With more than 1 million insect species described to date, it is perhaps unsurprising that at least some have come into conflict with humans. A variety of methods have been developed to control pest insects, but the application of synthetic insecticides remains one of the most widely used and effective approaches. Unfortunately, the inevitable result of the overuse of insecticides for the control of many pest species has been the evolution of resistance (1, 2). The evolutionary consequences of insecticide misuse are particularly well illustrated in the case of malaria-carrying Anopheles mosquitoes in Sub-Saharan Africa (2). Modern malaria control strategies rely heavily on the use of insecticides to suppress Anopheles populations, primarily applied as either insecticide-treated bed nets (ITNs) or as indoor residual sprays (IRSs). Between 2000 and 2015, the number of deaths due to malaria halved, 80% of which was attributed to the scale-up of these insecticide-based vector control interventions (3). These promising gains are now, however, under threat. The use of just a single insecticide class, the pyrethroids, in ITNs has created huge selection pressure for resistance to evolve, and resistance to pyrethroids in Anopheles mosquitoes is now widespread, reaching unprecedented levels in parts of West Africa (2). To make matters worse, although insecticides with different modes of action are available for use in IRSs, mosquito populations have now emerged that exhibit resistance to multiple classes (2, 4). In PNAS, Balabanidou et al. (5) use an impressive array of approaches to unravel the molecular basis of resistance in a strain of Anopheles gambiae from West Africa that exhibits such a multiresistance profile.
Over the past 50 y, significant advances have been made in our understanding of how insects evolve resistance to insecticides at the molecular level (6). Two mechanisms have been most frequently described: enhanced insecticide metabolism/sequestration by detoxification enzymes and mutations in insecticide target sites that alter the affinity of insecticide binding. Reduced penetration of insecticide through the insect cuticle has been proposed as an alternative resistance mechanism for some time, but much less frequently reported. Just a handful of studies have associated changes in insecticide penetration or cuticular thickness with resistance (7–9), and the precise mechanisms that result in changes in cuticular structure or composition are essentially unknown.
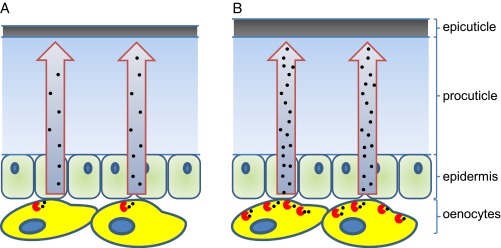
The insect cuticle is composed of the polysaccharide chitin, proteins, and lipids that form two primary layers: the inner procuticle, containing chitin, and the thin outer epicuticle, which is chitin-free (Fig. 1). Insects prevent desiccation by depositing cuticular hydrocarbons (CHCs), synthesized in specialized secretory cells in the epidermis called oenocytes, on their epicuticle as effective waterproofing agents. Previously in PNAS, Qiu et al. (10) demonstrated that in Drosophila and the housefly, Musca domestica, the final step of CHC synthesis is catalyzed by cytochrome P450 enzymes of the CYP4G subfamily (CYP4G1 and CYP4G2, respectively) that are highly expressed in oenocytes. This finding was of significant interest to the community working on insecticide resistance in anophelines because the potential functional orthologs of these genes in mosquitoes, CYP4G16 and CYP4G17, are overexpressed in resistant populations of both An. arabiensis and An. gambiae (11, 12), suggesting a possible link with cuticular-based resistance.
Fig. 1.
Hypothetical model for cuticular resistance in Anopheles mosquitoes. Cartoon cross-sections of the integument of an insecticide-susceptible mosquito and an insecticide-resistant mosquito, respectively, are shown. The cuticle comprises the relatively thin CHC-rich epicuticle and the thicker procuticle. CHCs (represented by black dots) are produced in specialized cells called oenocytes found associated with the epidermis or fat body. The final step of CHC synthesis is catalyzed by CYP4G P450s (represented by red circles), with CHCs then transported to the epicuticle. Resistant An. arabiensis mosquitoes (B) produce up to fourfold more CYP4G16 than susceptible mosquitoes (A), and this finding may explain the enhanced CHC synthesis and thickening of the epicuticle observed in resistant mosquitoes.
Balabanidou et al. (5) build on these studies by conducting one of the most comprehensive analyses of penetration resistance in insects described to date. The authors first demonstrated clear differences in the uptake of the pyrethroid deltamethrin through the cuticle of resistant and susceptible strains of An. gambiae. By measuring radiolabeled deltamethrin extracted from the cuticle with deltamethrin extracted from the whole mosquito body, they showed that the amount of internalized deltamethrin was ∼50% lower in resistant compared with susceptible mosquitoes. Changing from an oil- to acetone-based formulation of insecticide, to circumvent the lipid barriers of the cuticle, led to a 50% decrease in the resistance ratio between the two strains, suggesting differences in the lipid component of the cuticle are, at least partially, responsible for the observed resistance. Having established cuticular resistance in a multiple insecticide-resistant strain of An. gambiae, Balabanidou et al. (5) exploited a diverse range of methods to examine if differences in the structure or composition of the cuticle are associated with resistance. Transmission electron microscopy revealed a significantly enhanced thickness of the cuticle in resistant mosquitoes, with the outer epicuticle explaining much of the overall difference in total cuticle thickness. Crucially, gas chromatography and mass spectrometry showed that although there were no qualitative differences in CHC profiles between the two strains, a significant increase (29%) was observed in the CHC content of resistant mosquitoes. Taken together, these data provide significant advances in our understanding of cuticular resistance. First, they suggest the underlying basis may be due to quantitative rather than qualitative changes in chemical composition of the cuticle. Second, they highlight which parts of the insect cuticle might be most important in providing protection from insecticides by revealing that the greatest changes occur in the epicuticle, which contains the protective CHC-rich wax layer of the cuticle (Fig. 1).
What genetic changes underlie the increase in thickness of the epicuticle and the higher levels of CHCs observed in resistant An. gambiae mosquitoes? Because of prior work implicating CYP4G P450s in CHC biosynthesis and resistance (10, 12), Balabanidou et al. (5) then turned their focus to the two members of this P450 subfamily found in Anopheles mosquitoes. Using antibodies raised against CYP4G16 and CYP4G17 in immunofluorescence microscopy, they revealed that both proteins are highly abundant in oenocyte cells, the primary site of CHC synthesis. Interestingly, contrasting subcellular locations were observed for the two P450s; whereas CYP4G17 was found throughout the intracellular compartment of the cell, CYP4G16 showed a striking association with the cell periphery, predominantly associated with the cytoplasmic side of the cell membrane. The localization of CYP4G16 to the plasma membrane, or the distal endoplasmic reticulum (ER) associated with it, is intriguing and has not been reported previously for a P450. Balabanidou et al. (5) speculate that the location of CYP4G16 may be related to its role in hydrocarbon synthesis (discussed below), facilitating the swift export of hydrocarbons from the cell membrane of oenocytes for transport to the cuticle, and, given the novelty of this finding, this hypothesis clearly warrants further more detailed exploration.
Functional characterization of CYP4G16 and CYP4G17 by heterologous expression in an insect cell line demonstrated that CYP4G16 can catalyze a long-chain aldehyde substrate to hydrocarbon, and is thus a bona fide oxidative decarbonylase. Expression of insect P450s in vitro is nontrivial, especially in the case of CYP4 family P450s, and attempts to characterize CYP4G17 functionally were not possible due to inconsistent expression. Regardless, these findings, along with work by Qiu et al. (10), clearly highlight the important physiological role of this subfamily of P450s in CHC biosynthesis. More generally, such studies are important because although a great number of P450s have been implicated in the metabolism of natural and synthetic xenobiotics (i.e., detoxification), the endogenous substrates of the majority of insect P450s are unknown.
The work by Balabanidou et al. (5) represents a major advancement in our understanding of cuticular resistance, revealing the specific changes that occur in the cuticle of resistant mosquitoes and significantly progressing previous studies to provide additional evidence of a role for the CYP4G subfamily of P450s in this process. Given the complexity of cuticular biosynthesis, it is unsurprising that additional questions remain to be answered. First, the precise role of CYP4G16/17 in cuticular resistance in mosquitoes demands further investigation. In particular, the data provided in this study, as well as those data provided previously, are yet to link the overexpression of these P450s directly with resistance mediated by enhanced production of CHCs/thickness of the epicuticle. Balabanidou et al. (5) attempted to demonstrate causality using RNAi, but the results were inconclusive. An alternative route would be to exploit transgenic approaches to overexpress these genes and examine the effect on resistance. Overexpression could be achieved by exploiting the powerful genetic resources available in the model insect Drosophila melanogaster, such as the binary upstream activation sequence (UAS)/yeast transcriptional activator GAL4 expression system. The creation of transgenic Drosophila lines expressing mosquito CYP4G16/17 under the control of the UAS promoter and subsequent crossing with oenocyte-selective GAL4-promoter lines would allow expression of the transgenes at their native site. Although not yet routine, recent advances in the tools available for An. gambiae transgenesis may also make similar experiments possible in the native species (13, 14).
Synthesis of CHCs from long-chain fatty acids is complex and requires a combination of elongases, reductases, and dehydrogenases. It would be worthwhile, therefore, to explore if cuticular resistance involves enzymes from these families in addition to CYP4G16/17. Previous work on resistant An. arabiensis has identified several genes, co-upregulated with CYP4G16, that are involved in fatty acid metabolism (12). This finding suggests that a suite of genes involved in hydrocarbon synthesis may be co-upregulated to confer resistance, and the consistency of this finding warrants investigation.
Finally, the data described above strongly suggest a causal role for CYP4G16/17 in resistance. Once this role is definitely confirmed, it would be interesting to explore what drives their overexpression in resistant mosquitoes. No difference in CYPG16 copy number was observed in resistant An. arabiensis (12), and sequencing of the promoter regions of these genes would be a logical first step to examine if modification of a cis-acting element is associated with the enhanced expression of these genes. The identification of a genomic change linked to overexpression would facilitate the development of DNA-based diagnostics that can be used to determine the frequency and distribution of resistance conferred by this mechanism. Indeed, the extent to which cuticular resistance has arisen in Anopheles throughout Sub-Saharan Africa and the relative contribution of reduced insecticide penetration toward resistance phenotypes (in the presence of other resistance mechanisms) are outstanding and important questions for the management of resistance in these malaria vectors.
Footnotes
The authors declare no conflict of interest.
See companion article on page 9268.
References
- 1.Bass C, Denholm I, Williamson MS, Nauen R. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol. 2015;121:78–87. doi: 10.1016/j.pestbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32(3):187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 3.WHO 2015. World Malaria Report. Available at www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed July 18, 2016.
- 4.Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:146. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balabanidou V, et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc Natl Acad Sci USA. 2016;113:9268–9273. doi: 10.1073/pnas.1608295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feyereisen R, Dermauw W, Van Leeuwen T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol. 2015;121:61–77. doi: 10.1016/j.pestbp.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Juárez MP, Pedrini N, Girotti JR, Mijailovsky SJ. Pyrethroid resistance in Chagas disease vectors: The case of Triatoma infestans cuticle. Resistance Pest Management Newsletter. 2010;19(2):59–61. [Google Scholar]
- 8.Puinean AM, et al. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010;6(6):e1000999. doi: 10.1371/journal.pgen.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strycharz JP, et al. Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. Pestic Biochem Physiol. 2013;107(2):207–217. [Google Scholar]
- 10.Qiu Y, et al. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc Natl Acad Sci USA. 2012;109(37):14858–14863. doi: 10.1073/pnas.1208650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingham VA, et al. Dissecting the organ specificity of insecticide resistance candidate genes in Anopheles gambiae: Known and novel candidate genes. BMC Genomics. 2014;15:1018. doi: 10.1186/1471-2164-15-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CM, et al. The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit Vectors. 2013;6:343. doi: 10.1186/1756-3305-6-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynd A, Lycett GJ. Development of the bi-partite Gal4-UAS system in the African malaria mosquito, Anopheles gambiae. PLoS One. 2012;7(2):e31552. doi: 10.1371/journal.pone.0031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki Y, Niu G, Hughes GL, Rasgon JL. A viral over-expression system for the major malaria mosquito Anopheles gambiae. Sci Rep. 2014;4:5127. doi: 10.1038/srep05127. [DOI] [PMC free article] [PubMed] [Google Scholar]