Abstract
Despite the extensive in vitro characterization of CPAF (chlamydial protease/proteasome-like activity factor), its role in chlamydial infection and pathogenesis remains unclear. We now report that a Chlamydia trachomatis strain deficient in expression of CPAF (L2-17) is no longer able to establish a successful infection in the mouse lower genital tract following an intravaginal inoculation. The L2-17 organisms were cleared from the mouse lower genital tract within a few days, while a CPAF-sufficient C. trachomatis strain (L2-5) survived in the lower genital tract for more than 3 weeks. However, both the L2-17 and L2-5 organisms maintained robust infection courses that lasted up to 4 weeks when they were directly delivered into the mouse upper genital tract. The CPAF-dependent chlamydial survival in the lower genital tract was confirmed in multiple strains of mice. Thus, we have demonstrated a critical role of CPAF in promoting C. trachomatis survival in the mouse lower genital tracts. It will be interesting to further investigate the mechanisms of the CPAF-dependent chlamydial pathogenicity.
INTRODUCTION
Chlamydia trachomatis is a leading cause of sexually transmitted bacterial infection. Following the initial infection in the lower genital tract, the chlamydial organisms can ascend to the upper genital tracts. The upper genital tract infection may cause pathologies such as hydrosalpinx, leading to severe sequelae, including ectopic pregnancy and tubal factor infertility (1, 2). It remains unknown how C. trachomatis establishes a successful initial infection in the lower genital tract and achieves ascending infection. The mouse genital tract infection model has been used with either C. trachomatis (3–7) or C. muridarum (8–15) for studying chlamydial pathogenesis and immunity. C. trachomatis infection in the mouse genital tract, unlike C. muridarum infection, often fails to induce a long-lasting upper genital tract pathology such as hydrosalpinx. Furthermore, innate immunity alone appeared to be sufficient for controlling the infection (16). Nevertheless, following an intravaginal inoculation, the C. trachomatis organisms were found to survive in the lower genital tract for weeks and invasion of the uterine endometrial epithelia was also detected (3, 5). When C. trachomatis was directly introduced into the mouse endometrial epithelia via a transcervical or intrauterine inoculation (bypassing the cervical barrier), the organisms were able to induce more-robust inflammatory responses and immunity in the genital tract (7, 17). Thus, mouse genital tract infection with C. trachomatis via either intravaginal or intrauterine inoculation has been used for investigating C. trachomatis pathogenesis.
Using the C. trachomatis genital tract infection mouse model, Sturdevant et al. (3) correlated mutations in the hypothetical open reading frame (ORF) CT135 with the survival/infectivity of C. trachomatis serovar D in the mouse genital tracts. However, the mechanisms by which CT135 contributes to the pathogenesis of C. trachomatis serovar D remain unknown. Ramsey et al. (6) also used the mouse model for defining the role of the plasmid-encoded Pgp3 in the survival of C. trachomatis in the mouse genital tract. This finding was validated in a C. muridarum infection mouse model in which a Pgp3-deficient C. muridarum strain was no longer able to induce hydrosalpinx (18). Pgp3 is an immunodominant antigen that is secreted into the cytosol of the infected cells (19–21). Many other C. trachomatis proteins, including CT311 (22, 23), CT621/622 (24, 25), CT795 (26), and cHtrA (27) as well as GlgA (28), all encoded by hypothetical ORFs, have also been localized in the cytosol of the infected cells. However, the functions of these proteins are largely unknown.
Another well-characterized chlamydial secretion protein is the serine protease CPAF (chlamydial protease/proteasome-like activity factor). Although CPAF was initially discovered as a consequence of its ability to robustly degrade intracellular proteins of the infected cells (29–31), the extent to which these host targets are degraded during infection is a subject of debate (32–37). CPAF is a unique serine protease (38–42) that is secreted into the host cell cytosol via a sec-dependent type II secretion pathway (34, 43). We have recently validated the secretion of CPAF into the host cell cytosol (44). To further investigate the role of CPAF in chlamydial pathogenesis, we took advantage of chemically derived C. trachomatis L2 mutants (34, 45, 46) and compared a CPAF-deficient C. trachomatis strain to a control strain derived by lateral gene transfer (34) for survival in the mouse genital tract. We found that the CPAF-deficient C. trachomatis strain lost its ability to establish a successful infection in the mouse lower genital tract following an intravaginal inoculation whereas its ability to infect the upper genital tract was not significantly affected. This result indicates that CPAF preferentially promotes C. trachomatis survival in the mouse lower genital tract.
MATERIALS AND METHODS
Cell culture and chlamydial organisms.
HeLa cells (human cervical epithelial carcinoma cells) (ATCC CCL2; ATCC, Manassas, VA) were grown and maintained in Dulbecco modified Eagle medium (DMEM; Sigma, Saint Louis, MO) containing 10% fetal calf serum (FBS; Gemini Bio-Products, West Sacramento, CA) in a humidified incubator in the presence of 5% CO2. The C. trachomatis L2 wild-type organisms (strain L2/LGV-434/Bu [designated L2wt]) were purchased from ATCC. The CPAF-deficient C. trachomatis L2 organisms (designated L2-17) and the corresponding control CPAF-sufficient strain of L2-5 were provided by R. H. Valdivia (34). The pGFP::SW2 plasmid expressing a wild-type allele of CPAF or mCherry was transformed into L2-17 to produced L2-17/CPAF or L2-17/mCherry, respectively (44). All chlamydial organisms were propagated, purified, divided into aliquots, and stored as described elsewhere (47).
Mouse infection and live-organism shedding.
Female C57BL/6J (Jackson stock number 000664), C3H/HeJ (000659), and BALB/cJ (000651) mice were all purchased from Jackson Laboratories (Bar Harbor, ME). Mice were subjected to intravaginal or intrauterine inoculation with either 1.0 × 106 or 5.0 × 106 inclusion-forming units (IFUs) of the corresponding chlamydial organisms as indicated in individual experiments. Five days prior to infection, each mouse was injected subcutaneously with 2.5 mg medroxyprogesterone (Depo-Provera; Pharmacia Upjohn, Kalamazoo, MI). To monitor live-organism shedding from the lower genital tract, vaginal/cervical swabs were taken every 2 to 4 days for the first 3 weeks and weekly thereafter until negative shedding results were observed for 2 consecutive time points. The live chlamydial organisms in each swab were quantitated using an immunofluorescence assay as described previously (48). The calculated total number of IFUs per swab was converted into a log10 value and used to calculate the mean and standard deviation or median.
Genital tract pathology.
To evaluate the genital tract pathology, mice were sacrificed on days 21 to 60 postinfection for collecting genital tract tissues for histological scoring of inflammatory infiltration as described elsewhere (49). The hematoxylin-and-eosin (H&E)-stained sections were scored for inflammatory cell infiltrations by researchers who were blind to the mouse group designations, and the entire genital tract, including vaginal tissue, vaginal lumen, uterine tissue, uterine lumen, oviduct tissue, and oviduct lumen, was evaluated using scoring criteria described previously (10, 50) as follows: 0, no significant infiltration; 1, infiltration at a single focus; 2, infiltration at two to four foci; 3, infiltration at more than four foci; 4, confluent infiltration. Scores from left and right uterine horns or oviducts of the same mouse were added to represent the scores for a given mouse.
Statistical analyses.
The Wilcoxon rank sum test was used for analyzing both the log10 IFU shedding titers and the pathology scores.
RESULTS
CPAF is required for chlamydial survival in the mouse lower genital tract.
In the comparisons of L2-17 to L2-5 (34) or L2wt for determinations of survival rates in the lower genital tract following an intravaginal inoculation (Fig. 1), the L2-17 organisms were shown to have rapidly cleared from the lower genital tract with a minimal level of recoverable live organisms on days 3 and 7 (a) whereas L2-5 (b) and L2wt (c) continued to shed significant levels of live organisms for at least 14 days after inoculation. These results suggest that CPAF plays a significant role in promoting chlamydial survival in the mouse lower genital tracts of the mouse. When the genital tract tissues from the infected mice were examined for inflammatory infiltration (Fig. 2), mice intravaginally with L2-17 displayed a minimal level of inflammatory infiltration in the uterine tissues with an inflammatory score significantly lower than those displayed by mice infected with L2-5 or L2wt, indicating that the L2-17 organisms failed to stimulate inflammation in the uterine tissues, which is consistent with the observation that the L2-17 organisms were rapidly cleared in the mouse lower genital tract upon inoculation. It is worth noting that all mice developed an inflammatory infiltration score of ∼2 in the oviduct tissue regardless of the organisms used for infection. This universal level may reflect a general background in these mice and does not necessarily indicate tubal infection. It is not clear exactly how all of the tubal tissue sections came to display the elevated levels of inflammatory infiltrates. The similar distribution characteristics of the infiltrates in all mice suggest that the inflammation may not have been caused by the tubal infection since it is very difficult to obtain consistent tubal infection using intravaginal inoculation even with the wild-type L2 organisms.
FIG 1.
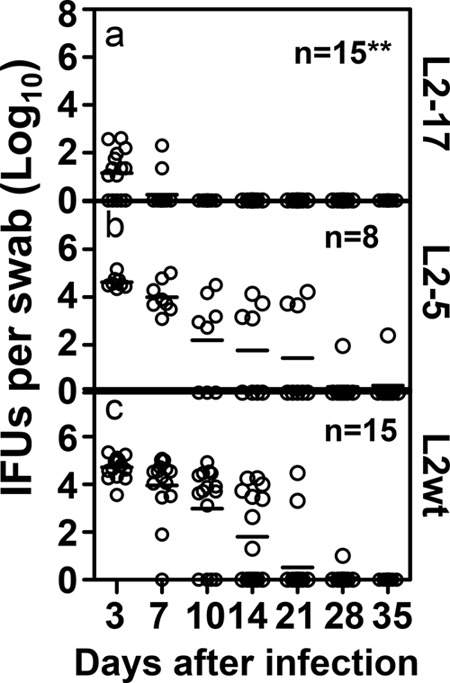
Effect of CPAF deficiency on chlamydial survival/infectivity in the lower genital tracts of mice following an intravaginal inoculation. Seven-week-old C3H/HeJ female mice were each inoculated with 5 × 106 inclusion-forming units (IFUs) of L2-17 (panel a, n = 15), L2-5 (panel b, n = 8), or L2wt (panel c, n = 15) intravaginally. On different days after inoculation as indicated along the x axis, vaginal swabs were taken for monitoring recovery of live chlamydial organism. The recovered live organisms were expressed as log10 IFUs per swab and presented individually (open circles) along the y axis. Each horizontal bar represents the median for each group. The data came from 3 independent experiments. Note that mice intravaginally inoculated with L2-17 displayed a significantly lower level of live-organism recovery than the mice similarly infected with L2-5 or L2wt (Wilcoxon; **, P < 0.01).
FIG 2.
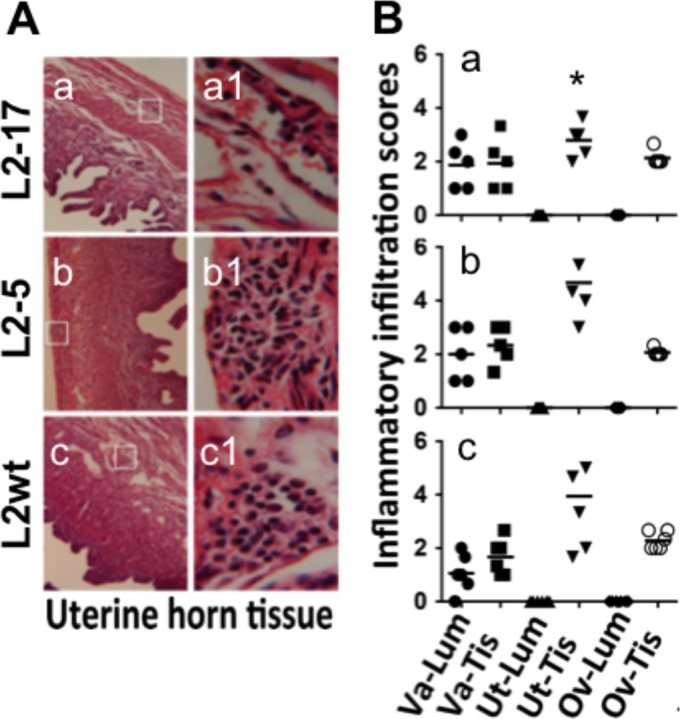
Effect of CPAF deficiency on inflammatory infiltration in the genital tracts of mice following an intravaginal inoculation. (A) The mice described in the Fig. 1 legend were sacrificed on days 21 to 60 postinfection, and the genital tracts were processed for microscopic detection of inflammatory infiltration. Five mice from each group sacrificed on or close to day 21 were selected for the pathology analysis. The mouse sacrifice dates were matched among the 3 groups. Representative images of uterine horn tissue from each group of mice were taken under 10× (a to c) and 100× (a1 to c1) objective lenses. The areas covered under the 100× lens are marked with white rectangles in the corresponding 10× images. (B) Different sections of the genital tract, including the lumen (Lum) and tissue (Tis) of vagina (Va), uterine/uterine horns (Ut), and oviducts (Ov), were semiquantitatively scored for inflammatory infiltration based on the criteria described in Materials and Methods. The inflammatory scores from each mouse were plotted individually along the y axis. Solid circles stand for the inflammatory scores from Va-Lum, solid squares for scores from Va-Tis, solid standard triangles for scores from Ut-Lum, solid inverted triangles for scores from Ut-Tis, solid diamonds for scores from Ov-Lum, and open circles for scores from Ov-Tis. Note that the inflammatory scores indicated for the uterine/uterine horn tissues (Ut-Tis) of mice intravaginally inoculated with L2-17 (a) are significantly lower (Wilcoxon; *, P < 0.05) than those indicated for mice similarly inoculated with either L2-5 (b) or L2wt (c).
CPAF is not important for chlamydial survival in the mouse upper genital tract.
When the L2-17 or L2-5 organisms were directly delivered into the upper genital tract via an intrauterine inoculation (bypassing the cervical barrier), all mice continuously shed live organisms for at least 4 weeks (Fig. 3). Although the infection represented by the overall shedding time course of L2-17 was less robust than that seen with L2-5 following the intrauterine inoculation, the reduction was not significant. Thus, we can conclude that CPAF is a more significant contributor to chlamydial survival in the lower than in the upper genital tract. Consistently, after an intrauterine inoculation, both L2-17 and L2-5 induced robust inflammation in the endometrial tissue (Fig. 4), confirming that CPAF was not required for chlamydial survival and stimulation of inflammation after the organisms were delivered to the endometrial tissue.
FIG 3.
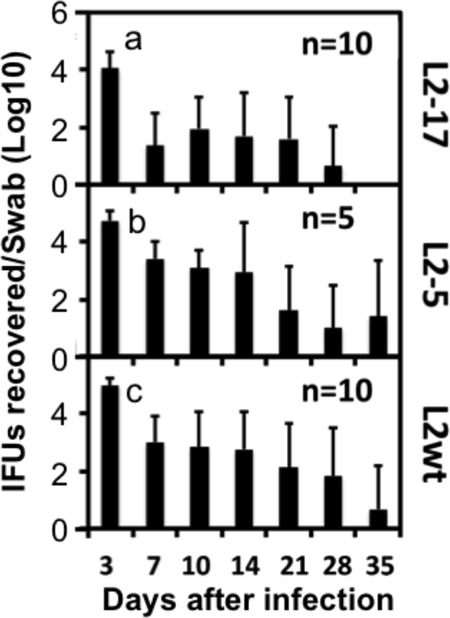
Effect of CPAF deficiency on chlamydial survival in the lower genital tracts of mice following an intrauterine inoculation. Seven-week-old C3H/HeJ female mice were each infected with 5 × 106 IFUs of L2-17 (panel a; n = 10), L2-5 (panel b; n = 5), or L2wt (panel c; n = 10) via an intrauterine inoculation. On different days after inoculation as indicated along the x axis, vaginal swabs were taken for monitoring recovery of live chlamydial organisms. The recovered levels of live organisms are expressed as the number of log10 inclusion-forming units (IFUs) per swab as shown along the y axis. No significant difference in levels of live-organism shedding was found between the groups (Wilcoxon).
FIG 4.
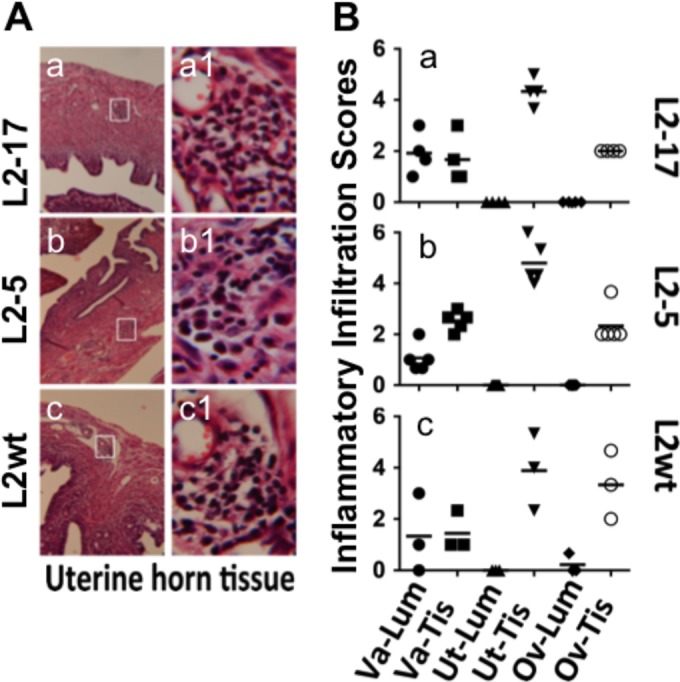
Effect of CPAF deficiency on inflammatory infiltration in the mouse genital tracts following an intrauterine inoculation. (A) The mice described in the Fig. 3 legend were sacrificed on days 21 to 60 postinfection, and the genital tracts were processed for microscopic detection of inflammatory infiltration. Representative images of uterine horn tissue from each group of mice were taken under 10× (a to c) and 100× (a1 to c1) objective lenses. The areas covered under the 100× lens are marked with white rectangles in the corresponding 10× images. (B) Different sections of the genital tract, including the lumen (Lum) and tissue (Tis) of vagina (Va), uterine/uterine horns (Ut), and oviducts (Ov), were semiquantitatively scored for inflammatory infiltration based on the criteria described in Materials and Methods. The inflammatory scores from each mouse were plotted individually along the y axis. Solid circles stand for the inflammatory scores from Va-Lum, solid squares for scores from Va-Tis, solid upright triangles for scores from Ut-Lum, solid upside triangles for scores from Ut-Tis, solid diamonds for scores from Ov-Lum, and open circles for scores from Ov-Tis. No significant differences in inflammatory infiltration scores were found between the groups (Wilcoxon).
The CPAF-dependent chlamydial survival in the lower genital tract is reproduced in multiple mouse strains, and the CPAF deficiency is rescued with a plasmid-encoded CPAF.
C57BL/6J mice are known to be more resistant to chlamydial infection than C3H/HeJ mice (51). C57BL/6J mice tend to develop Th1-dominant adaptive immunity upon chlamydial infection, while BALB/c mice develop Th2-dominant adaptive immunity (52). We then compared the survival rates seen with L2-17 and L2-5 in the lower genital tracts of C57BL/6J, C3H/HeJ, and BALB/c mice (Fig. 5). The L2-17 organisms failed to survive in the lower genital tracts regardless of the mouse strains tested. The organisms were cleared from the mouse lower genital tracts within a few days of the inoculation. These observations suggest that the lower genital tract factors responsible for clearing L2-17 infection are shared among different mouse strains. To further validate whether the failure of L2-17 to survive in the mouse lower genital tract is mainly due to lack of CPAF, we complemented L2-17 with a plasmid-encoded wild-type CPAF to produce the transformant L2-17/CPAF. We found that the L2-17/CPAF organisms showed significantly increased survival in the mouse lower genital tract (Fig. 6). As a control, the L2-17 organisms were similarly transformed with a mCherry-expressing plasmid. The L2-17/mCherry organisms remained defective in survival in the mouse lower genital tract.
FIG 5.
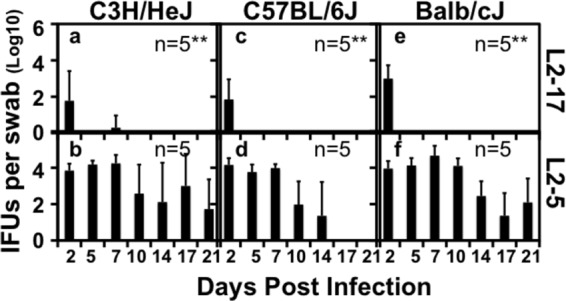
CPAF-dependent chlamydial survival in the lower genital tracts of multiple strains of mice. Six-week-old female C3H/HeJ (a and b; n = 5 for each group), C57BL/6J (c and d; n = 5), and BALB/cJ (e and f; n = 5) mice were intravaginally inoculated with 106 IFUs of L2-17 (a, c, and e) or L2-5 (b, d, and f). Vaginal/cervical swabs were taken on different days after the inoculation as indicated along the x axis for monitoring live-organism recovery as shown along the y axis. Note that the L2-17 organisms displayed significantly lower levels of live-organism recovery than the L2-5 organisms regardless of the strains of mice infected (Wilcoxon; **, P < 0.01).
FIG 6.
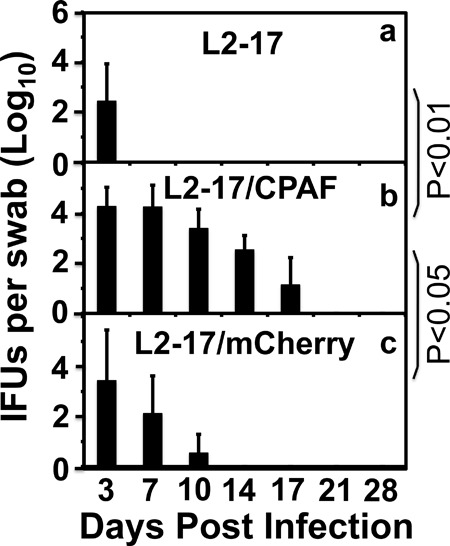
CPAF complementation effect on chlamydial survival in mouse lower genital tract. Seven-week-old C57BL/6J female mice were each inoculated with 1 × 106 IFUs of L2-17 (a; n = 5), L2-17/CPAF (b; n = 5), or L2-17/mCherry (c; n = 5) intravaginally. On different days after inoculation as indicated along the x axis, vaginal swabs were taken for monitoring recovery of live chlamydial organisms. The recovered live organisms were expressed as log10 inclusion-forming units (IFUs) per swab as shown along the y axis. Note that L2-17/CPAF developed more-extensive live-organism shedding than either L2-17 or L2-17/mCherry (P < 0.01 or P < 0.05, respectively; Wilcoxon).
DISCUSSION
In the current report, we have presented the first experimental evidence demonstrating that CPAF promotes chlamydial survival in the mouse lower genital tract but not in the upper. First, the CPAF-deficient C. trachomatis L2-17 strain failed to infect the mouse lower genital tract whereas the CPAF-sufficient L2-5 control strain was able to cause a 2-week-long infection, indicating that CPAF plays a critical role in promoting C. trachomatis survival in the mouse lower genital tract tissues. Second, the uterine tissue of mice intravaginally inoculated with L2-17 lacked significant inflammatory infiltration whereas the uterine tissue of mice similarly inoculated with L2-5 developed significant inflammatory infiltration, suggesting that L2-17 either failed to reach to the uterine tissue or was unable to provoke uterine inflammation. Third, both L2-17 and L2-5 developed robust infections in the mouse genital tracts and induced significant inflammatory infiltration in the uterine tissue when the organisms were directly delivered into the upper genital tract. These observations have confirmed that CPAF is not essential for the C. trachomatis survival and induction of inflammation in the upper genital tracts. The result described above further suggests that lack of uterine inflammation in mice intravaginally infected with L2-17 is probably due to the failure of L2-17 to reach the uterine tissue, since L2-17 is able to activate uterine inflammation when directly delivered to the uterine tissue. Fourth, the requirement for CPAF to aid in chlamydial survival in the mouse lower genital tract was reproduced in 3 different strains of mice despite their different local cytokine profiles and varied adaptive immunity phenotypes (52, 53). Finally, complementation of the L2-17 organisms with a CPAF-expressing plasmid partially restored their survival in the mouse lower genital tract whereas a similar transformation performed with a mCherry-expressing control plasmid failed to do so. The significant but partial rescue of the chlamydial infectivity by the CPAF complementation may have been a consequence of either the interference from the excessive amount of the incompletely processed CPAF fragments in the complemented strain (38, 40–42, 44) or a CPAF-independent defect inherited in the parental L2-17 strain (34). Nevertheless, the complementation experiments have demonstrated that the failure of L2-17 to survive in the mouse lower genital tract was at least partially due to the lack of CPAF.
The previously published controversial findings on CPAF degradation of cellular proteins in cell culture systems (32, 33) motivated us to use the L2-17 strain (34) for evaluating the potential functions of CPAF in the mouse genital tracts, which led us to discover an essential role of CPAF in promoting chlamydial survival in the mouse lower genital tract. This mouse model may also allow us to further investigate the mechanisms by which CPAF aids in chlamydial survival. For example, questions on whether CPAF promotes chlamydial ascension from the lower to the upper genital tract and whether CPAF aids in chlamydial infectivity in different genital tract tissues can now be addressed by simultaneously monitoring the numbers of infectious chlamydial organisms and the numbers of genome copies in multiple genital tract tissues. The next issue is that of how CPAF promotes chlamydial survival/ascension/infectivity. It has been hypothesized that CPAF accumulated in the infected host cell cytosol at the late stage of intracellular chlamydial growth (29) may be released to confront the extracellular mucosal effectors before the intrainclusion organisms are exposed to extracellular environments during host cell lysis and chlamydial spreading (33). The fact that L2-17 was cleared within a few days after inoculation suggests that the innate immunity effectors of the lower genital tract are effective for controlling the infection and that these same effectors may be targeted by CPAF. This hypothesis is consistent with the finding that innate immunity is sufficient for controlling C. trachomatis infection in the mouse genital tract (16). The CPAF-dependent chlamydial survival in the mouse lower genital tract was reproduced in 3 different strains of mice, suggesting that CPAF may target host factors in the lower genital tract shared by the different strains. It is unlikely that the different H-2 haplotypes (C57BL/6J, H-2b; C3H/HeJ, H-2k; BALB/c, H-2d) and the other strain-specific factors (51–53) are targeted by CPAF. We recently reported that CPAF selectively degraded cathelicidin LL-37 and neutralized its antichlamydial activity (54). It will be interesting to use mice deficient in CRAMP (55), a cathelin-related antimicrobial peptide and the mouse homolog of human LL-37 that is produced in all mouse genital tracts (56), to test whether the CRAMP deficiency can rescue L2-17 in the mouse lower genital tract.
When CPAF was initially discovered, attention was mainly focused on its potential role in dealing with the intracellular proteins of the infected cells (57, 58). Recent studies suggest that CPAF and other chlamydial proteins secreted into the cytoplasm of the infected cells may target extracellular molecules (33, 54). However, caution should be taken in interpreting the interactions of chlamydial factors with host factors detected in in vitro systems. The biological relevance of the in vitro observations has to be validated using mutant chlamydial organisms in animal models as demonstrated in the current study. As generation of chlamydial mutants becomes easier and animal models continue to be optimized, it is expected that more biologically relevant chlamydial pathogenic mechanisms will be uncovered. A caveat is that some of these chlamydial mutants may be highly attenuated in their growth properties, including attachment, entry, intracellular replication, and exit. The reduced survival/infectivity of the mutants in the mouse genital tract may reflect only their slow/inefficient or defective growth abilities. Indeed, L2-17 produced 3-fold-fewer infectious progeny elementary bodies (EBs) in cultured cells than L2-5 (34), which may have contributed to the significantly shortened survival of L2-17 in the mouse lower genital tract. However, when L2-17 and L2-5 were directly delivered to the upper genital tract, both maintained robust infection courses. Although the infection course of L2-17 was less robust than that of L2-5, probably due to the reduced ability of L2-17 to produce EBs (34), the difference was not significant. Thus, CPAF deficiency preferentially reduced chlamydial survival in the mouse lower but not upper genital tract. We hypothesize that in addition to the growth defect, the L2-17 organisms may also be more susceptible to the lower genital tract effectors. A test of this hypothesis is under way.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma M, Sethi S, Daftari S, Malhotra S. 2003. Evidence of chlamydial infection in infertile women with fallopian tube obstruction. Indian J Pathol Microbiol 46:680–683. [PubMed] [Google Scholar]
- 3.Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, Carlson JH, Goheen MM, Selleck EM, Martens C, Caldwell HD. 2010. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun 78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares-Zavaleta N, Whitmire W, Gardner D, Caldwell HD. 2010. Immunization with the attenuated plasmidless Chlamydia trachomatis L2(25667R) strain provides partial protection in a murine model of female genitourinary tract infection. Vaccine 28:1454–1462. doi: 10.1016/j.vaccine.2009.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigar IM, Schripsema JH, Wang Y, Clarke IN, Cutcliffe LT, Seth-Smith HM, Thomson NR, Bjartling C, Unemo M, Persson K, Ramsey KH. 2014. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog Dis 70:61–69. doi: 10.1111/2049-632X.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey KH, Schripsema JH, Smith BJ, Wang Y, Jham BC, O'Hagan KP, Thomson NR, Murthy AK, Skilton RJ, Chu P, Clarke IN. 2014. Plasmid CDS5 influences infectivity and virulence in a mouse model of Chlamydia trachomatis urogenital infection. Infect Immun 82:3341–3349. doi: 10.1128/IAI.01795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gondek DC, Olive AJ, Stary G, Starnbach MN. 2012. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol 189:2441–2449. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connell CM, Ingalls RR, Andrews CW Jr, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 13.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igietseme JU, He Q, Joseph K, Eko FO, Lyn D, Ananaba G, Campbell A, Bandea C, Black CM. 2009. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis 200:926–934. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. 2011. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun 79:2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturdevant GL, Caldwell HD. 2014. Innate immunity is sufficient for the clearance of Chlamydia trachomatis from the female mouse genital tract. Pathog Dis 72:70–73. doi: 10.1111/2049-632X.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Chen D, Zhong Y, Wang S, Zhong G. 2008. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun 76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, Zhong G. 2010. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J Bacteriol 192:6017–6024. doi: 10.1128/JB.00847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galaleldeen A, Taylor AB, Chen D, Schuermann JP, Holloway SP, Hou S, Gong S, Zhong G, Hart PJ. 2013. Structure of the Chlamydia trachomatis immunodominant antigen Pgp3. J Biol Chem 288:22068–22079. doi: 10.1074/jbc.M113.475012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei L, Dong X, Li Z, Zhong G. 2013. Identification of a novel nuclear localization signal sequence in Chlamydia trachomatis-secreted hypothetical protein CT311. PLoS One 8:e64529. doi: 10.1371/journal.pone.0064529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei L, Qi M, Budrys N, Schenken R, Zhong G. 2011. Localization of Chlamydia trachomatis hypothetical protein CT311 in host cell cytoplasm. Microb Pathog 51:101–109. doi: 10.1016/j.micpath.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong S, Lei L, Chang X, Belland R, Zhong G. 2011. Chlamydia trachomatis secretion of hypothetical protein CT622 into host cell cytoplasm via a secretion pathway that can be inhibited by the type III secretion system inhibitor compound 1. Microbiology 157:1134–1144. doi: 10.1099/mic.0.047746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobolt-Pedersen AS, Christiansen G, Timmerman E, Gevaert K, Birkelund S. 2009. Identification of Chlamydia trachomatis CT621, a protein delivered through the type III secretion system to the host cell cytoplasm and nucleus. FEMS Immunol Med Microbiol 57:46–58. doi: 10.1111/j.1574-695X.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi M, Lei L, Gong S, Liu Q, DeLisa MP, Zhong G. 2011. Chlamydia trachomatis secretion of an immunodominant hypothetical protein (CT795) into host cell cytoplasm. J Bacteriol 193:2498–2509. doi: 10.1128/JB.01301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Lei L, Gong S, Chen D, Flores R, Zhong G. 2011. The chlamydial periplasmic stress response serine protease cHtrA is secreted into host cell cytosol. BMC Microbiol 11:87. doi: 10.1186/1471-2180-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu C, Lei L, Peng B, Tang L, Ding H, Gong S, Li Z, Wu Y, Zhong G. 2013. Chlamydia trachomatis GlgA is secreted into host cell cytoplasm. PLoS One 8:e68764. doi: 10.1371/journal.pone.0068764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong G, Fan P, Ji H, Dong F, Huang Y. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med 193:935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong G, Fan T, Liu L. 1999. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J Exp Med 189:1931–1938. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong G, Liu L, Fan T, Fan P, Ji H. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J Exp Med 191:1525–1534. doi: 10.1084/jem.191.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen AL, Johnson KA, Lee JK, Sütterlin C, Tan M. 2012. CPAF: a chlamydial protease in search of an authentic substrate. PLoS Pathog 8:e1002842. doi: 10.1371/journal.ppat.1002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad TA, Yang Z, Ojcius DM, Zhong G. 2013. A path forward for the chlamydial virulence factor CPAF. Microbes Infect 15:1026–1032. doi: 10.1016/j.micinf.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snavely EA, Kokes M, Dunn JD, Saka HA, Nguyen BD, Bastidas RJ, McCafferty DG, Valdivia RH. 16 May 2014. Reassessing the role of the secreted protease CPAF in Chlamydia trachomatis infection through genetic approaches. Pathog Dis doi: 10.1111/2049-1632X.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong G. 2014. Question the questions on CPAF. Pathog Dis 72:3–4. doi: 10.1111/2049-632X.12205. [DOI] [PubMed] [Google Scholar]
- 36.Häcker G. 2014. The chlamydial protease CPAF: important or not, important for what? Microbes Infect 16:367–370. doi: 10.1016/j.micinf.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Johnson KA, Lee JK, Chen AL, Tan M, Sütterlin C. 2015. Induction and inhibition of CPAF activity during analysis of Chlamydia-infected cells. Pathog Dis 73:1–8. doi: 10.1093/femspd/ftv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Feng Y, Chen D, Wu X, Huang S, Wang X, Xiao X, Li W, Huang N, Gu L, Zhong G, Chai J. 2008. Structural basis for activation and inhibition of the secreted Chlamydia protease CPAF. Cell Host Microbe 4:529–542. doi: 10.1016/j.chom.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Chen D, Chai J, Hart PJ, Zhong G. 2009. Identifying catalytic residues in CPAF, a Chlamydia-secreted protease. Arch Biochem Biophys 485:16–23. doi: 10.1016/j.abb.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen D, Lei L, Flores R, Huang Z, Wu Z, Chai J, Zhong G. 2010. Autoprocessing and self-activation of the secreted protease CPAF in Chlamydia-infected cells. Microb Pathog 49:164–173. doi: 10.1016/j.micpath.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong F, Pirbhai M, Zhong Y, Zhong G. 2004. Cleavage-dependent activation of a chlamydia-secreted protease. Mol Microbiol 52:1487–1494. doi: 10.1111/j.1365-2958.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 42.Dong F, Sharma J, Xiao Y, Zhong Y, Zhong G. 2004. Intramolecular dimerization is required for the chlamydia-secreted protease CPAF to degrade host transcriptional factors. Infect Immun 72:3869–3875. doi: 10.1128/IAI.72.7.3869-3875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen D, Lei L, Lu C, Flores R, DeLisa MP, Roberts TC, Romesberg FE, Zhong G. 2010. Secretion of the chlamydial virulence factor CPAF requires the Sec-dependent pathway. Microbiology 156:3031–3040. doi: 10.1099/mic.0.040527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z, Tang L, Sun X, Chai J, Zhong G. 2015. Characterization of CPAF critical residues and secretion during Chlamydia trachomatis infection. Infect Immun 83:2234–2241. doi: 10.1128/IAI.00275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kokes M, Dunn JD, Granek JA, Nguyen BD, Barker JR, Valdivia RH, Bastidas RJ. 2015. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe 17:716–725. doi: 10.1016/j.chom.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen B, Valdivia R. 2014. A chemical mutagenesis approach to identify virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis. Methods Mol Biol 1197:347–358. doi: 10.1007/978-1-4939-1261-2_20. [DOI] [PubMed] [Google Scholar]
- 47.Su H, McClarty G, Dong F, Hatch GM, Pan ZK, Zhong G. 2004. Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for chlamydial acquisition of host glycerophospholipids. J Biol Chem 279:9409–9416. doi: 10.1074/jbc.M312008200. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. doi: 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. 2014. Lack of long-lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun 82:2688–2696. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Yang Z, Sun X, Tang L, Ding Y, Xue M, Zhou Z, Baseman J, Zhong G. 13 April 2015. Intrauterine infection with plasmid-free Chlamydia muridarum reveals a critical role of the plasmid in chlamydial ascension and establishes a model for evaluating plasmid-independent pathogenicity. Infect Immun doi: 10.1128/IAI.00353-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darville T, Andrews CW Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun 65:3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, HayGlass KT, Brunham RC. 1996. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol 156:4338–4344. [PubMed] [Google Scholar]
- 53.Darville T, Andrews CW Jr, Sikes JD, Fraley PL, Rank RG. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun 69:3556–3561. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang L, Chen J, Zhou Z, Yu P, Yang Z, Zhong G. 2015. Chlamydia-secreted protease CPAF degrades host antimicrobial peptides. Microbes Infect 17:402–408. doi: 10.1016/j.micinf.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 56.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. 2006. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 57.Zhong G. 2009. Killing me softly: chlamydial use of proteolysis for evading host defenses. Trends Microbiol 17:467–474. doi: 10.1016/j.tim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong G. 2011. Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways. Front Microbiol 2:14–19. doi: 10.3389/fmicb.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]


