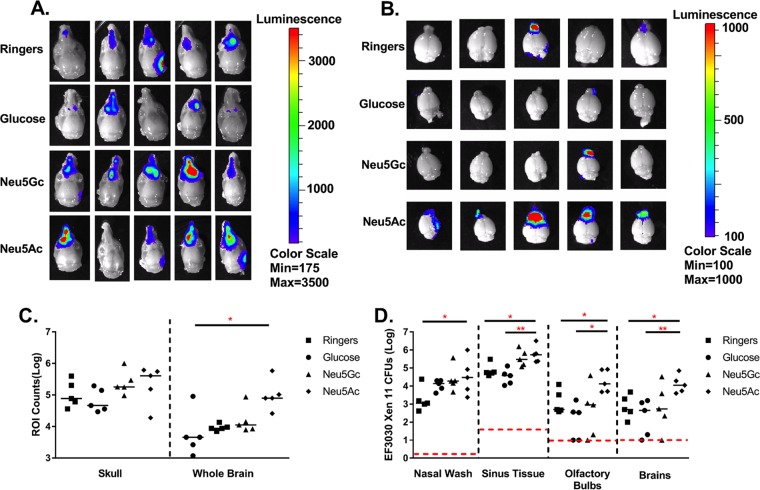
Neu5Ac is the most abundant sialic acid found in humans and is ubiquitously expressed, while Neu5Gc is considered an “oncofetal” antigen and is associated primarily with fetal development and human tumors (21). To determine if increased pneumococcal invasion into the CNS is a phenomenon specific to Neu5Ac, we included Neu5Gc as well as another carbon source metabolized by pneumococci (glucose) in our infection model and compared the effects on bacterial dissemination (Fig. 3). The heads of infected animals were removed and skinned before being imaged to increase the sensitivity of luciferase detection in the sinuses. Imaging revealed that luciferase expression levels in the heads were not different among Ringer's solution-, glucose-, Neu5Ac-, and Neu5Gc-treated mice; however, there was a modest (but not significant) increase in expression levels in Neu5Ac-treated animals (Fig. 3A and C). The greatest differences were seen when the exposed brains of infected animals were imaged directly. Neu5Ac-treated mice had significantly higher luciferase expression levels (P < 0.05) in the olfactory bulbs and brain than did control mice treated with Ringer's solution (Fig. 3B and C). This increase in the signal from Neu5Ac-treated mice was confirmed by comparing numbers of CFU recovered among the different groups (Fig. 3D). Mice treated with Neu5Gc and glucose had approximately the same number of bacteria recovered from the nasopharynx, with a slight increase in CFU recovery in sinus tissue from Neu5Gc-treated mice. The olfactory bulbs and whole brains of Neu5Ac-treated mice had significantly higher numbers of CFU recovered than did glucose-treated animals (P < 0.05 and P < 0.01, respectively) and Ringer's solution-treated animals (P < 0.05 and P < 0.01, respectively). Although Neu5Gc and Neu5Ac treatments were not significantly different in terms of luciferase expression (Fig. 3B) or CFU recovery (Fig. 3C), in both cases, Neu5Ac-treated animals showed higher CFU levels in the nasal wash specimens, olfactory bulbs, and brains than those of mice treated with Neu5Gc. The median total luciferase expression levels in the whole brains of Neu5Ac-treated animals were over half a log higher than those for Neu5Gc-treated animals (4.77 log total counts and 4.05 log total counts, respectively), and the median level of CFU recovery for the whole-brain homogenate was 1.31 logs higher in Neu5Ac-treated animals (4.04 log CFU versus 2.73 log CFU). This provides evidence that invasion into the CNS is increased by treatment with Neu5Ac compared to glucose or even other sialic acids like Neu5Gc.
FIG 3.
Treatment with N-acetylneuraminic acid but not N-glycolylneuraminic acid leads to increased invasion into the brain. (A and B) Six- to eight-week-old BALB/cByJ were intranasally infected with 107 CFU of EF3030 Xen 11 without anesthesia. After 24 h, mice were treated with lactated Ringer's solution, 1 mg of N-acetylneuraminic acid (Neu5Ac), 1 mg of N-glycolylneuraminic acid (Neu5Gc), or 1 mg of glucose (GLU). Animals were then perfused, and skinned heads (A) and brains (B) were imaged at 4 days postinfection. (C) An ROI was identified, and light emission was measured (counts) minus the background. The whole brain refers to brain and olfactory bulbs. (D) CFU from tissue homogenates enumerated from infected animals. The dashed line indicates the minimum level of detection, and the solid bar represents the median. Statistical significance was determined by one-way ANOVA (C) and a Mann-Whitney rank sum test (D) (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [indicating significant differences between populations]).
Increased cytokine/chemokine expression in intranasally challenged sialic acid-treated mice.
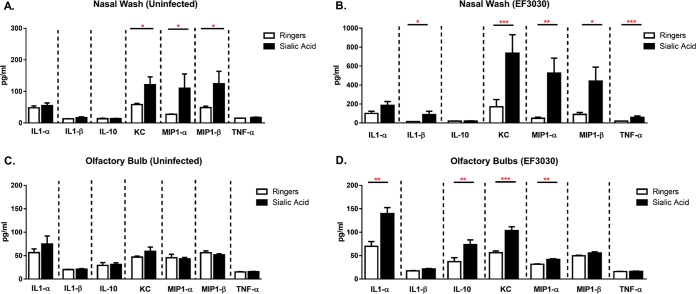
The inflammatory response and, thus, the expression of proinflammatory cytokines are some of the indicators of the severity of pneumococcal meningitis (43). We wished to determine if nonhematogenous CNS invasion induced a cytokine/chemokine profile similar to that seen with other animal models of pneumococcal meningitis (Fig. 4). The expression levels of the proinflammatory cytokines IL-1α, IL-1β, and TNF-α; the anti-inflammatory cytokine IL-10; and the chemoattractants KC, MIP-1α, and MIP-1β in nasal wash specimens and olfactory bulbs of infected animals were examined. In the nasal wash specimens after infection, protein expression levels of IL-1β, KC, MIP-1α, MIP-1β, and TNF-α were significantly increased after treatment with sialic acid compared to Ringer's solution-treated animals, with no change being seen in IL-1α and IL-10 expression levels. In the olfactory bulbs after infection, IL-1α, IL-10, KC, and MIP-1α showed significant changes in expression associated with sialic acid, while IL-1β, MIP-1β, and TNF-α expression levels were unchanged. The cytokines/chemokines that showed increased expression levels were not the same for both the nasal wash specimens and olfactory bulbs. While both the nasal wash specimens and the olfactory bulbs showed increased KC and MIP-1α levels, the nasopharynx additionally showed increased IL-1β, MIP-1β and TNF-α expression levels, while the olfactory bulbs showed increased expression levels of IL-1α and IL-10.
FIG 4.
Increased cytokine/chemokine expression in sialic acid-treated mice. Eight- to 12-week-old C57BL/6J mice were treated intranasally with lactated Ringer's solution (A and C) or intranasally challenged at 24 h with 1 × 107 CFU of EF3030 (B and D). At 4 days postinfection, cytokine/chemokine expression levels in the olfactory bulbs and nasal lavage fluid were determined by a multiplex expression assay (n = 10 to 19 mice/group). Statistical significance was determined by a Mann-Whitney rank sum test (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [indicating significant differences between populations]).
Sialic acid treatment also caused increases in the expression levels of the leukocyte chemoattractants KC, MIP-1α, and MIP-1β in nasal wash specimens of uninfected animals but had no statistically significant effect on cytokine/chemokine expression in the olfactory bulbs of uninfected animals. Furthermore, the sialic acid-dependent increases in the expression levels of KC, MIP-1α, and MIP-1β in uninfected mice were one-seventh, one-fifth, and one-quarter of those in sialic acid-treated infected mice, respectively. The increased proinflammatory cytokine/chemokine levels in the olfactory bulbs correlate with the more robust infection seen in sialic acid-treated mice and are consistent with the hypothesis that sialic acid enhances bacterial dissemination.
Neuraminidase is important for central nervous system invasion.
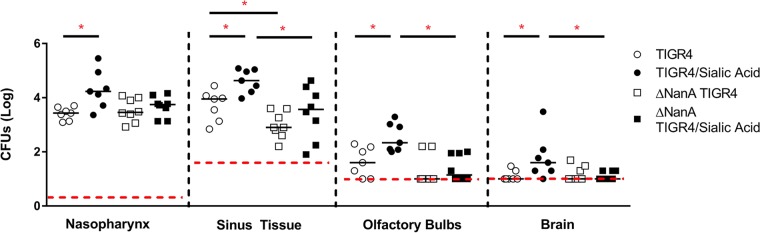
Streptococcus pneumoniae can extensively modify glycans on host cells, and it is thought that this gives pneumococci an advantage during colonization (19). Pneumococci can express up to 10 glycosidases, with one of the most prominent being neuraminidase, which cleaves sialic acid-containing substrates and is expressed by most pneumococcal serotypes (19, 20). Previous reports have shown that neuraminidase A (NanA) is important for the ability of pneumococci to cross the blood-brain barrier and that neuraminidase activity may contribute to the severity of pneumococcal meningitis (44, 45). In addition, it has been reported that pneumococci grown in the presence of sialic acid (Neu5Ac) can have up to 12 times the neuraminidase activity of pneumococci grown with glucose (24). We therefore decided to investigate the effect of disabling the production of NanA on bacterial dissemination from the nasopharynx to the brain. To study this, we used a TIGR4 NanA deletion mutant and compared its pathogenesis to that of wild-type TIGR4 with and without added sialic acid (Fig. 5). Mice infected with the wild type typically had higher numbers of CFU recovered from the sinus tissue and the olfactory bulbs than mice infected with the NanA mutant. The NanA mutant showed a decreased ability to infect the sinus tissue, and generally, the addition of sialic acid had less of an effect on the TIGR4 mutant than it did on the wild type. Most notably, sialic acid seemed to have little to no effect in helping the TIGR4 mutant invade the CNS. Thus, the ability of sialic acid to stimulate the invasion of the CNS seems to be hampered by the lack of one of the major pneumococcal sialidases.
FIG 5.
Neuraminidase is important for central nervous system invasion. Eight- to 12-week-old C57BL/6J mice were intranasally challenged with 3 × 106 CFU of pneumococcal strain TIGR4 or the TIGR4 ΔNanA strain without anesthesia. After 24 h, mice were treated with 1 mg of Neu5Ac or an equal volume of lactated Ringer's solution. CFU were counted from tissue homogenates at 4 days postinfection. The horizontal red dashed line represents the lower limit of detection in each assay, which is where CFU-negative samples were plotted. The solid horizontal lines represent the medians for each data set. Statistical significance was determined by a two-tailed Mann-Whitney rank sum test (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [indicating significant differences between populations]).
DISCUSSION
In this study, we investigated the ability of sialic acid to induce CNS invasion without peripheral blood infection in a mouse colonization model. This was done to help address the question of which events may promote this type of infection in human populations. Our results also add validity to reports that pneumococci can invade the CNS and cause meningitis through a nonhematogenous route. We demonstrated that while pneumococci have the ability to invade the CNS without a stimulant, the addition of Neu5Ac postinfection increases the frequency of CNS invasion as well as bacterial loads in the sinus tissue, olfactory bulbs, and brain without resulting in detectable bacteremia in the blood.
It has been reported that other pathogens can also invade the CNS directly from nasopharyngeal colonization, and evidence has shown that the cribriform plate and the olfactory nerve are some of the portals of entry into the CNS (6, 9–11, 46, 47). Our findings are consistent with data from these reports because we have shown the localization of pneumococci to the olfactory bulbs in the brains of infected animals. This result plus the concurrent infection of the nasopharynx and sinus tissue suggest a cascade of events that can lead to invasion and possibly that the severity of the sinus infection is a precursor to CNS invasion. This, along with recent evidence of the association and manipulation of the olfactory ensheathing cells by pneumococci (48, 49), provides credence to the idea that the olfactory nerves are the portals of entry into the CNS.
Pathogen movement through the cribriform plate and along the olfactory nerve is not the only explanation. Filippidis and Fountas cited the nasal lymphatics as a plausible source of access to the CNS (46). Nevertheless, our findings strongly support data from our previous report and are consistent with infection disseminating from the olfactory epithelium, along the olfactory nerve, and to the olfactory bulb of the brain (6). It should be noted that when using the IVIS system, we occasionally detected a fluorescent signal from the area of the brain near the ear. We suspect that our intranasal infection caused secondary otitis media that resulted in CNS invasion. Otitis media is often caused when respiratory pathogens or colonizers of the nasopharynx infect the Eustachian tube, which connects directly to the middle ear. This result was seen sporadically during our experiments but is consistent with other previously reported data (7). Further research is needed to understand factors that lead to otitis media in our model.
Sialic acid has long been studied as a major contributor to disease severity in bacterial pathogenesis and is considered an important factor in coinfection by pneumococci and influenza virus (27, 50). Previous research has shown that Neu5Ac and its derivatives can be used as a sole carbon source for pneumococci and can function as a regulator of the nanAB locus (24). Therefore, we wanted to determine whether pneumococci have the ability to respond to another sialic acid in the same manner as Neu5Ac and if another carbon source, glucose, which is known to be metabolized by pneumococci, could promote CNS invasion. In our studies, glucose-treated animals had recoverable CFU in the olfactory bulb and the brain but never to the same extent as when animals were treated with Neu5Ac. The presence of a carbon source (glucose) was not enough to explain the increased dissemination of pneumococci into the CNS. This suggests that Neu5Ac has an effect that is not due to solely carbon availability. The addition of Neu5Gc, a sialic acid which is similar to Neu5Ac, was also not adequate to reproduce this effect. This suggests that physiological factors (pH, charge, and inflammation) of treatment of infected animals with sialic acid are not as important as the addition of Neu5Ac itself.
A limitation of our study is the amount of sialic acid that was given to each animal. The initial concentration that we used stems from data reported previously by Trappetti et al. (22), who gave 1 mg of sialic acid per mouse intranasally and showed significant increases in pneumococcal colonization and lung invasion. We ended up using this concentration only after we determined that 1 mg of sialic acid per mouse gave the best results. We realize that a 1-mg concentration is not physiologically relevant and is not indicative of how much sialic acid the pneumococci were actually exposed to. We estimate that a small percentage of the free sialic acid given to the mice actually stayed in the nasopharynx. In future studies, we hope to accurately measure the amount of sialic acid in regions of bacterial colonization. Additionally, we want to see if the reduction in the amount of bound sialic acid in the sinus tissue in the nasopharynx would affect our result.
We also found that a neuraminidase mutant had a decreased ability to invade the CNS. It is possible that the ability of sialic acid to induce the expression of neuraminidase contributes to the invasion of the CNS or that the continued cleavage of sialic acid in vivo is needed for invasion. Additional studies are needed to further explore this finding.
Cytokines and chemokines play an important role in the pathogenesis of bacterial meningitis. In clinical and research settings, they are often used as an indication of meningitis. In our study, we analyzed the expression of key cytokines that are important indicators of pneumococcal meningitis. TNF-α and IL-1β are important early-response proinflammatory cytokines that have been shown to be present at increased levels in the CSF of patients with bacterial meningitis and linked to attracting leukocytes to the subarachnoid space (4, 41). We did not see an increased expression level of TNF-α or IL-1β (Fig. 5D) in the olfactory bulbs of infected animals. It is possible that the lack of an increase is due to timing, as the expression levels of TNF-α and IL-1β are highest in the first 24 h postinfection, or due to the elevated levels of IL-10, which can directly inhibit IL-1β and TNF-α (4). This can also be due to the intact blood-brain barrier. With less leukocyte infiltration, one would see less of the cascade that promotes IL-1β and TNF-α expression. We saw increased KC, MIP-1α, and IL-1α levels. IL-1α contains a nuclear localization sequences and is found in the nucleus of cells. IL-1α is released during cell damage or death and initiates early inflammatory responses. KC and MIP are functional homologs of IL-8, and their levels are increased during acute-phase pneumococcal meningitis in mice (4, 39, 41). The cytokine profiles have some differences from what has been reported previously. The discrepancies could be an artifact of nonhematogenous infection, but more information is needed before a definitive conclusion can be drawn.
It is important to note that we do not feel that nonhematogenous invasion of the CNS is the only way, or even the primary way, in which bacterial meningitis can be established. Previous studies revealed that in most cases, patients with meningitis are bacteremic, which suggests that the majority of cases of bacterial meningitis occur through hematogenous infection (51–53). However, our results support the existence of an alternative pathway by which pneumococci can reach the brain and that this pathway is somehow activated by sialic acid.
In conclusion, our findings suggest that sialic acid, specifically Neu5Ac, may prompt Streptococcus pneumoniae to leave its niche in the nasopharynx and invade sterile sites such as the sinus tissue and olfactory bulbs. It seems likely that pneumococci may pass from the nasal olfactory epithelium, present in our nasal wash samples, to the olfactory bulbs and then the remainder of the brain. This work corroborates evidence that physiological changes can prompt pneumococci to change from an asymptomatic colonizer to an invasive pathogen (16). It is possible that coinfection with viruses or bacteria that also possess a neuraminidase could create a high level of free sialic acid in the environment, which could be a precursor for more severe disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kurt Zinn, Division Director of Advanced Medical Imaging Research at the University of Alabama at Birmingham, for training and the use of the in vivo imaging systems. We also thank Chad Steele, University of Alabama at Birmingham, for his help with the cytokine assay. Finally, we thank Frederik W. “Frits” van Ginkel, who passed away suddenly this year. This work would not have been possible without his pioneering vision, and he will surely be missed.
These studies were supported by NIH grant R01 AI021458 (D.E.B.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01514-15.
REFERENCES
- 1.Shak JR, Vidal JE, Klugman KP. 2013. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol 21:129–135. doi: 10.1016/j.tim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox CM, Link-Gelles R. 2014. Chapter 11, Pneumococcal. In Roush SW, Baldy LM (ed), Manual for the surveillance of vaccine-preventable diseases. CDC, Atlanta, GA: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html Accessed 27 May 2016. [Google Scholar]
- 3.Iovino F, Orihuela CJ, Moorlag HE, Molema G, Bijlsma JJ. 2013. Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS One 8:e68408. doi: 10.1371/journal.pone.0068408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. 2011. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 24:557–591. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams AE, Blakemore WF. 1990. Pathogenesis of meningitis caused by Streptococcus suis type 2. J Infect Dis 162:474–481. doi: 10.1093/infdis/162.2.474. [DOI] [PubMed] [Google Scholar]
- 6.van Ginkel FW, McGhee JR, Watt JM, Campos-Torres A, Parish LA, Briles DE. 2003. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc Natl Acad Sci U S A 100:14363–14367. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra A, Brigham D. 2001. Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect Immun 69:7318–7325. doi: 10.1128/IAI.69.12.7318-7325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjolinder H, Jonsson AB. 2010. Olfactory nerve—a novel invasion route of Neisseria meningitidis to reach the meninges. PLoS One 5:e14034. doi: 10.1371/journal.pone.0014034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbert RP, Harris J, Chong KP, Chapman J, West AK, Chuah MI. 2012. Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised primary olfactory pathway. J Neuroinflammation 9:109. doi: 10.1186/1742-2094-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarolim KL, McCosh JK, Howard MJ, John DT. 2000. A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J Parasitol 86:50–55. doi: 10.1645/0022-3395(2000)086[0050:ALMSOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 12.Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K. 1999. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. QJM 92:151–157. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]
- 13.Garges HP, Moody MA, Cotten CM, Smith PB, Tiffany KF, Lenfestey R, Li JS, Fowler VG Jr, Benjamin DK Jr. 2006. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 117:1094–1100. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 14.Coant PN, Kornberg AE, Duffy LC, Dryja DM, Hassan SM. 1992. Blood culture results as determinants in the organism identification of bacterial meningitis. Pediatr Emerg Care 8:200–205. doi: 10.1097/00006565-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Tacon CL, Flower O. 2012. Diagnosis and management of bacterial meningitis in the paediatric population: a review. Emerg Med Int 2012:320309. doi: 10.1155/2012/320309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marks LR, Davidson BA, Knight PR, Hakansson AP. 2013. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 4:e00438-13. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer R, Camilli A. 2007. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol Microbiol 66:1–13. doi: 10.1111/j.1365-2958.2007.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer R, Baliga NS, Camilli A. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J Bacteriol 187:8340–8349. doi: 10.1128/JB.187.24.8340-8349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King SJ. 2010. Pneumococcal modification of host sugars: a major contributor to colonization of the human airway? Mol Oral Microbiol 25:15–24. doi: 10.1111/j.2041-1014.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 20.Coats MT, Murphy T, Paton JC, Gray B, Briles DE. 2011. Exposure of Thomsen-Friedenreich antigen in Streptococcus pneumoniae infection is dependent on pneumococcal neuraminidase A. Microb Pathog 50:343–349. doi: 10.1016/j.micpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, Suzuki A, Wynshaw-Boris A, Ryan AF, Gallo RL, Varki N, Varki A. 2007. N-Glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol 27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trappetti C, Kadioglu A, Carter M, Hayre J, Iannelli F, Pozzi G, Andrew PW, Oggioni MR. 2009. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J Infect Dis 199:1497–1505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- 23.Marion C, Burnaugh AM, Woodiga SA, King SJ. 2011. Sialic acid transport contributes to pneumococcal colonization. Infect Immun 79:1262–1269. doi: 10.1128/IAI.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gualdi L, Hayre JK, Gerlini A, Bidossi A, Colomba L, Trappetti C, Pozzi G, Docquier JD, Andrew P, Ricci S, Oggioni MR. 2012. Regulation of neuraminidase expression in Streptococcus pneumoniae. BMC Microbiol 12:200. doi: 10.1186/1471-2180-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel SJ, Roche AM, Weiser JN. 2014. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe 16:55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short KR, Reading PC, Brown LE, Pedersen J, Gilbertson B, Job ER, Edenborough KM, Habets MN, Zomer A, Hermans PW, Diavatopoulos DA, Wijburg OL. 2013. Influenza-induced inflammation drives pneumococcal otitis media. Infect Immun 81:645–652. doi: 10.1128/IAI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullers JA, Bartmess KC. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 28.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog 23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 29.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun 60:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yother J, Handsome GL, Briles DE. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol 174:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avery OT, Macleod CM, McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med 79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaniel LS, Yother J, Vijayakumar M, McGarry L, Guild WR, Briles DE. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med 165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry AM, Paton JC. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun 68:133–140. doi: 10.1128/IAI.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lock RA, Paton JC, Hansman D. 1988. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog 4:33–43. doi: 10.1016/0882-4010(88)90046-0. [DOI] [PubMed] [Google Scholar]
- 35.Daniels CC, Coan P, King J, Hale J, Benton KA, Briles DE, Hollingshead SK. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect Immun 78:2163–2172. doi: 10.1128/IAI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis 190:1661–1669. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- 37.Zinn KR, Chaudhuri TR, Szafran AA, O'Quinn D, Weaver C, Dugger K, Lamar D, Kesterson RA, Wang X, Frank SJ. 2008. Noninvasive bioluminescence imaging in small animals. ILAR J 49:103–115. doi: 10.1093/ilar.49.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M Jr, Verma S. 2013. Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in West Nile virus encephalitis. J Virol 87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. 2015. The interleukin (IL)-1 cytokine family—balance between agonists and antagonists in inflammatory diseases. Cytokine 76:25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Barichello T, Pereira JS, Savi GD, Generoso JS, Cipriano AL, Silvestre C, Petronilho F, Dal-Pizzol F, Vilela MC, Teixeira AL. 2011. A kinetic study of the cytokine/chemokines levels and disruption of blood-brain barrier in infant rats after pneumococcal meningitis. J Neuroimmunol 233:12–17. doi: 10.1016/j.jneuroim.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Mook-Kanamori B, Geldhoff M, Troost D, van der Poll T, van de Beek D. 2012. Characterization of a pneumococcal meningitis mouse model. BMC Infect Dis 12:71. doi: 10.1186/1471-2334-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briles DE, Hollingshead SK, Paton JC, Ades EW, Novak L, van Ginkel FW, Benjamin WH Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J Infect Dis 188:339–348. doi: 10.1086/376571. [DOI] [PubMed] [Google Scholar]
- 43.Coutinho LG, Grandgirard D, Leib SL, Agnez-Lima LF. 2013. Cerebrospinal-fluid cytokine and chemokine profile in patients with pneumococcal and meningococcal meningitis. BMC Infect Dis 13:326. doi: 10.1186/1471-2334-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchiyama S, Carlin AF, Khosravi A, Weiman S, Banerjee A, Quach D, Hightower G, Mitchell TJ, Doran KS, Nizet V. 2009. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med 206:1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Toole RD, Goode L, Howe C. 1971. Neuraminidase activity in bacterial meningitis. J Clin Invest 50:979–985. doi: 10.1172/JCI106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filippidis A, Fountas KN. 2009. Nasal lymphatics as a novel invasion and dissemination route of bacterial meningitis. Med Hypotheses 72:694–697. doi: 10.1016/j.mehy.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 47.McGavern DB, Kang SS. 2011. Illuminating viral infections in the nervous system. Nat Rev Immunol 11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macedo-Ramos H, Campos FS, Carvalho LA, Ramos IB, Teixeira LM, De Souza W, Cavalcante LA, Baetas-da-Cruz W. 2011. Olfactory ensheathing cells as putative host cells for Streptococcus pneumoniae: evidence of bacterial invasion via mannose receptor-mediated endocytosis. Neurosci Res 69:308–313. doi: 10.1016/j.neures.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz-Mendoza S, Macedo-Ramos H, Santos FA, Quadros-de-Souza LC, Paiva MM, Pinto TC, Teixeira LM, Baetas-da-Cruz W. 2016. Streptococcus pneumoniae infection regulates expression of neurotrophic factors in the olfactory bulb and cultured olfactory ensheathing cells. Neuroscience 317:149–161. doi: 10.1016/j.neuroscience.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Severi E, Hood DW, Thomas GH. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 153:2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 51.Fuglsang-Damgaard D, Pedersen G, Schonheyder HC. 2008. Positive blood cultures and diagnosis of bacterial meningitis in cases with negative culture of cerebrospinal fluid. Scand J Infect Dis 40:229–233. doi: 10.1080/00365540701642161. [DOI] [PubMed] [Google Scholar]
- 52.Kim KS, Wass CA, Cross AS. 1997. Blood-brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp Neurol 145:253–257. doi: 10.1006/exnr.1997.6458. [DOI] [PubMed] [Google Scholar]
- 53.Saez-Llorens X, McCracken GH Jr. 2003. Bacterial meningitis in children. Lancet 361:2139–2148. doi: 10.1016/S0140-6736(03)13693-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.