Abstract
Extracellular vesicles (EVs) released by various cells are small phospholipid membrane-enclosed entities that can carry miRNA. They are now central to research in many fields of biology because they seem to constitute a new system of cell–cell communication. Physical and chemical characteristics of many EVs, as well as their biogenesis pathways, resemble those of retroviruses. Moreover, EVs generated by virus-infected cells can incorporate viral proteins and fragments of viral RNA, being thus indistinguishable from defective (noninfectious) retroviruses. EVs, depending on the proteins and genetic material incorporated in them, play a significant role in viral infection, both facilitating and suppressing it. Deciphering the mechanisms of EV-cell interactions may facilitate the design of EVs that inhibit viral infection and can be used as vehicles for targeted drug delivery.
Keywords: extracellular vesicles, exosomes, viruses, defective viruses, infection
The earth hath bubbles as the water has…
William Shakespeare, Macbeth
Act I, Scene 3
Cells in vivo and ex vivo release membrane vesicles. These extracellular vesicles (EVs) are 50- to 100-nm-sized lipid bilayer-enclosed entities containing proteins and RNA. Not long ago, EVs were considered to be “cellular dust” or garbage and did not attract much attention. However, it has recently been found that EVs can have important biological functions and that in both structural and functional aspects they resemble viruses. This resemblance becomes even more evident with EVs produced by cells productively infected with viruses. Such EVs contain viral proteins and parts of viral genetic material. In this article, we emphasize the similarity between EVs and viruses, in particular retroviruses. Moreover, we emphasize that in the specific case of virus-infected cells, it is almost impossible to distinguish EVs from (noninfectious) viruses and to separate them.
Let us start with definitions. Although EVs were discovered decades ago, EV research emerged as a separate field relatively recently and currently lacks sufficient practical nomenclature. In full analogy with viral biogenesis, some of these vesicles are generated inside cells and on release into the extracellular milieu are called “exosomes,” whereas others pinch off from the plasma membrane and are generally referred to as “microvesicles” (1). Most commonly, the general term EVs is used to refer to any membrane vesicle of a type that is released into the extracellular space. However, use of this general term not only masks the fact that EVs are highly heterogeneous in size, structure, and biogenesis but may also lead to apparent controversies when different studies deal with different entities but call them by the same name. The diversity of EVs may also underlie the large variety of roles ascribed to them in normal cell function and in pathologies (2).
In contrast to EVs, the definition of viruses developed by 20th century virologists was quite precise: both the Encyclopedia Britannica and the Oxford English Dictionary define virus as “an infectious agent of small size that can multiply only in living cells.” EVs do not fall under this definition, because despite their resemblance to viruses in many aspects, they are fundamentally different, as they do not replicate. However, contemporary virology has distanced itself from this strict definition of virus by its wide use of the terms noninfectious and defective virus. Therefore, EVs generated by retrovirus-infected cells that carry viral proteins and even fragments of viral genomes essentially fall under the definition of noninfectious viruses.
Based on current knowledge, there are many aspects in which EVs resemble viruses, in particular retroviruses. First, although some EVs may be up to a micrometer in size, the majority of EVs are <300 nm, the size of a typical RNA virus. Like enveloped viruses, EVs are surrounded by a lipid membrane that also contains cell membrane proteins. Like many viruses, EVs are formed in the endosomal system or at the plasma membrane via defined biogenesis pathways, for example, involving the endosomal sorting complexes required for transport (ESCRT) machinery (1). Like viruses, EVs can bind to the plasma membranes of other cells, enter them either through fusion or endocytosis, and trigger specific reactions from these recipient cells (1). Finally, EVs carry genetic material, and this genetic material can change functions of the recipient cells (2, 3). Especially in the case of retroviruses, EVs generated in infected cells contain selected molecules of viral origin (4) and can be so similar to noninfectious defective viruses that have lost their ability to replicate that the difference between them becomes blurred. In other cases, EVs provide an “envelope” to nonenveloped viruses, e.g., hepatitis A, and these EV-encapsulated viruses can infect cells (5). Similarly, EV released by hepatitis C-infected cells can carry fully infectious viral genomes that in target cells generate new infectious viral particles (6).
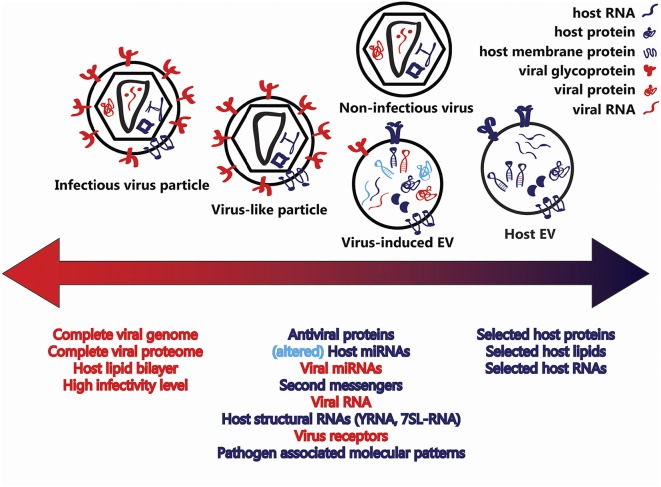
In this Perspective, we suggest that in retrovirus infections a variety of diverse vesicles is released, such that on one extreme there are EVs consisting entirely of host cell components and on the other replication-capable viruses. In between these extremes are nonreplicating particles that can be considered both as defective viruses and as EVs containing various amounts of virus-specific molecules (Fig. 1).
Fig. 1.
Structural similarities between EVs and virions. Cells infected with enveloped RNA (retro)viruses release vesicles containing a variety of host and viral factors. On one extreme, there are EVs consisting entirely of host cell components (blue), and on the other extreme there are infectious viruses surrounded by a host lipid bilayer and containing all of the virus-specific molecules (red) necessary for infectivity. In virus-infected cells, EVs incorporate fragments of the viral genome and viral (glyco)proteins. Moreover, virus infections modify the incorporation of host proteins and RNAs into EVs (light blue). Such infection-induced EVs and the so-called defective viruses and virus-like particles are intermediate entities, and the border between them seems not to exist.
Obviously, unlike true viruses, EVs that contain viral proteins and fragments of viral genomes do not cause outbreaks and epidemics. However, EVs can either directly interact with retroviruses or modulate host cells, thereby affecting the infection. Studies on other virus infections in which EVs were shown to affect antiviral immune responses [e.g., human herpesviruses, in particular Epstein-Barr virus (EBV)] or in which EVs were shown to entrap nonenveloped viruses (like hepatitis A virus and hepatitis E virus) have been reviewed elsewhere (7, 8).
EVs and Viruses Cross Paths in Biogenesis
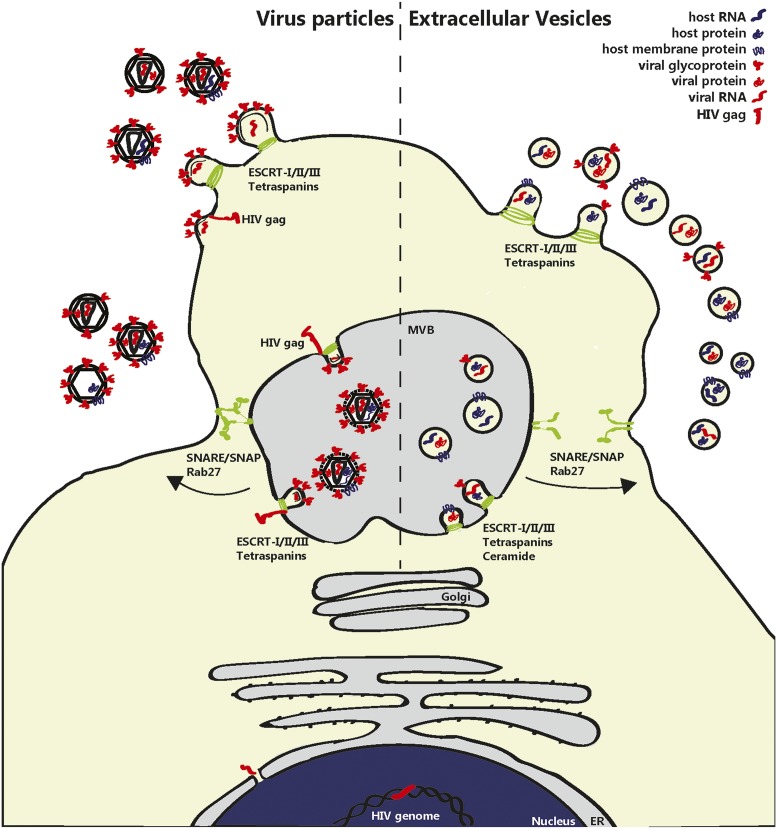
Early discussions on relationships between EVs and viruses (9, 10) were largely based on the fact that both EVs and retroviruses use the cellular vesiculation machinery, which explained striking similarities between EVs and retroviruses in lipid composition (high cholesterol and glycosphingolipids) and protein content (tetraspanins, GPI proteins, and cytoplasmic proteins). Moreover, it was hypothesized that retroviruses exploit preexisting pathways for intracellular vesicle trafficking (The Trojan exosome hypothesis) (9) and could be regarded as “modified or mutated exosomes.” Others disputed the idea, because in contrast to retroviruses, there was little evidence for an active role of EVs in functional modification of target cells via transport of bioactive proteins, lipids, and genetic material (10). Later, it was found that EVs do contain genetic material, mainly in the form of small RNAs (3,11,12). Besides the involvement of molecular mechanisms for sorting of specific proteins into EVs (13), numerous studies also indicate that the RNA content of EVs doesn’t simply reflect the RNA content of the EV-producing cell. Although some RNAs may passively diffuse into EVs in the course of their biogenesis, active sorting of specific RNAs has been shown to depend on defined RNA-binding proteins (14). Moreover, EV-associated miRNAs and mRNAs have been found to be enriched in certain sorting motifs (14–16). Recent scientific breakthroughs have shown that EV-associated proteins, lipids, and genetic material can be functionally transferred to target cells (13, 17–19), strongly implying that EVs and (retro)viruses have in common not only structural but also some functional aspects. This similarity is a reflection of the similarity in biogenesis of EVs and viruses (Fig. 2).
Fig. 2.
Similarities between biogenesis of EVs and virions. EVs and enveloped retrovirus particles (e.g., HIV) are simultaneously released by infected cells and share pathways for biogenesis at the plasma membrane or at multivesicular bodies (MVBs). For example, proteins of the ESCRT complex and tetraspanins are involved in both virion and EV formation. Viral RNA (red) enters the cytoplasm, after which Gag-mediated virion assembly takes place in the MVB or at the plasma membrane. MVB can contain both virions and EVs and are released from the cell after fusion of the MVB with the plasma membrane through the action of Rab, SNARE, and SNAP proteins. Defective viruses are also formed but are noninfectious because of the lack of essential viral components. Whereas specific host proteins and RNAs (blue), such as CD63 and APOBEC3G, can be incorporated into virions, viral components (red) are also incorporated in the plethora of EV types released by the cells. These include fragments of the viral genome, viral miRNAs, and viral (glyco)proteins, such as Nef and Gag. This intertwining of their pathways for biogenesis blurs the distinction between virions and EVs.
“Mister Postman”: What Do EVs and Viruses Deliver
Published data indicate that EVs share with viruses an important function that played a critical role in evolution, namely delivering bioactive material from one cell to another (7, 8, 20, 21). Specific combinations of lipids and proteins, in particular, tetraspanins (22), in the EV membrane can mediate specific targeting of vesicles to recipient cells and may determine the ability of vesicles to fuse with cellular membranes. These molecules, as well as genetic material and proteins enclosed in EVs (e.g., transcription factors and cytokines), constitute molecular signals that can affect the function of recipient cells. It is exactly this trait of being multicomponent transport units that EVs share with enveloped viruses. Below, we discuss further characteristics shared by EVs and viruses.
As suggested from the above, like viral envelope proteins, EV surface proteins can determine adhesion to the plasma membrane of specific target cells. The intercellular adhesion molecule 1 (ICAM 1), present in dendritic cell (DC)-derived EVs, for example, mediates EV recruitment by other DCs and activated T cells (23, 24). Interestingly, the combination of integrin proteins on tumor cell EVs was recently shown to determine their delivery to specific target organs, where these EVs prepare the site of metastasis (25). A number of cellular proteins are incorporated both in EVs and in virions. Tetraspanins, for example, are associated with EVs and have also been reported to be incorporated into retroviruses, in which these host proteins can play a role in infectivity (26). Other EV membrane proteins can act as ligands for receptors on the target cell plasma membrane. MHC class II–peptide complexes on DC-derived EVs, for example, can bind or target T-cell receptors (27). Besides proteins associated with the EV surface, lipids too can mediate signaling to target cells. Examples of EV-associated bioactive lipids include prostaglandin E2, which can play a role in tumor evasion and immune suppression, and lysophosphatidylcholine, which also affects membrane fusion and induces immune cell activation and chemotaxis (28, 29). EV surface proteins and lipids may also determine the ability of vesicles to fuse with cellular membranes, as they do in the case of viruses (30). Fusion of EVs with target cells allows EV-entrapped signaling molecules to exert effects on target cell functioning. These molecules include cytosolic proteins such as transcription factors and also cytokines such as IL-1β that lack an N-terminal signal peptide and that are released via alternative secretion routes (31). Moreover, EVs can carry specific enzymes, such as metalloproteinases and leukotriene-synthesis enzymes (32). Interestingly, DNA polymerase can also be transported by EVs. Whereas early studies reported the association of a DNA polymerase that catalyzed ribonuclease-sensitive DNA synthesis (thus resembling viral reverse transcriptase but not proving its identity) with particulate structures in the cytoplasm (27), more recent data show that tumor EVs can display endogenous reverse transcriptase activity (28). This suggests that under certain conditions, reverse transcriptase can be incorporated into EVs.
Some data indicate that EVs, although less effectively than virions themselves, can transfer cytosolic proteins involved in antiviral responses, such as APOBEC3G and cGAMP (33–36), to recipient cells. However, the relative efficiency of virions and EVs in transferring these proteins may be dependent on cell type and environmental conditions.
In some cases, EVs can also deliver genetic material into target cells. After the initial discovery that EVs carry protein-encoding mRNAs and small noncoding RNAs involved in regulation of gene expression [microRNA (miRNA)] (3), several groups demonstrated alterations in target cell gene expression due to the transfer of such RNAs via EVs (2). Besides miRNAs, EVs also contain a large variety of other small noncoding RNAs, such as fragments of protein-coding regions and repeat sequences, which could also act as regulatory RNAs by influencing gene expression (11). Although the most of genetic material enclosed in virions encodes for viral proteins that are essential for virus replication, viruses and EVs unite in their capacity to transfer RNAs that can trigger pathogen recognition receptors (PRRs) in target cells. Fragments of the viral genome, as well as virus-encoded small RNAs, such as those encoded by EBV, and certain host cell miRNAs, have been shown to trigger target cell PRRs. Although triggering of the PRR system results in complex responses, in some cases it may induce an increased activation status of these cells (37–39). Most of the described EV-mediated effects on the function of other cells are restricted to in vitro systems or occur within the same organism. Whereas viruses transfer between organisms as well as from cell to cell within an organism, the functional transfer of EVs from one individual to the other, as has been suggested for semen- or mother’s milk-derived EVs, has not been proven (12).
Mission (Almost) Impossible: Separation of Virions from EVs
Because EVs are produced by virtually all cells, probably every viral preparation is in fact a mixture of virions and EVs. To study their respective functions, it is necessary to separate EVs and virions. This is very difficult with some viruses, such as retroviruses, because both EVs and retroviruses are comparable in size (EVs ranging from 50 to 100 nm, virions being ∼100 nm) and buoyant density (EVs: 1.13–1.18 g/L; most retroviruses: 1.16–1.18 g/L). Other membrane-derived materials may also have similar characteristics. Therefore, density gradients, which are often used to separate EVs from contaminating protein aggregates on the basis of differences in buoyant densities (40), are not always reliable for separation of EVs from viral particles. Similar technical hurdles were also experienced at the early stages of retrovirus research, when there were long-lasting disagreements and controversies regarding replication-incompetent oncoviral particles causing cancer and their dependence on competent helper viruses for propagation (41). In those early days, electron microscopists observed that ultracentrifuged viruses copelleted with other 100-nm-sized membrane-enclosed particles. In the case of mouse erythroleukemia, pseudorabies, and polio virus these particles were termed “defective interfering particles” (42). Such particles were found to be functionally active, e.g., in repressing virus infection or oncogenic transformation (43), and would nowadays perhaps be classified as “virus-induced EVs.” At this time, it was discovered that these noninfectious viruses could be separated from their infectious counterparts (helper virus) on the basis of their slower migration in density gradients (42). Interestingly, a similar method has more recently been reported for separation of EVs from HIV virions that are produced in HEK 293 T cells or present in the plasma of HIV-1–positive individuals (44, 45). Virus particles and EVs were separated on the basis of migration in velocity gradients and distinguished on the basis of the presence of p24 in virus particles and, for example, acetylcholinesterase and CD45 in EVs but not in HIV. Although it has been reported that in contrast to EVs, HIV particles do not incorporate CD45 (46) or acetylcholinesterase (44), it is not clear if this is universal, and of course, these markers may not be carried by all of EVs. The very criteria for purity of the isolated preparations become murky with the realization that the border between retrovirus virions, like HIV, and EVs is blurry (Fig. 1). This is obviously different in the case of EV-enclosed nonenveloped viruses, such as hepatitis A, which can be distinguished from nonenveloped virions using neutralizing antibodies. This approach cannot be applied to enveloped viruses, because viral envelope proteins to which neutralizing antibodies are formed can be incorporated into EVs. Unless more specifically defined, it is currently virtually impossible to specifically separate and identify EVs that carry viral proteins, host proteins, and viral genomic elements from enveloped viral particles that carry the same molecules. Nevertheless, high-throughput methods to analyze individual nano-sized particles may facilitate discrimination of different particles in the EV–virus continuum in the future. For example, recent developments in flow cytometry-based techniques have opened up the possibility to quantify and characterize particles 50–200 nm in size. Such developments include not only hardware adaptations in high-end flow cytometers to improve signal-to-noise ratios, but also optimized staining protocols for general labeling of EVs and the use of magnetic nanoparticles to screen the surface antigenic composition of EVs (47, 48).
To Be or Not to Be Infected: EVs in Pro- and Antiviral Strategies
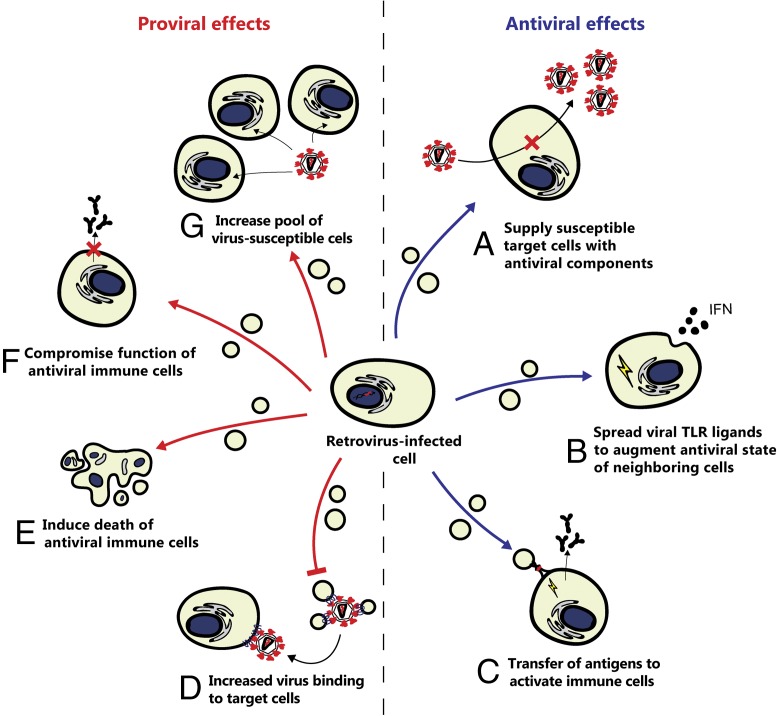
In vivo, EVs can interact with viruses and with each other either directly or via modulation of host responses, thus participating in a “War and Peace” between viruses and host (49, 50). Some viruses induce the infected cells to release modified EVs that facilitate infection by increasing the pool of susceptible target cells (e.g., by increasing the number of activated cells) or their susceptibility to viral infection or by serving as decoys that absorb antiviral antibodies, thereby compromising antiviral immunity. In contrast, EVs carrying viral proteins can also be beneficial to the host, for example, by providing dendritic cells with viral antigens to facilitate the initiation of adaptive immune responses. Hypothetically, the capacity of EVs to regulate the lifespan of permissive cells and to modify antiviral immune responses may give additional flexibility to the host in responding to viral infection. Thus, EVs formed during viral infection may play either pro- or counter-viral roles (Fig. 3). It is currently unknown whether the diverse functions ascribed to virus-induced EV may in part be explained by differences in the purity of EV populations used in various studies. A general understanding of parameters that determine the net effect of EVs on viral infections is therefore still lacking.
Fig. 3.
Proviral and antiviral effects of EVs released by retrovirus-infected cells. Retrovirus infection can lead to the release of modified EVs that either facilitate or suppress infection. Potential antiviral effects include (A) EV-mediated delivery of antiviral components, such as APOBEC3G, to increase resistance to infection; (B) spread of TLR ligands, such as viral RNA, via EVs to warn nonsusceptible neighboring cells of the presence of viral infection; and (C) provision of antigen presenting cells with viral antigen to facilitate the initiation of adaptive immune responses. Potential proviral effects include (D) inhibition of the neutralizing effect of EV, leading to decreased binding of EV to virions and an increase in the number of virions that may infect other cells; (E) EV-mediated delivery of viral components (e.g., Nef) that induce induce cell senescence or death of antiviral immune cells; (F) EV-mediated delivery of viral components that suppress the function of immune cells (e.g., Nef-induced down-regulation of antibody production by B cells); and (G) increase of the pool of virus-susceptible cells, e.g., by transference of coreceptors for virus binding to other cells.
EVs Facilitate Viral Infection.
Several HIV proteins and RNAs have been detected in EVs released from HIV-infected cells. One of the viral components released via EVs is the HIV transactivation response element (TAR) RNA (51). TAR is an RNA stem-loop structure located at the 5′ ends of HIV-1 transcripts, which in infected cells can be bound by Tat, thereby facilitating recruitment of elongation factors and increased production of viral RNA (52). When transferred via EVs, TAR RNA can increase the population of susceptible target cells. Inside EV-targeted cells, the full-length TAR RNA is processed into miRNAs, which silence mRNA coding for Bcl-2 Interacting Protein. The consequent increase in resistance to apoptosis allows the cell to produce virus for a longer period, thereby facilitating HIV infection (51).
In addition, EVs released by HIV-infected cells selectively incorporate the HIV virulence factor Nef via interaction of the Nef secretion modification region with mortalin, a member of the Hsp70 family of chaperones involved in cellular protein trafficking (53). Delivery of the EV-associated Nef to T cells affects these cells in several ways. First, the transferred Nef may activate T cells, rendering them more susceptible to HIV infection (54). Second, EVs can deliver Nef to some of the bystander CD4+ T cells and induce cell senescence or death (55). This mechanism can contribute to the high level of T-cell deaths during the early stages of HIV infection, when viral load is still low (55). Finally, intercellular transfer of Nef by EVs may facilitate evasion of the humoral immune response by suppressing IgG2 and IgA production in B cells, as has also been shown for Nef transfer by HIV-infected macrophages to B cells via intercellular conduits (56). In in vitro systems, it has been shown that EVs can transfer the HIV coreceptors CCR5 and CXCR4 to other cells, thus making them prone to HIV infection (57, 58). This EV-mediated process may expand the spectra of HIV-infected cells, but it is yet unknown whether such a phenomenon plays an important role in vivo.
EVs Suppress Viral Infection.
In in vitro experiments, it has been shown that T cells can produce EVs containing the HIV receptor CD4. These EVs can attach to viral particles, thereby decreasing the numbers of virions that would otherwise infect CD4+ T cells (59). However, HIV can counteract this by stimulating the incorporation of HIV-Nef into these EVs, leading to the inhibition of CD4 incorporation in EVs and a decreased effectiveness of the above-described host antiviral response (59).
Another EV-mediated host cell protection mechanism against HIV involves the EV-mediated transport of the host antiviral protein APOBEC3G. This cytidine deaminase is usually incorporated into virions together with retroviral RNA and inhibits viral replication by creating G-to-A mutations in the transcribed viral DNA (60). The antiviral action of APOBEC3G is counteracted by the HIV-encoded protein Vif, which interferes with APOBEG3G incorporation into virions. Delivery of APOBEC3G without Vif via EVs can counteract the effect of Vif and thus increase resistance of EV-targeted cells to HIV infection (34). Similarly, recent data indicate that the second messenger cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) (induced by cGAMP synthase) is enclosed both in HIV particles and in EVs that are released from infected cells. Intercellular transfer of cGAMP, although accomplished more efficiently by viruses than by EV, triggers antiviral IFN responses in newly infected cells in a stimulator of interferon genes (STING)-dependent manner (35, 36).
EVs from virus-infected cells not only contain endogenous (mi)RNAs but have also been shown to be selectively enriched in viral RNAs (e.g., in the case of HCV-induced EVs) (38). The PRRs in EV-targeted cells may recognize such RNAs as pathogen-associated molecular patterns (PAMPs) and respond by triggering the innate antiviral response (38, 61). HIV-infected macrophages also release EV containing viral RNAs (viral miRNAs vmiR88 and -99) that trigger endosomal TLR8 and NF-κB signaling in EV-targeted bystander macrophages (61). The subsequent production of proinflammatory cytokines (e.g., TNFα) contributes to the initiation of the immune response against HIV. Dissemination of viral RNA via EVs provides a strategy to warn nonsusceptible neighboring cells of the presence of viral infection. During HCV infection, for example, plasmacytoid DC are targeted by viral RNA containing EVs and, as a result, initiate an inflammatory response (38). In addition, EVs containing host miRNA produced by virus-resistant cells can confer resistance to other cells. This has been demonstrated for trophoblasts, which are largely resistant to infection by various viruses, including HIV, probably contributing to in vivo fetus protection. EVs produced by these cells in vitro carry host miRNAs and deliver them to virus-susceptible cells, making them resistant to virus infection (62).
Conclusions: Prospects for EV Therapy
A growing body of evidence indicates that cells infected with enveloped or nonenveloped viruses release EVs that contain viral components. Here, we aimed to create awareness that virus preparations may never be pure but rather are contaminated with diverse subpopulations of EVs, and some of these EVs may be either indistinguishable from or very similar to so-called defective viruses. Because of their common biogenesis paths, viruses and EVs may be close relatives, although only the former can replicate in cells. Importantly, EVs generated by infected cells are not neutral, as they can either facilitate virus propagation or enhance the antiviral response. Understanding of the structure of EVs produced by infected cells, determining their cargo, and deciphering the fine mechanisms by which they affect viral infection are required not only for basic virology but also for translation into therapy. Below, we present three examples of potential utilizations of EVs in immunotherapy, vaccine development, and drug delivery:
(i) EVs with viral proteins can serve as decoys for antiviral antibodies by binding them, leaving infectious virions partially undetected. Eliminating these EVs (e.g., with immunoadsorption based on their nonviral markers) may enhance antiviral immune responses. (ii) Understanding the roles of EVs in antiviral immune reactions may guide engineering of EVs that have strong antiviral properties. (iii) Knowledge of phenotypes and functions of EVs generated in response to viral inoculation can in the future be applied to improve virus vaccines by eliminating or adding defined subsets of EVs.
Targeted drug delivery is one of the most important and unresolved problems in pharmacology. By contrast, viruses are highly targeted: in the course of evolution they have acquired high specificity toward their cellular targets by incorporating specific binding proteins. Incorporation of such viral proteins onto the EV membrane may facilitate EV-mediated delivery of drugs to specific cells (63).
However, to achieve these goals several important questions need to be answered regarding the role of EVs in intercellular communication in the steady state and during viral infections:
-
i)
What are the exact mechanisms by which EVs affect viral infection at both cellular and systemic levels?
-
ii)
Can we use new technologies, some of which are described in this report, to obtain viral preparations free of contaminating EVs and, reciprocally, EV preparations produced by infected cells and free of contaminating viruses? Only after we can obtain clean populations, can question # 1 be addressed experimentally.
-
iii)
How can we predict either in vitro or in vivo net biological activity when viruses and EVs are mixed?
-
iv)
Can we obtain EVs with specific (viral) surface proteins to target vesicles to particular cells and organs?
-
v)
Can we efficiently scale up the production of EVs so that we have sufficient quantities to test their in vivo effects and even perform clinical trials in the future?
-
vi)
Can we design and engineer EVs that block newly evolving viruses? Can we, for example, use EVs to block Zika viral infection developing in fetuses or to enhance antiviral activity to new influenza strains?
Answers to these questions will show whether the newly emergent field of extracellular vesicle research will become important for understanding fundamental mechanisms of virus infections and be translated into anti-viral therapeutic strategies.
Acknowledgments
E.N.-t.H. receives funding from the European Research Council (ERC) under the European Union's Seventh Framework Programme (FP/2007–2013)/ERC grant agreement 337581; the work of L.B.M. is funded by the National Institute of Child Health and Human Development/NIH Intramural Program; the work of R.C.G. is funded by the Gates Foundation, the National Institute of Allergy and Infectious Diseases, and the University of Maryland School of Medicine.
Footnotes
The authors declare no conflict of interest.
See Core Concepts on page 9126.
This article is a PNAS Direct Submission.
References
- 1.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 2.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 4.Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 2015;7(6):3204–3225. doi: 10.3390/v7062770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Z, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496(7445):367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10(10):e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meckes DG, Jr, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85(24):12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meckes DG., Jr Exosomal communication goes viral. J Virol. 2015;89(10):5200–5203. doi: 10.1128/JVI.02470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould SJ, Booth AM, Hildreth JEK. The Trojan exosome hypothesis. Proc Natl Acad Sci USA. 2003;100(19):10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelchen-Matthews A, Raposo G, Marsh M. Endosomes, exosomes and Trojan viruses. Trends Microbiol. 2004;12(7):310–316. doi: 10.1016/j.tim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Nolte-’t Hoen EN, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vojtech L, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42(11):7290–7304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarroya-Beltri C, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol Direct. 2013;8:12. doi: 10.1186/1745-6150-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koppers-Lalic D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Reports. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Ridder K, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12(6):e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zomer A, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segura E, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 24.Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, et al. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J Virol. 2008;82(2):1021–1033. doi: 10.1128/JVI.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Théry C, et al. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 28.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 30.Dumas F, Preira P, Salomé L. Membrane organization of virus and target cell plays a role in HIV entry. Biochimie. 2014;107(Pt A):22–27. doi: 10.1016/j.biochi.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKenzie A, et al. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15(5):825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 32.Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 33.Harris RS, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113(6):803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 34.Khatua AK, Taylor HE, Hildreth JEK, Popik W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol. 2009;83(2):512–521. doi: 10.1128/JVI.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridgeman A, et al. Viruses transfer the antiviral second messenger cGAMP between cells. Science. 2015;349(6253):1228–1232. doi: 10.1126/science.aab3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentili M, et al. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science. 2015;349(6253):1232–1236. doi: 10.1126/science.aab3628. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. microRNAs are ligands of Toll-like receptors. RNA. 2013;19(6):737–739. doi: 10.1261/rna.036319.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dreux M, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baglio SR, et al. Sensing of latent EBV infection through exosomal transfer of 5'pppRNA. Proc Natl Acad Sci USA. 2016;113(5):E587–E596. doi: 10.1073/pnas.1518130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda N, Fan H, Yoshikai Y. Oncogenesis by retroviruses: Old and new paradigms. Rev Med Virol. 2008;18(6):387–405. doi: 10.1002/rmv.592. [DOI] [PubMed] [Google Scholar]
- 42.Eckner RJ, Hettrick KL. Defective Friend spleen focus-forming virus: Interfering properties and isolation free from standard leukemia-inducing helper virus. J Virol. 1977;24(1):383–396. doi: 10.1128/jvi.24.1.383-396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang AS. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- 44.Cantin R, Diou J, Bélanger D, Tremblay AM, Gilbert C. Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J Immunol Methods. 2008;338(1-2):21–30. doi: 10.1016/j.jim.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Konadu KA, et al. Isolation of exosomes from the plasma of HIV-1 positive individuals. J Vis Exp. 2016 doi: 10.3791/53495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esser MT, et al. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: Implications for viral pathogenesis and immune regulation. J Virol. 2001;75(13):6173–6182. doi: 10.1128/JVI.75.13.6173-6182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolte-’t Hoen EN, et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine (Lond) 2012;8(5):712–720. doi: 10.1016/j.nano.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arakelyan A, Ivanova O, Vasilieva E, Grivel J-C, Margolis L. Antigenic composition of single nano-sized extracellular blood vesicles. Nanomedicine (Lond) 2015;11(3):489–498. doi: 10.1016/j.nano.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisco A, Vanpouille C, Margolis L. War and peace between microbes: HIV-1 interactions with coinfecting viruses. Cell Host Microbe. 2009;6(5):403–408. doi: 10.1016/j.chom.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Bhattarai N, McLinden JH, Xiang J, Kaufman TM, Stapleton JT. Conserved motifs within hepatitis C virus envelope (E2) RNA and protein independently inhibit T cell activation. PLoS Pathog. 2015;11(9):e1005183. doi: 10.1371/journal.ppat.1005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayanan A, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013;288(27):20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He N, et al. HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol Cell. 2010;38(3):428–438. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shelton MN, Huang M-B, Ali SA, Powell MD, Bond VC. Secretion modification region-derived peptide disrupts HIV-1 Nef’s interaction with mortalin and blocks virus and Nef exosome release. J Virol. 2012;86(1):406–419. doi: 10.1128/JVI.05720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arenaccio C, et al. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J Virol. 2014;88(19):11529–11539. doi: 10.1128/JVI.01712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenassi M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10(9):1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mack M, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6(7):769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 58.Rozmyslowicz T, et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17(1):33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 59.de Carvalho JV, et al. Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS One. 2014;9(11):e113691. doi: 10.1371/journal.pone.0113691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soros VB, Yonemoto W, Greene WC. Newly synthesized APOBEC3G is incorporated into HIV virions, inhibited by HIV RNA, and subsequently activated by RNase H. PLoS Pathog. 2007;3(2):e15. doi: 10.1371/journal.ppat.0030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernard MA, et al. Novel HIV-1 miRNAs stimulate TNFα release in human macrophages via TLR8 signaling pathway. PLoS One. 2014;9(9):e106006. doi: 10.1371/journal.pone.0106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delorme-Axford E, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110(29):12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]