Significance
Allergic asthma is a chronic airway disease, and the number of individuals with asthma continues to grow. Eosinophils recruited to allergic airways contribute significantly to airway inflammation via release of proinflammatory mediators that cause epithelial tissue damage, bronchoconstriction, and airway remodeling. Here we show that galectin-1 (Gal-1), an endogenous immunoregulatory lectin, binds to eosinophil-expressed surface glycans to inhibit cell migration and induce apoptosis. Using a mouse model of allergic asthma, we show that mice lacking Gal-1 exhibit increased airway eosinophils and airway hyperresponsiveness compared with wild-type mice. Because Gal-1 plays an important role in regulating airway inflammation, identifying pathways to induce Gal-1 synthesis and/or favor its biological activity might enable exploitation of its proresolving function to suppress allergic asthma.
Keywords: galectin-1, eosinophils, apoptosis, migration, allergic airway inflammation
Abstract
Galectin-1 (Gal-1), a glycan-binding protein with broad antiinflammatory activities, functions as a proresolving mediator in autoimmune and chronic inflammatory disorders. However, its role in allergic airway inflammation has not yet been elucidated. We evaluated the effects of Gal-1 on eosinophil function and its role in a mouse model of allergic asthma. Allergen exposure resulted in airway recruitment of Gal-1–expressing inflammatory cells, including eosinophils, as well as increased Gal-1 in extracellular spaces in the lungs. In vitro, extracellular Gal-1 exerted divergent effects on eosinophils that were N-glycan– and dose-dependent. At concentrations ≤0.25 µM, Gal-1 increased eosinophil adhesion to vascular cell adhesion molecule-1, caused redistribution of integrin CD49d to the periphery and cell clustering, but inhibited ERK(1/2) activation and eotaxin-1–induced migration. Exposure to concentrations ≥1 µM resulted in ERK(1/2)-dependent apoptosis and disruption of the F-actin cytoskeleton. At lower concentrations, Gal-1 did not alter expression of adhesion molecules (CD49d, CD18, CD11a, CD11b, L-selectin) or of the chemokine receptor CCR3, but decreased CD49d and CCR3 was observed in eosinophils treated with higher concentrations of this lectin. In vivo, allergen-challenged Gal-1–deficient mice exhibited increased recruitment of eosinophils and CD3+ T lymphocytes in the airways as well as elevated peripheral blood and bone marrow eosinophils relative to corresponding WT mice. Further, these mice had an increased propensity to develop airway hyperresponsiveness and displayed significantly elevated levels of TNF-α in lung tissue. This study suggests that Gal-1 can limit eosinophil recruitment to allergic airways and suppresses airway inflammation by inhibiting cell migration and promoting eosinophil apoptosis.
Allergic asthma is an inflammatory disease of the airways that is associated with increased pulmonary recruitment of inflammatory cells, especially eosinophils, elevated levels of Th2 cytokines, proinflammatory chemokines, and growth factors that together contribute to the overall pathogenesis of the disease including the development of bronchoconstriction and airway hyperresponsiveness (AHR) (1). This disease is to a large extent driven by activation of Th2 cells and airway eosinophilia. Further, decreased number and/or function of regulatory T cells (Tregs) is also thought to contribute to atopic allergic disease and asthma in patients (2). Although various therapeutic approaches targeting T cells or cytokines and chemokines released by these cells have been developed, positive outcomes are either limited to small subpopulations (3) or vary significantly among patient groups, thus warranting the need for identification of novel or alternate therapeutic approaches.
Galectin-1 (Gal-1) is a glycan-binding protein that binds to N-acetyl-lactosamine (Gal-β1-3/4-N-acetyl-d-glucosamine or LacNAc) residues of complex N- and O-glycans on cell-surface glycoconjugates (4). Gal-1 has wide distribution in adult tissue including the lung, and is expressed by various cell types such as polymorphonuclear cells (PMNs), macrophages, dendritic cells (DCs), activated T cells, stromal cells, endothelial cells, and epithelial cells (5, 6). At a cellular level, Gal-1 may act either intracellularly or extracellularly, and is profoundly involved in resolving acute and chronic inflammation by affecting processes such as immune cell adhesion, migration, activation, signaling, proliferation, differentiation, and apoptosis (5, 7). Increasing evidence from multiple chronic inflammatory disease models supports the critical antiinflammatory role of exogenous and endogenous Gal-1 in limiting or resolving inflammation (6). Additionally, Gal-1 showed proresolving effects in models of acute inflammation where neutrophil recruitment (8) and extravasation (9) as well as mast cell degranulation were suppressed (9). The immunosuppressive role of Gal-1 was further supported by studies with Tregs where cells from Gal-1 null mice exhibited reduced regulatory activity (10). More recent studies emphasized the immunosuppressive effect of Gal-1 in macrophages and cells of the microglia compartment, which showed a shift toward an M2 phenotype and reduced secretion of proinflammatory cytokines upon exposure to this lectin (11).
Given the critical antiinflammatory and proresolving function of Gal-1 in innate and adaptive immune compartments and its broad expression in lung tissue, we hypothesized that this endogenous lectin might regulate eosinophil function and airway inflammation. Clinical studies have shown that sputum leukocytes (largely macrophages) from asthmatic patients have decreased intracellular Gal-1 relative to cells from healthy subjects, suggesting that decreased Gal-1 expression might favor exacerbation of asthma (12). In the present study, we examined the regulated expression and biological relevance of endogenous and exogenous/extracellular Gal-1 in eosinophil function in vitro and in a model of allergic asthma.
Results
Expression of Gal-1 Is Induced in Allergic Lungs.
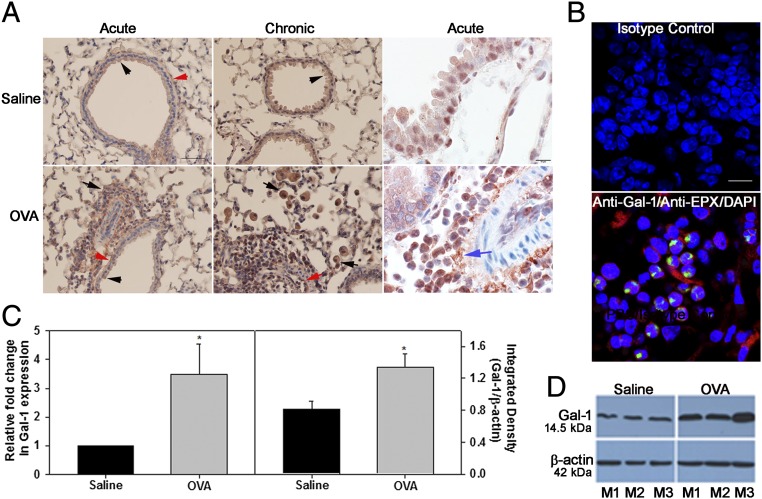
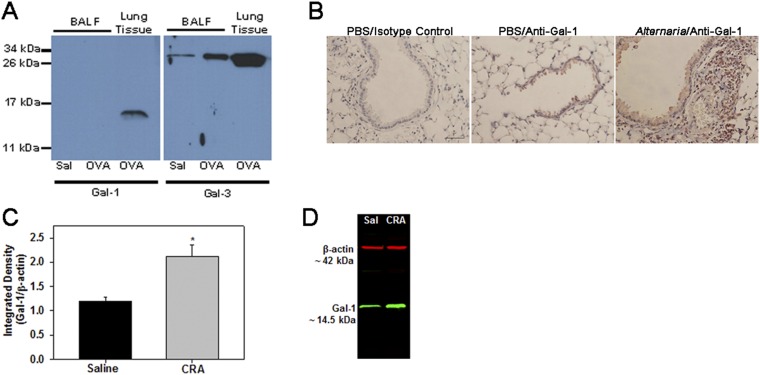
We examined the expression of Gal-1 in the lungs during allergic inflammation in a mouse model. In control mice, baseline Gal-1 expression was observed mostly in airway epithelial cells, smooth muscle cells, and endothelial cells by immunohistochemistry (IHC; Fig. 1A, Upper, Left and Middle). Acute or chronic ovalbumin (OVA) exposure resulted in increased Gal-1 expression in the lungs that was largely observed in the recruited inflammatory cells in addition to expression in airway epithelial cells, smooth muscle cells, and endothelial cells (Fig. 1A, Lower, Left and Middle). Further, increased Gal-1 expression was detected in extracellular spaces after allergen exposure (Fig. 1A, Right, Upper and Lower). Dual immunofluorescence (IF) staining of lung sections from allergen-challenged mice for Gal-1 and eosinophil peroxidase (EPX), an eosinophil-specific marker, revealed several cells that stained positively for Gal-1 (red) and EPX (green) in addition to cells that were positive for Gal-1 but negative for EPX (Fig. 1B, Lower). These findings indicate that eosinophils, the major inflammatory cells recruited to allergic airways, express Gal-1. Total lung Gal-1 expression was significantly higher in acute OVA-challenged mice compared with saline-exposed controls at the level of mRNA and protein (Fig. 1 C and D). Soluble Gal-1 was not detectable in the bronchoalveolar lavage fluid (BALF) of OVA-challenged mice, whereas Gal-3 was easily detected in these samples (Fig. S1A). Exposure to other allergens such as Alternaria alternata extract (Fig. S1B) or cockroach antigen (CRA; Fig. S1 C and D) also resulted in increased Gal-1 expression in the lungs of mice similar to OVA. Thus, allergic airway inflammation irrespective of the nature of allergen exposure results in up-regulated expression of Gal-1.
Fig. 1.
Expression of Gal-1 is induced in allergic lungs. (A) Gal-1 expression in lungs of mice exposed to allergen challenge (or saline alone as control) by IHC. Gal-1 expression in airway epithelial cells (black arrowheads), endothelial cells (red arrow), smooth muscle cells (red arrowheads), inflammatory cells (black arrows), and extracellular spaces (blue arrow) is shown. [Scale bars, 50 µm (Left) and 10 µm (Right).] (B) Lung sections from OVA-exposed mice dual-stained with antibodies against Gal-1 (red) and eosinophil-specific mouse anti-human EPX (green) (Bottom) or rabbit and mouse IgG as controls (Top). (Scale bar, 10 µm.) (C) Gal-1 mRNA (Left) and protein (Right) expression in lungs of OVA (acute)-challenged or saline-exposed mice. (D) Gal-1 expression in lung tissue of three representative mice (M1–M3) for each group. Data are representative of n = 6 mice per group in A and n = 3 mice per group in B. Combined data (mean ± SEM) of n = 5–7 mice per group are shown in C. *P < 0.05 (Left) and *P < 0.01 (Right) in C for control versus allergen-challenged group.
Fig. S1.
Expression of Gal-1 in allergen-challenged mice. (A) Expression of Gal-1 (Left) and Gal-3 (Right) in the BALF from saline- (Sal) and OVA-challenged mice by Western blot analysis on a 15% (for Gal-1) or 12% (for Gal-3) gel under denatured conditions using anti–Gal-1 or anti–Gal-3, respectively. (B) Gal-1 expression in lung sections from mice exposed to PBS (control) or Alternaria alternata extract by IHC. (Scale bar, 50 µm.) (C and D) Gal-1 expression in lung tissue from saline- and CRA-challenged mice by Western blot. Representative data of n = 3 mice per group in A and B and n = 4 mice per group in C (mean ± SEM) are shown. *P < 0.02 in C for control versus allergen-challenged group.
Gal-1 Binds to Eosinophils in a Carbohydrate-Dependent Manner.
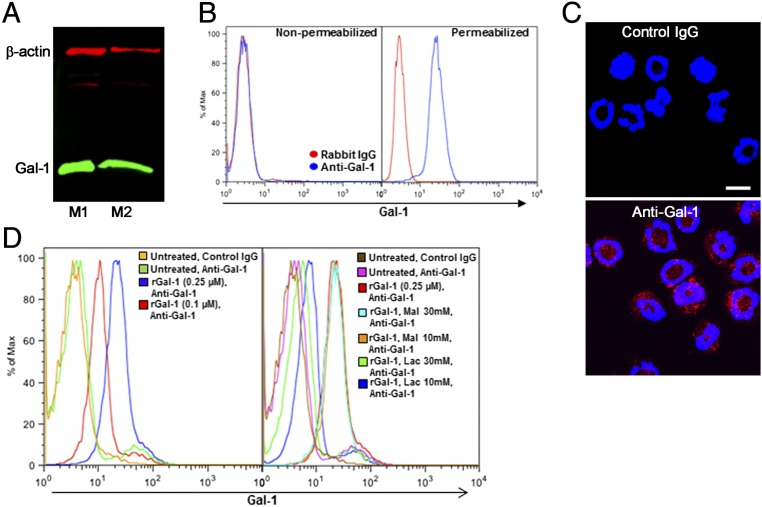
We examined Gal-1 expression at the cellular level using bone marrow (BM)-derived murine eosinophils and identified a protein band of ∼14.5 kDa corresponding to Gal-1 in its monomeric form (13) (Fig. 2A). Interestingly, flow cytometry studies revealed that Gal-1 expression by eosinophils (nonactivated) was detectable only when cells were permeabilized (Fig. 2B, Right), with no positive staining for this lectin under nonpermeabilized conditions (Fig. 2B, Left). This suggests that in resting eosinophils, Gal-1 is expressed intracellularly but is undetectable on the cell surface. Further, IF staining of permeabilized eosinophils indicated that Gal-1 was predominantly expressed in the cytosolic compartment (Fig. 2C).
Fig. 2.
Murine eosinophils express Gal-1 intracellularly and bind to Gal-1 on the cell surface. (A) Expression of Gal-1 in lysates of BM eosinophils from two representative mice (M1 and M2) by Western blot analysis. (B) Expression of Gal-1 in BM-derived eosinophils by flow cytometry. Note that Gal-1 expression is detected only in permeabilized cells. (C) IF staining of permeabilized eosinophils with anti–Gal-1 (Bottom) or rabbit IgG (Top). (Scale bar, 10 µm.) (D) Dose-dependent Gal-1 binding to nonpermeabilized eosinophils in the absence (Left) or presence (Right) of increasing concentrations of lactose (Lac) or maltose (Mal). Data are representative of three independent experiments with eosinophils from different mice.
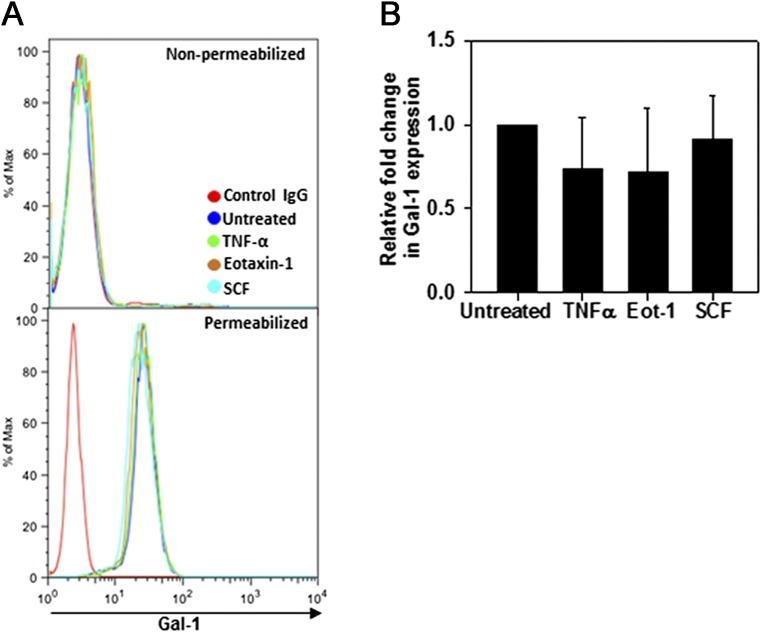
Because allergen exposure results in increased Gal-1 expression in extracellular spaces (Fig. 1A), it is likely that eosinophils recruited to the airways are exposed to Gal-1 extracellularly. We found that soluble recombinant Gal-1 (rGal-1) binds to the surface of eosinophils in a dose-dependent fashion (Fig. 2D, Left). Exposure to rGal-1 in the presence of increasing concentrations of lactose, a specific disaccharide inhibitor, but not maltose (negative control), inhibited Gal-1 binding to the cell surface, indicating that Gal-1 binds to surface ligands on eosinophils in a carbohydrate-dependent manner (Fig. 2D, Right). Intracellular (protein and mRNA) and cell-surface expression of Gal-1 were not altered in eosinophils after stimulation/activation with TNF-α, eotaxin-1 (CCL11), or stem cell factor-1 (SCF-1) (Fig. S2 A and B). Further, Western blot analysis of culture supernatant concentrates (∼10-fold) from these activated eosinophils was negative for Gal-1 immunoreactivity, suggesting that Gal-1 may not be secreted in detectable levels by eosinophils.
Fig. S2.
Effect of cytokines on Gal-1 expression by BM-derived eosinophils. Expression of Gal-1 protein by flow cytometry (A) and Gal-1 mRNA by qPCR (B) by eosinophils stimulated with murine eotaxin-1 (100 nM), TNF-α (100 ng/mL), SCF-1 (200 ng/mL), or medium alone for 24 h. Data in A are representative of three independent experiments with eosinophils from different mice. Combined data (mean ± SEM) of three experiments in triplicate are shown in B.
Eosinophils Express the Repertoire of Cell-Surface Glycans Required for Gal-1 Binding and Function.
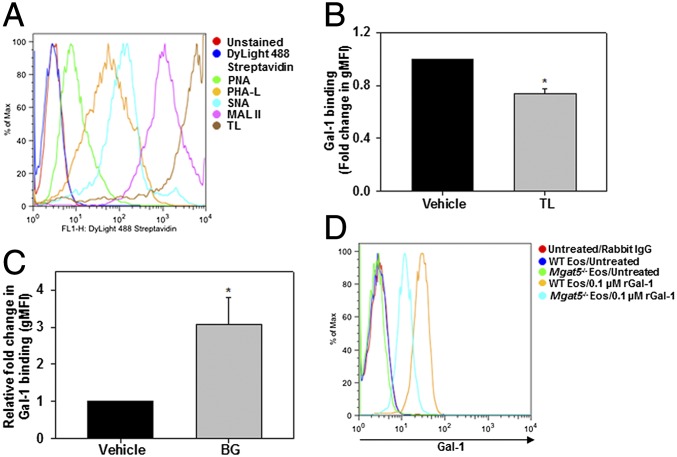
Gal-1 is well-known for its ability to recognize complex N- and O-glycans containing unmodified or terminally modified (α2,3-sialylated) LacNAc residues (5). To identify Gal-1–binding carbohydrate partners on eosinophils, we analyzed the glycophenotype of these cells using an array of labeled plant lectins with known carbohydrate-binding specificities by flow cytometry. Murine eosinophils express complex N-glycans [β1-6 branched N-linked carbohydrates, based on Phaseolus vulgaris leucoagglutinin (PHA)-L reactivity] as well as O-glycans [asialo core-1 O-glycans bearing terminal galactose residues (Galβ1-3GalNAcαSer-Thr), based on peanut agglutinin (PNA) reactivity] (Fig. 3A). Based on staining intensity, eosinophils expressed predominantly glycans containing poly-LacNAc [Lycopersicon esculentum (tomato) lectin (TL) reactivity] and to a lesser extent glycans containing α2,3-linked sialic acid residues [Maackia amurensis lectin-II (MAL-II) reactivity] and α2,6-linked sialic acid residues [Sambucus nigra lectin (SNA) reactivity], thus demonstrating the presence of many potential Gal-1–binding partners on their cell surface. When pretreated with these lectins, only TL showed partial, albeit significant, reduction in binding of rGal-1 to the cell surface (Fig. 3B), substantiating the involvement of LacNAc residues in Gal-1 binding. Studies with eosinophils where O-linked glycosylation was specifically inhibited using benzyl-α-GalNAc (BG), an inhibitor of O-linked oligosaccharide chain elongation, demonstrated significantly increased Gal-1 binding (Fig. 3C). This suggests that, at least in the case of eosinophils, O-glycosylation may mask Gal-1 effects. However, binding of Gal-1 to eosinophils required complex branched N-glycans. Gal-1 binding to BM-derived eosinophils from mice deficient in UDP-N-acetylglucosamine:α-6-d-mannoside β1,6 N-acetylglucosaminyltransferase V (Mgat5, Mgat5−/−), which lack tetraantennary N-glycans, was substantially lower than binding to their WT counterparts (Fig. 3D). These findings indicate that Gal-1 binding to eosinophils is mediated in part via interaction with complex N-linked glycans and does not require the presence of O-glycans.
Fig. 3.
Gal-1 binding to eosinophil-expressed glycans. (A) Glycophenotype analysis of BM eosinophils. Cells were incubated with specific plant lectins followed by streptavidin-conjugated DyLight 488 and then analyzed by flow cytometry. (B) Gal-1 binding to eosinophils pretreated with TL or vehicle (PBS) examined by flow cytometry with Gal-1 antibody. gMFI, geometric mean fluorescence intensity. (C) Role of O-glycans in Gal-1 binding. Eosinophils cultured in medium containing BG (2 mM) or vehicle (DMSO) were treated with rGal-1 and examined for Gal-1 binding. (D) Requirement of complex branched N-glycans for Gal-1 binding. WT and Mgat5−/− eosinophils (nonpermeabilized) were treated with rGal-1 and examined for Gal-1 binding. Data in A and D are representative of three independent experiments with eosinophils from different mice. Combined data (mean ± SEM) of four experiments are shown in B and C. *P < 0.01 in B and *P < 0.03 in C versus vehicle-treated cells.
Exogenous Gal-1 Induces Apoptosis in Eosinophils.
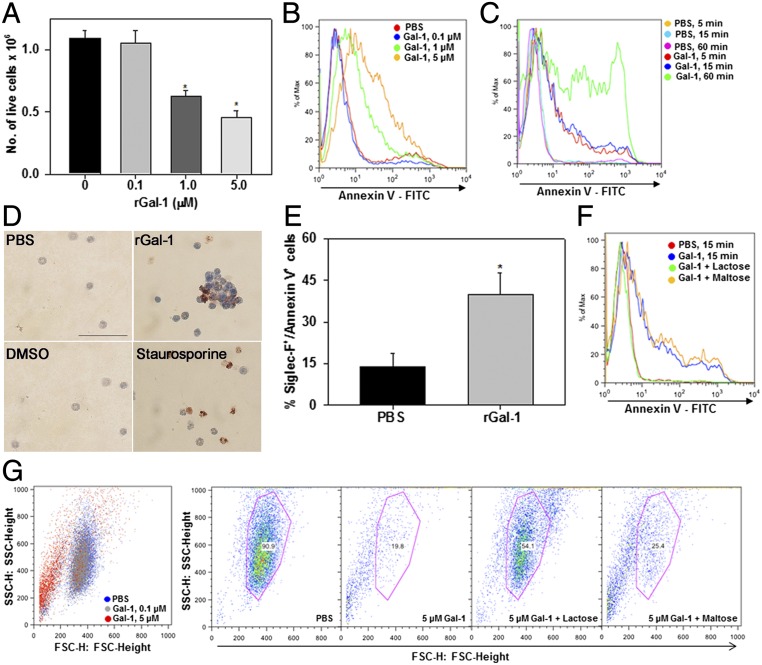
Given the role of extracellular Gal-1 in promoting apoptosis of activated as well as fully differentiated T cells (14, 15) and modulating phosphatidylserine exposure (without engaging the full apoptotic program) in PMNs (16), we examined the direct effects of rGal-1 on eosinophil survival. Significant loss of cell viability (based on trypan blue dye staining) was observed when eosinophils were exposed to rGal-1 at concentrations of 1.0 µM or higher for 15 min, whereas no effect was noted at lower concentrations (Fig. 4A). Gal-1 induced phosphatidylserine exposure in eosinophils, an early apoptotic event, in a dose-dependent manner (Fig. 4B) at time points as early as 5 min at a concentration of 5 µM (Fig. 4C), as shown by annexin V staining. Under these conditions, rGal-1 also modulated later events in the apoptosis cascade, as shown by TUNEL assay. A substantial number of rGal-1–treated eosinophils were positive for TUNEL staining similar to cells treated with staurosporine, a well-known apoptotic stimulus, whereas control cells [PBS-treated (for Gal-1) and DMSO-treated (for staurosporine)] were negative for TUNEL staining (Fig. 4D). Treatment with rGal-1 also resulted in clustering of cells relative to control cells or cells treated with staurosporine, an effect commonly observed in the presence of exogenous Gal-1 (17). Further, rGal-1 exerted a similar effect on peripheral blood eosinophils. Treatment of peripheral blood leukocytes from allergen-challenged mice with 1.0 µM rGal-1 resulted in an approximately threefold increase in the number of Siglec-F–positive cells (eosinophils) that stained positive for annexin V (Fig. 4E). The glycan-binding requisite for Gal-1–induced eosinophil apoptosis was established by studies wherein treatment of cells with rGal-1 in the presence of lactose but not maltose completely inhibited apoptosis (Fig. 4F). In addition, a substantial number of eosinophils exposed to rGal-1 at 5 µM were found to undergo shape change, potentially cell shrinkage, based on the decrease in mean forward scatter (FSC) detected compared with untreated cells (Fig. 4G, Left). This decrease in FSC was inhibited by lactose but not maltose (Fig. 4G, Right).
Fig. 4.
Exogenous Gal-1 induces eosinophil apoptosis. (A) Effect of rGal-1 on the absolute number of live cells by trypan blue dye exclusion at the indicated doses. (B and C) Flow cytometric analysis after annexin V staining of eosinophils exposed to different concentrations of rGal-1 and eosinophils treated with 5.0 µM rGal-1 for different durations of time, respectively. (D) Analysis of cell death in eosinophils (stained brown) after rGal-1 or staurosporine (positive control) treatment by TUNEL assay. (Scale bar, 50 μM.) (E) Annexin V staining of peripheral blood leukocytes exposed to rGal-1. Annexin V positivity of cells gated for Siglec-F–positive staining (eosinophils) is shown. (F) Inhibition of Gal-1–induced apoptosis by lactose but not maltose (negative control). (G) Effect of rGal-1 on eosinophil shape change. FSC versus side scatter dot plots are shown. (G, Left) Dose-dependent cell shrinkage in Gal-1–treated eosinophils. (G, Right) Blockade of this effect by lactose but not maltose. Combined data (mean ± SEM) of three independent experiments with cells from n = 3–7 mice are shown in A and E. Data shown in B–D, F, and G are representative of two or three experiments with eosinophils from different mice. *P < 0.01 in A and E for comparison with PBS-treated cells.
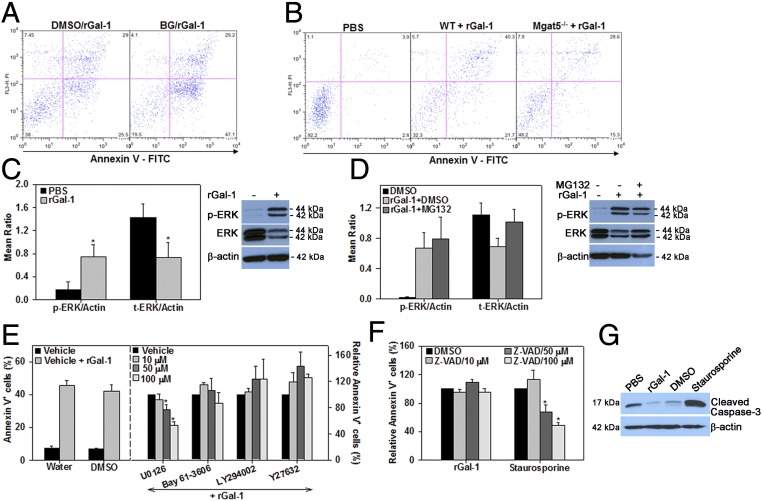
To examine whether Gal-1–induced eosinophil apoptosis is mediated via cell-surface elongated O-glycans and/or complex branched N-glycans, cells treated with BG as described earlier or Mgat5−/− eosinophils were exposed to rGal-1. rGal-1–induced cell death was higher in BG-treated cells compared with corresponding vehicle-treated cells (Fig. 5A), confirming the fact that O-glycans may mask Gal-1 effects. On the other hand, Mgat5−/− eosinophils showed reduced Gal-1–induced apoptosis relative to WT eosinophils (Fig. 5B), suggesting that Gal-1–induced eosinophil apoptosis is partly mediated by complex β1-6 branched N-glycans. Exposure of cells to rGal-1 at proapoptotic concentrations (1–5 µM) resulted in a marked increase in phosphorylated ERK(1/2) relative to untreated cells (Fig. 5C, Left and Right). Surprisingly, this effect was associated with a significant decrease in the levels of total ERK(1/2). Interestingly, pretreatment of eosinophils with MG132, a proteasome inhibitor that inhibits degradation of ubiquitin-tagged proteins, before exposure to rGal-1 prevented the reduction in total ERK levels (Fig. 5D, Left and Right). These studies suggest that rGal-1–induced apoptosis is mediated via a rapid and strong activation of ERK(1/2) and is associated with degradation of ERK(1/2) by the proteasome.
Fig. 5.
Gal-1–induced apoptosis is mediated by complex branched N-glycans and involves activation of the ERK(1/2) signaling pathway. (A) Involvement of O-glycans in Gal-1–induced apoptosis. Eosinophils cultured in medium containing BG or vehicle (DMSO) were treated with rGal-1 (5 µM) and examined for annexin V positivity. (B) Requirement of complex branched N-glycans for Gal-1–induced apoptosis. WT and Mgat5−/− eosinophils were treated with rGal-1 and examined for annexin V positivity. (C) Induction of ERK(1/2) phosphorylation in Gal-1–treated eosinophils determined by densitometric analysis of Western blots. (D) Inhibition of ERK(1/2) degradation by proteasome inhibitor MG132 determined by densitometric analysis of Western blots. (E) Inhibition of Gal-1–induced apoptosis by MEK1/2 inhibitor. (E, Right) Annexin V staining of eosinophils pretreated with the indicated inhibitors of signaling molecules and then with rGal-1 relative to vehicle alone is shown. (E, Left) Annexin V positivity in vehicle-treated versus rGal-1–treated cells is shown. (F) Caspase-independent apoptosis of eosinophils by Gal-1. Cells were pretreated with caspase inhibitor (pan; Z-VAD-FMK) at the indicated doses (or vehicle) followed by rGal-1 or staurosporine and examined for annexin V positivity. (G) Western blot of Gal-1–treated eosinophils with antibodies against cleaved caspase-3. Data shown in A and B are representative of three experiments and in G of two experiments with eosinophils from different mice. Combined data (mean ± SEM) of three or four independent experiments in C and D and four to six independent experiments in E and F are shown. *P < 0.05 in C, *P < 0.05 for 50 µM and *P < 0.01 for 100 µM U0126 in E, and *P < 0.01 for 50 and 100 µM Z-VAD in F for comparison with PBS or vehicle-treated cells.
To further investigate the signaling pathway(s) implicated in this effect, eosinophils were pretreated with inhibitors of signaling molecules such as Syk, Rho-associated protein kinase-1 (ROCK1), phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase kinase (MEK), and caspases before incubation with rGal-1 and then analyzed for annexin V binding. Significant dose-dependent inhibition of annexin V binding was noted only with the MEK inhibitor U0126, not with inhibitors of Syk, ROCK1, or PI3K (Fig. 5E). Because Gal-1–induced apoptosis in T cells is modulated via activation of caspases (18), we examined the involvement of caspases in Gal-1–induced apoptosis of eosinophils. Pretreatment with the pan-caspase inhibitor Z-VAD-FMK up to 100 µM did not inhibit Gal-1–induced eosinophil apoptosis. In contrast, this inhibitor substantially reduced staurosporine-induced apoptosis, which is known to be caspase-mediated at this concentration (Fig. 5F). These studies suggest that Gal-1–induced apoptosis of eosinophils involves activation of the ERK(1/2) signaling pathway and may be independent of caspase activation. This is further supported by the lack of caspase-3 activation in rGal-1–treated eosinophils. Unlike staurosporine, rGal-1 treatment did not result in an increase in expression of cleaved caspase-3 (∼17 kDa) by Western blot analysis (Fig. 5G).
Exposure to Gal-1 Differentially Controls Eosinophil Adhesion and Migration.
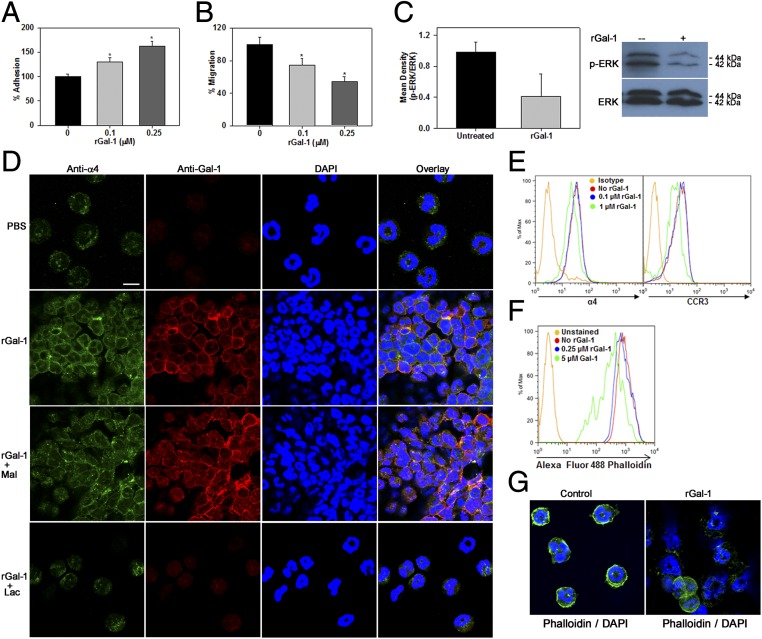
We examined the effect of rGal-1 on the ability of eosinophils to adhere to vascular cell adhesion molecule-1 (VCAM-1), which supports eosinophil trafficking and recruitment to sites of inflammation. Because rGal-1 induces apoptosis of eosinophils at concentrations of 1.0 µM or higher, these studies were carried out at lower doses of rGal-1. Cells exposed to lower concentrations of rGal-1 exhibited increased adhesion to recombinant murine VCAM-1 in a dose-dependent manner compared with untreated cells under static conditions (Fig. 6A). However, despite the increased adhesion, eosinophils treated with rGal-1 at these concentrations demonstrated significantly reduced migration toward eotaxin-1 in in vitro chemotaxis assays (Fig. 6B), an effect that was accompanied by inhibition of ERK(1/2) activation (Fig. 6C). This is in contrast to the increased activation of ERK(1/2) observed in eosinophils exposed to higher concentrations of rGal-1 in the apoptosis studies described above.
Fig. 6.
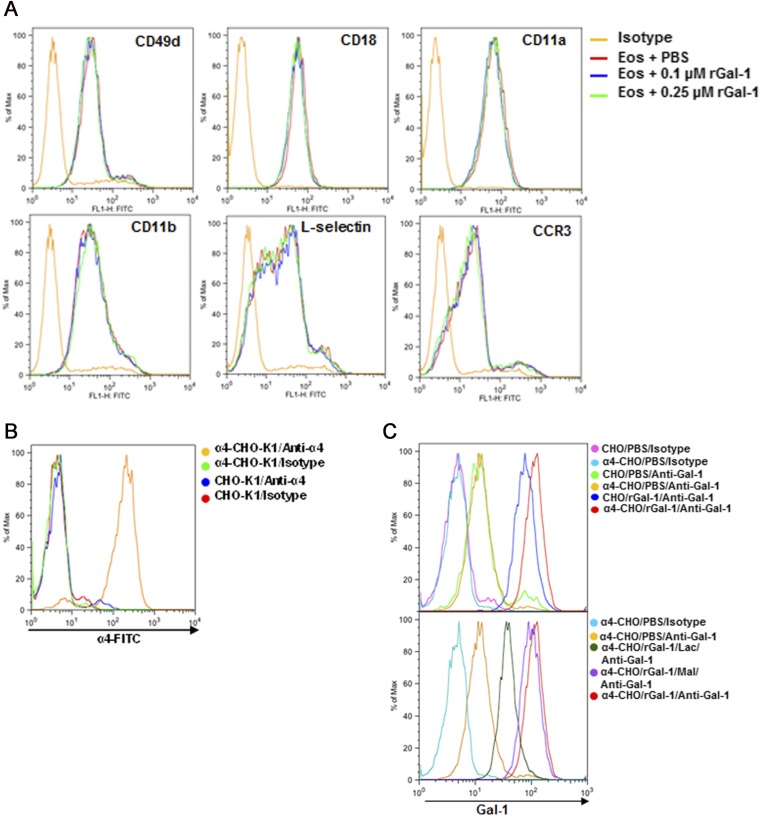
Extracellular Gal-1 differentially regulates eosinophil adhesion and migration. (A and B) Effect of rGal-1 at subapoptotic concentrations (<0.25 µM) on eosinophil adhesion to VCAM-1–coated coverslips (A) and eotaxin-1–induced migration (B). (C) Inhibition of ERK activation by Gal-1 at subapoptotic concentrations by Western blot analysis. (D) Clustering of eosinophils, redistribution of CD49d (α4), and colocalized expression of rGal-1 with α4-integrin by dual IF staining with anti-α4 (green) and anti–Gal-1 (red). (Scale bar, 10 µm.) (E) Effect of rGal-1 at proapoptotic concentrations on expression of α4 and CCR3 by flow cytometry. (F and G) Total F-actin and actin cytoskeleton in eosinophils treated with rGal-1 at proapoptotic concentrations. Combined data (mean ± SEM) of three experiments in duplicate in A, four experiments in triplicate in B, and three experiments in C (Left) are shown. Data shown in D–G are representative of three or four independent experiments with eosinophils from different mice. *P < 0.05 for 0.1 μM and *P < 0.01 for 0.25 μM rGal-1–treated cells compared with untreated in A and B.
We investigated whether treatment with Gal-1 alters the expression of cell-surface adhesion molecules that mediate eosinophil adhesion and migration by flow cytometry. Exposure to Gal-1 up to 0.25 μΜ did not appear to alter the level of expression of α4 (CD49d), β2-, αL-, or αM-integrins, L-selectin, or the eotaxin receptor CCR3 on the cell surface (Fig. S3A). However, confocal microscopy following dual IF staining with antibodies against α4 and Gal-1 indicated that relative to the more dispersed α4 expression noted in untreated cells, treatment of eosinophils with rGal-1 not only induced cell clustering but also resulted in redistribution of α4 to the periphery/edge of individual cells in the cluster, where strong Gal-1 expression was also observed (Fig. 6D). Further, several cells exhibited colocalization of α4-integrin and Gal-1. This Gal-1–mediated effect was inhibited when cells were treated with lactose but not maltose. The ability of Gal-1 to interact with α4-integrins was investigated using human α4-transfected Chinese hamster ovary (α4β1-CHO-K1) cells that express functional α4β1 relative to control CHO-K1 cells that do not (Fig. S3B). α4-CHO-K1 and control CHO-K1 cells were found to inherently express Gal-1 on their cell surface. However, increased cell-surface binding of exogenous rGal-1 to α4-CHO-K1 cells was noted relative to nontransfected cells (Fig. S3C, Upper). Further, this increased binding of exogenous rGal-1 to α4-CHO-K1 was glycan-dependent and inhibited by lactose (Fig. S3C, Lower). Because Gal-1 exerts differing effects on eosinophils depending on the concentration (alteration of adhesion and migration at lower concentrations versus apoptosis at higher concentrations), we next examined the effect of Gal-1 at a higher concentration (1.0 µM, proapoptotic) on cell-surface expression of these receptors. Interestingly, only expression of α4 and CCR3 was partially reduced at this concentration (Fig. 6E). Finally, because Gal-1 alters eosinophil motility and survival, its effect on the actin cytoskeleton was evaluated based on phalloidin staining (indicative of actin polymerization). Gal-1 at lower concentrations (0.25 µM) had no effect, whereas apoptosis-inducing concentrations resulted in a marked reduction in phalloidin binding relative to untreated cells by flow cytometry (Fig. 6F). Further, confocal microscopy revealed distinct phalloidin binding predominantly at the cell periphery/margin of control cells (Fig. 6G, Left), whereas Gal-1 treatment at proapoptotic concentrations caused disruption of this organized actin cytoskeleton in many cells, with an overall decrease in phalloidin binding (Fig. 6G, Right).
Fig. S3.
Adhesion molecule expression in rGal-1–treated eosinophils and Gal-1–α4 interaction. (A) Eosinophils were treated with rGal-1 (0.1 or 0.25 µM) or buffer alone (15 min, 37 °C) and then examined for expression of the identified adhesion molecules or CCR3 by flow cytometry. Representative data of three independent experiments with eosinophils from different mice are shown. (B) Flow cytometric analysis of α4-CHO-K1 and control CHO-K1 cells for expression of α4. (C) Interaction of rGal-1 with α4. Control and α4-CHO-K1 cells were examined for baseline Gal-1 expression and rGal-1 binding by flow cytometry with Gal-1 antibody (Upper). rGal-1 binding to α4-CHO-K1 cells in the presence and absence of lactose (Lac) and maltose (Mal) is also shown (Lower). Data shown in B and C are representative of three or four independent experiments.
Gal-1 Deficiency Results in Increased Allergen-Induced Airway Inflammation.
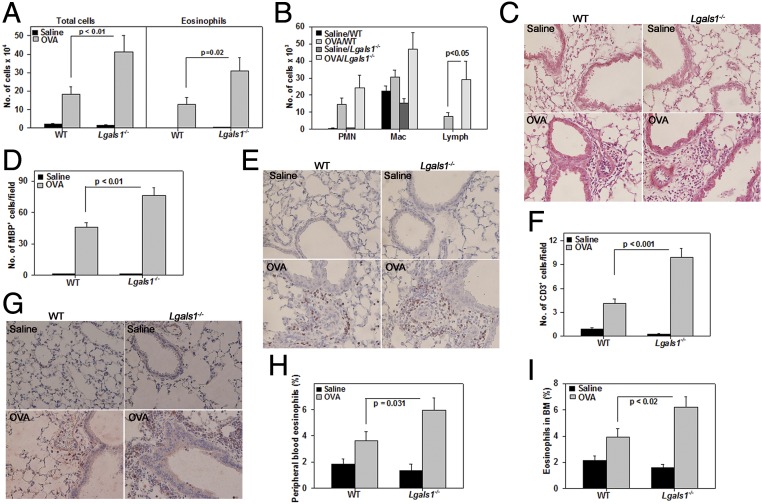
To understand the pathophysiological relevance of Gal-1 in allergic airway inflammation, WT and Gal-1–deficient (lectin galactoside-binding soluble 1 [Lgals1−/−]) mice were sensitized and challenged with OVA. OVA challenge induced a marked influx of inflammatory leukocytes in the BALF of WT and Lgals1−/− mice. However, the total number of inflammatory cells and specifically of eosinophils and lymphocytes was significantly higher in the BALF of OVA-challenged Lgals1−/− mice (Fig. 7 A and B). Consistent with this finding, H&E staining showed evidence of greater lung tissue inflammation in OVA-challenged Lgals1−/− mice relative to WT mice (Fig. 7C) and a significantly increased number of lung tissue eosinophils and T cells based on IHC for major basic protein (MBP), an eosinophil-specific protein (Fig. 7 D and E), and CD3, a T cell-specific marker (Fig. 7 F and G), respectively. Along with increased airway eosinophils, peripheral blood and BM differential cell counts indicated a significantly higher percentage of eosinophils in OVA-challenged Lgals1−/− mice relative to WT counterparts (Fig. 7 H and I).
Fig. 7.
Gal-1 deficiency leads to increased airway inflammation after allergen exposure. (A and B) Number of total cells (A, Left) and eosinophils (A, Right) and number of PMNs, macrophages (Mac), and lymphocytes (Lymph) (B) in the BALF from saline- and OVA-challenged WT and Lgals1−/− mice. (C) Cellular infiltration in lung tissue after H&E staining. Representative images are shown. (Scale bar, 50 µm.) (D and E) Quantitation of lung tissue eosinophils and representative images after MBP IHC (stained reddish brown). (Scale bar, 50 µm.) (F and G) Quantitation of lung tissue T cells and representative images after CD3ε IHC (stained brown). (Scale bar, 50 µm.) (H and I) Quantitation of eosinophils in peripheral blood and BM, respectively, after Hema 3 staining. Combined data (mean ± SEM) of mice from three independent experiments (n = 8–9 mice for OVA groups and n = 6 mice for saline groups in A, B, D, and F; n = 7–8 mice for OVA groups and n = 7 mice for saline groups in H and I) are shown.
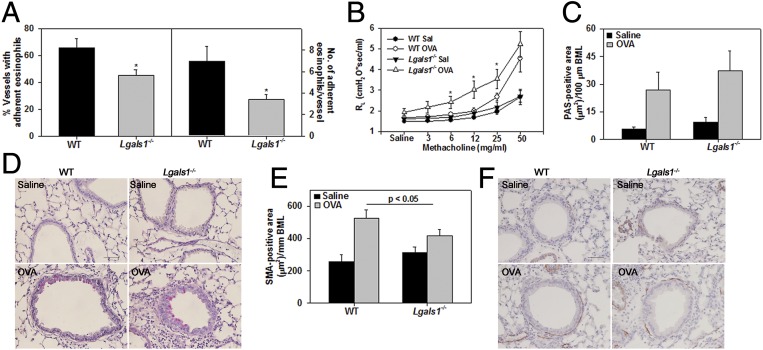
A larger number of blood vessels in the lungs of WT OVA-challenged mice exhibited eosinophils (MBP-positive cells) adherent along the vessel wall relative to lung blood vessels of Lgals1−/− mice (Fig. 8A, Left). Further, the number of adherent eosinophils per vessel was also significantly higher in WT OVA-challenged mice compared with Lgals1−/− counterparts (Fig. 8A, Right). AHR in control and OVA-challenged WT and Lgals1−/− mice exposed to increasing concentrations of aerosolized methacholine was evaluated (Fig. 8B). Control mice of both genotypes displayed only a marginal increase in pulmonary resistance (RL) with increasing doses of methacholine. In OVA-challenged Lgals1−/− mice, airway resistance was significantly higher compared with corresponding control mice even at low doses of methacholine challenge (i.e., 6 mg/mL), and remained elevated at higher doses. OVA-challenged WT mice, on the other hand, exhibited increased airway resistance relative to WT control mice only at higher doses of methacholine (>25 mg/mL). More importantly, airway resistance in OVA-challenged Lgals1−/− mice was significantly higher than in WT counterparts at 6, 12, and 25 mg/mL methacholine. These findings are indicative of a higher tendency to develop AHR in OVA-challenged Lgals1−/− and are consistent with the increased cellular inflammation observed in these mice relative to WT allergen-challenged mice. In addition to cellular inflammation, airway mucus accumulation and increased airway smooth muscle mass are important factors that contribute to increased airway resistance. OVA challenge increased airway mucus secretion and airway smooth muscle mass in WT and Lgals1−/− mice compared with corresponding control mice (Fig. 8 C–F). Whereas there was no significant difference between the OVA-challenged groups with respect to airway mucus secretion (Fig. 8 C and D), smooth muscle mass in OVA-challenged Lgals1−/− mice was lower than in WT mice (Fig. 8 E and F).
Fig. 8.
Increased airway reactivity in allergen-challenged Gal-1–deficient mice. (A) Quantitation of the number of lung blood vessels with eosinophils (MBP-positive cells) and the number of adherent eosinophils in the blood vessels of OVA-challenged WT and Lgals1−/− mice. (B) Pulmonary resistance in saline- and OVA-challenged WT and Lgals1−/− mice following exposure to aerosolized methacholine. (C and D) Quantitation of mucus secretion (stained pink) in airways of saline- and OVA-challenged WT and Lgals1−/− mice along with representative images. BML, basement membrane length; PAS, periodic acid–Schiff reagent. (Scale bar, 50 µm.) (E and F) Quantitation of airway smooth muscle mass based on α-SMA IHC (stained brown). Representative images are shown in F. (Scale bar, 50 µm.) Combined data (mean ± SEM) of mice from two independent experiments (n = 7–8 mice per group) in A and three independent experiments (n = 7–8 mice for OVA groups and n = 6–7 mice for saline groups) in B, C, and E are shown. *P < 0.025 in A and *P < 0.05 at 6 and 25 mg/mL and *P < 0.03 at 12 mg/mL methacholine in B for comparison of OVA-challenged groups.
Modulation of Cytokines and Chemokines in Airways of Allergen-Challenged WT and Lgals1−/− Mice.
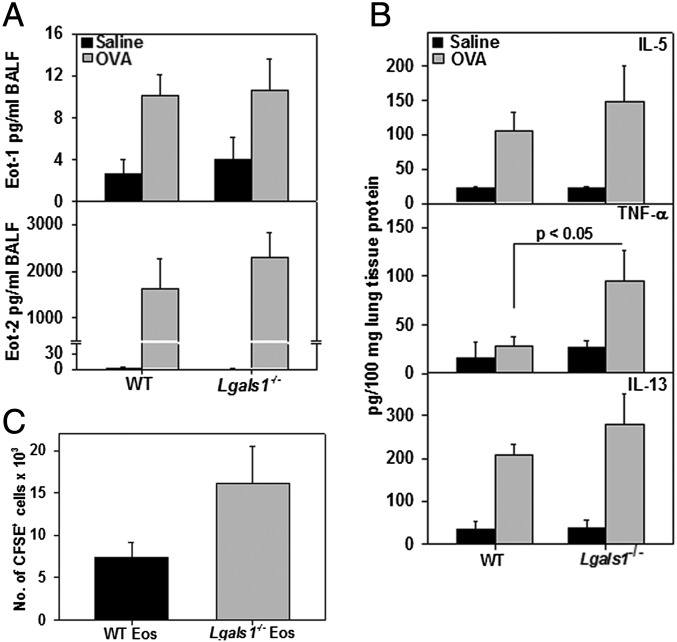
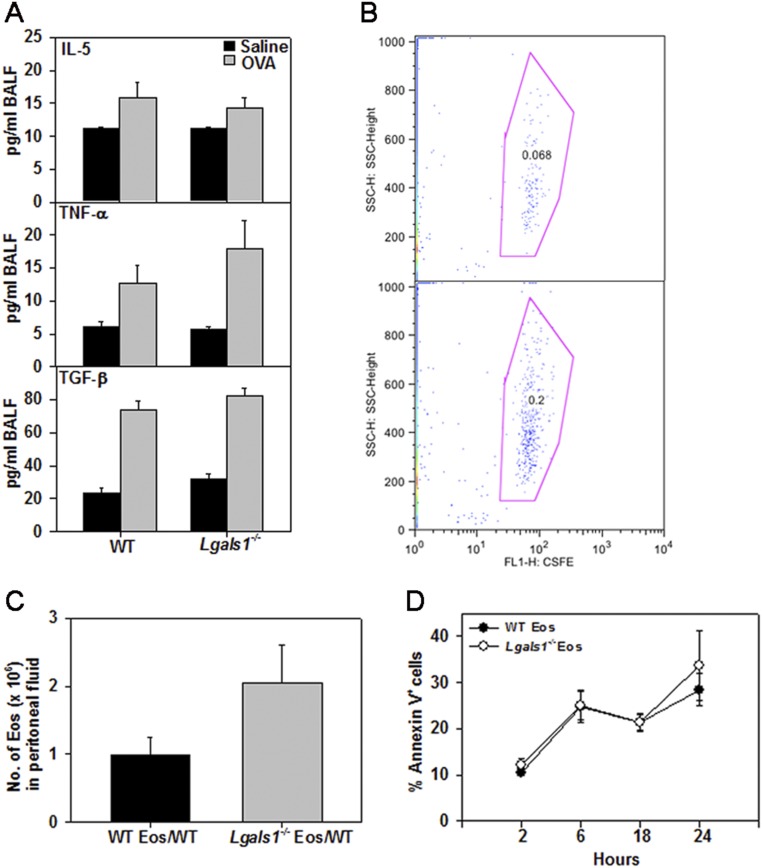
Consistent with the asthma phenotype, IL-5, TNF-α, TGF-β, eotaxin-1, and eotaxin-2 (CCL24) were all significantly elevated in the BALF of OVA-challenged mice of both genotypes compared with corresponding control mice. No significant difference in the levels of any of these mediators was noted between OVA-challenged Lgals1−/− and WT mice (Fig. 9A and Fig. S4A). However, TNF-α levels were significantly higher in lung tissue from OVA-challenged Lgals1−/− mice than in WT counterparts, and IL-5 and IL-13 also tended to be higher (Fig. 9B).
Fig. 9.
Inflammatory cytokines and chemokines in airways of allergen-challenged WT and Gal-1–deficient mice. (A) Eotaxin-1 and -2 (Eot-1, Eot-2) levels in the BALF of saline- and OVA-challenged WT and Lgals1−/− mice. (B) IL-5, TNF-α, and IL-13 levels in lung lysates from the above groups of mice. Combined data (mean ± SEM) of mice from three independent experiments (n = 7–9 mice for OVA groups and n = 6–7 mice for saline groups) are shown in A and B. (C) Recruitment of i.v. infused CFSE-labeled BM eosinophils from WT or Lgals1−/− mice to the peritoneum of mice with TG-induced inflammation. Combined data (mean ± SD) of two out of three experiments in duplicate mice with similar results are shown.
Fig. S4.
BALF cytokines in allergen-challenged WT and Lgals1−/− mice and recruitment of WT versus Lgal1s−/− eosinophils to sites of inflammation. (A) IL-5, TNF-α, and TGF-β levels in the BALF of saline- and OVA-challenged WT and Lgals1−/− mice. (B) CFSE-labeled BM eosinophils from WT (WT Eos) or Lgals1−/− (Lgals1−/− Eos) mice were infused (i.v.) into WT mice with TG-induced peritoneal inflammation as described in the main text. A representative dot plot of CFSE-labeled BM eosinophils recruited to the peritoneum in each case is shown. (C) Total number of eosinophils (infused and endogenous) in peritoneal fluid collected 2 h after infusion of WT or Lgals1−/− cells based on differential cell counts. (D) Survival of WT versus Lgals1−/− Eos. BM Eos from WT or Lgals1−/− mice were cultured up to 24 h in medium containing low serum (5%) and assessed for constitutive apoptosis based on annexin V staining at the indicated time points by flow cytometry. Combined data (mean ± SEM) of three independent experiments (n = 7–9 mice for OVA and n = 6–7 mice for saline group) in A and for BM-derived eosinophils from n = 3 different mice per group in C and D are shown.
The increased recruitment of eosinophils to the airways and peripheral blood of allergen-challenged Lgals1−/− mice relative to WT mice (Fig. 7) is unlikely to be entirely due to elevated eotaxin-1 and -2 because levels of these chemokines were not found to be substantially different between the two allergen-challenged groups. Thus, we examined inherent differences between WT and Lgals1−/− eosinophils with respect to migration in vivo. BM eosinophils from WT and Lgals1−/− mice were carboxyfluorescein succinimidyl ester (CFSE)-labeled and infused into WT mice with thioglycollate (TG)-induced peritoneal inflammation. There was a twofold increase in the number of CFSE-labeled Lgals1−/− eosinophils recruited to the inflamed peritoneum relative to WT eosinophils based on flow cytometry analysis of the peritoneal fluid (Fig. 9C and Fig. S4B). This was reflected in a larger number of total eosinophils (endogenous eosinophils plus infused eosinophils) in the peritoneum of WT mice that received Lgals1−/− eosinophils compared with WT mice that were infused with WT eosinophils (Fig. S4C). We also compared survival of WT versus Lgals1−/− eosinophils in culture medium containing low serum up to 24 h but found no inherent difference in constitutive apoptosis based on annexin V staining (Fig. S4D), suggesting that endogenous Gal-1 did not regulate eosinophil viability. Collectively, these findings suggest that Gal-1–deficient eosinophils exhibit increased ability to recruit to sites of inflammation compared with WT eosinophils, which could also contribute to the increased eosinophilia observed in allergen-challenged Lgals1−/− mice.
Discussion
Eosinophils recruited to allergic airways serve as a reservoir for proinflammatory cytokines, chemokines, growth factors, and cytotoxic granule proteins (MBP, EPX, and eosinophil cationic protein) that cause significant epithelial tissue damage and contribute to bronchoconstriction, mucus production, vascular permeability, and airway remodeling (19). Given the homeostatic/antiinflammatory role of Gal-1 in multiple chronic disease models as well as acute inflammation models (5, 6), we examined the role played by Gal-1 in allergic airway inflammation and asthma. Exposure to various allergens (OVA, Alternaria, and CRA) resulted in increased Gal-1 expression in the lungs due to the recruitment of Gal-1–expressing inflammatory cells, including eosinophils, as well as increased expression of Gal-1 in the basement membrane of the airway epithelium and extracellular spaces beneath the epithelium and blood vessel wall. Expression of Gal-1 has been demonstrated in the nucleus, cytosol, and intracellular side of cell membranes as well as extracellularly in culture supernatants and in the ECM of tissues despite the absence of a secretion signal sequence (5, 20). Based on techniques used in the current study, soluble Gal-1 was not detectable in the BALF of OVA-challenged mice. Gal-1 could be secreted at concentrations below the detection limit of our assay or the secreted Gal-1 may rapidly bind to cell-surface glycans or the ECM and thus not remain in a soluble form at concentrations high enough to be detected.
Studies with BM-derived eosinophils confirmed that eosinophils (nonactivated) express Gal-1 intracellularly but not on the cell surface. These findings are consistent with Gal-1 expression in activated T cells where only intracellular expression was noted, with no detectable cell-surface expression or secretion (15). Further, intracellular Gal-1 expression or expression on the cell surface was not induced after stimulation/activation with asthma-inducing mediators such as TNF-α, eotaxin-1, or SCF-1. Despite the lack of endogenous cell-surface Gal-1 expression, murine eosinophils were found to rapidly bind to exogenous/extracellular Gal-1 on the cell surface in a carbohydrate-dependent manner. Gal-1 is known to bind preferentially to complex N- and O-glycans containing terminal modified or unmodified LacNAc residues (5), and can therefore bind to a host of cell-surface glycoproteins that contain these glycan components. Glycophenotyping of murine eosinophils confirmed the expression of complex N- and O-glycans bearing LacNAc residues on the cell surface that could potentially bind to Gal-1. Further, lectin inhibition studies demonstrated the involvement of LacNAc residues of eosinophil-expressed cell-surface glycans in binding to Gal-1. Studies to determine the relative requirement of N- versus O-glycans for Gal-1 binding suggest that eosinophil-expressed complex N-glycans are involved in this interaction. Although O-glycans do not appear to be required for Gal-1 binding, based on increased binding of exogenous Gal-1 in the absence of complex O-glycans, it is possible that O-glycans may play a role in regulating Gal-1 binding to N-glycans by masking N-glycans that participate in binding to Gal-1. This is in contrast to activated T cells, where both N- and O-glycans participate in Gal-1 binding (14).
Although eosinophils did not appear to secrete Gal-1 in response to stimulation with TNF-α, eotaxin-1, or SCF-1 in vitro, these mediators may play a different role in vivo and induce Gal-1 secretion, which could potentially exert autocrine effects. Further, in vivo, eosinophils are likely to be exposed to varying concentrations of soluble Gal-1 released by other cells such as endothelial cells (20) or to Gal-1 presented by the ECM (21) during inflammation. Our in vitro studies demonstrate that Gal-1 rapidly induces apoptosis of eosinophils at high concentrations, typical of inflammatory reactions. The proapoptotic effect of Gal-1 has previously been demonstrated in other cell types such as T cells (14), B cells (22), epithelial cells (23), and endothelial cells (24) but not macrophages, DCs, or mast cells (25). Gal-1–induced apoptosis of both BM-derived as well as peripheral blood eosinophils from allergen-challenged mice occurred at lower concentrations and more rapidly (≥1 µM by 15 min) than in T cells (14), B cells (22), and endothelial cells (24). Further, Gal-1 treatment caused cell shrinkage in eosinophils, a hallmark feature of apoptosis (26). These Gal-1–mediated effects were significantly inhibited in the presence of lactose, establishing their carbohydrate-dependent nature. Gal-1 at higher concentrations also caused a marked reduction in total F-actin levels (indicative of F-actin depolymerization) and disruption of the cytoskeleton, consistent with cell disintegration into apoptotic bodies (26). Previous studies investigating the effect of immobilized Gal-1 (coated on plastic supports) on adhesion and migration of human eosinophils reported altered actin polymerization/depolymerization dynamics with a prevalence of glomerular actin (27), suggesting that the actin cytoskeleton is a target of Gal-1–mediated signaling. Eosinophils exposed to proapoptotic concentrations of Gal-1 exhibited reduced cell-surface expression of α4 and CCR3. It is possible that these receptors are rapidly recycled or shed, a phenomenon previously described in other cell types during apoptosis (28) that may serve as a mechanism to suppress eosinophil activation and recruitment.
In activated T cells, Gal-1–induced cell death has been shown to involve the participation of AP-1 (4), NF-κB (29), and Bcl-2 (4) as well as activation of caspases (18), although caspase-independent T-cell apoptosis has also been shown to occur (30). In the current study, Gal-1–induced apoptosis of eosinophils did not appear to involve activation of caspases, similar to the effects observed in endothelial cells (24). However, a role for ERK(1/2) was identified. Exposure to rGal-1 at concentrations >1 μΜ caused a marked increase in phosphorylation of ERK(1/2), and eosinophil apoptosis was inhibited by the MEK inhibitor U0126. Whereas activation of the ERK(1/2) pathway is typically known to govern cell proliferation and differentiation and is protective against apoptosis (31), recent studies indicate that activation of ERK by certain stimuli can indeed act as a cell-death signal and facilitate engagement of apoptotic programs (32). Interestingly, in the current study, increased ERK(1/2) activation was associated with a drastic reduction/degradation of ERK(1/2) by the proteasome. Previous studies have demonstrated that, in addition to functioning as an upstream activator of ERK(1/2), MEKK1 has E3 ubiquitin ligase activity and can thus mediate ubiquitination and degradation of ERK(1/2), which may serve as a negative regulatory mechanism for decreasing ERK(1/2) activity in stress-induced cells (33).
Apart from its ability to induce apoptosis, Gal-1 has been shown to affect immune cell motility. Whereas PMNs were less migratory toward IL-8 after treatment with Gal-1 (34), DCs demonstrated increased chemokine-induced migration (35) in vitro. Further, at lower concentrations (subapoptotic), Gal-1 has been shown to inhibit T-cell adhesion to ECM proteins in a dose-dependent manner (36). Our studies show that Gal-1 treatment at subapoptotic concentrations (up to 0.25 µM) causes eosinophils to form clusters, increases their adhesion to VCAM-1, and alters distribution of α4-integrin to the cell periphery but does not affect the expression level of adhesion receptors (α4- or β2-integrins and L-selectin). In this regard, Gal-1 has been reported to directly bind to β1-integrins on vascular smooth muscle cells, transiently increasing the availability of its active form on the cell surface and thus modulating adhesion (37). Treatment of eosinophils with Gal-1 at the same concentrations that increased adhesion resulted in decreased eotaxin-1–induced migration, with the expression level of CCR3 remaining unaltered. Exogenous Gal-1–induced clustering of cells along with increased adhesion to VCAM-1 and inhibition of ERK(1/2) activation [a signaling molecule required for eotaxin-1–induced eosinophil migration (38)] may result in the cells being less motile to undergo directed movement toward eotaxin-1. These in vitro findings are further supported by observations in our model of allergic airway inflammation where eosinophils adherent in lung blood vessels were more commonly noted in allergen-challenged WT mice than in Lgals1−/− mice, potentially due to exposure to Gal-1 secreted by or presented on the surface of endothelial cells. Indeed, endothelial cells in allergen-challenged mice express Gal-1 based on our IHC data. Additionally, allergen-challenged Lgals1−/− mice exhibited increased lung tissue eosinophils relative to WT counterparts despite similar levels of the eosinophil-specific chemokines eotaxin-1 and -2, which could be due to the lack of the inhibitory/limiting effects of Gal-1 on eosinophil migration that was noted in vitro.
Consistent with the inhibitory effect of exogenous Gal-1 on eosinophil migration in vitro and the increased number of blood vessels with adherent eosinophils in allergen-challenged WT mice relative to Gal-1–deficient mice, eosinophil recruitment in the BALF and lung tissue after allergen challenge was significantly lower in WT mice than in Gal-1–deficient counterparts. Further, these mice had lower peripheral blood and BM eosinophilia. In a WT setting, depending on concentrations in the local milieu, extracellular Gal-1 may regulate eosinophilia by inducing apoptosis of differentiated eosinophils in the BM or inhibit their migration/mobilization from the BM to sites of inflammation. Indeed, previous studies have shown that administration of Gal-1 can prevent cyclophosphamide- and G-CSF–induced migration of progenitor cells from the BM in vivo (39). Along with the increased eosinophilia, allergen-challenged Gal-1–deficient mice exhibited significantly elevated T cells and AHR. However, airway smooth muscle mass was somewhat lower in allergen-challenged Gal-1–deficient mice despite more inflammation. Gal-1 is secreted by vascular smooth muscle cells and is known to induce vascular smooth muscle proliferation, attachment, spreading, and migration (37). It is conceivable that it may have a similar effect on airway smooth muscle cells, which might explain the decrease in α-smooth muscle actin (α-SMA)–positive cells in allergen-challenged Gal-1–deficient mice relative to WT counterparts. Finally, TNF-α in the lung tissue of allergen-challenged Gal-1–deficient mice was significantly higher relative to WT counterparts. TNF-α is secreted by eosinophils and T cells, both of which are elevated in allergen-challenged Gal-1–deficient mice. It is known to promote migration of inflammatory cells as well as induction of AHR (40) and inhibit the suppressive function of both naturally occurring and induced Tregs in asthmatic patients (41). In this regard, studies have shown that Gal-1 is required for the regulatory activity of CD4+CD25+ Tregs (10). The inherently increased ability of Gal-1–deficient eosinophils to recruit to sites of inflammation versus WT cells as noted in the peritonitis model, suggestive of a role for intracellular Gal-1 in regulating eosinophil migration independent of the extracellular effect of Gal-1, could also be an important player responsible for the increased eosinophilia in allergen-challenged Gal-1–deficient mice. In a recent study, administration of rGal-1 to mice with IgE-mediated allergic conjunctivitis resolved clinical signs as well as reduction in Th2 cytokines, eotaxin, RANTES, and MAPK signaling. However, this was associated with increased Gal-1 expression and eosinophilia in the conjunctiva relative to untreated mice (42).
Overall, our studies demonstrate that Gal-1, which is induced in the lung in response to allergen challenge, is important in regulating airway inflammation. This is supported by our in vitro findings that extracellular Gal-1 can bind to eosinophil-expressed cell-surface glycans in a carbohydrate-dependent manner to inhibit cell migration and induce apoptosis as well as our in vivo studies demonstrating increased eosinophilia and airway inflammation in the absence of the endogenous lectin. Targeting eosinophils is an important strategy for alleviating not only allergic asthma but potentially even other eosinophil-driven disorders.
Materials and Methods
Mice.
Mice with a deletion of the gene encoding Gal-1 (Lgals1−/−), originally provided by F. Poirier, Institut Jacques Monod, Paris, France, or Mgat5 (Mgat5−/−; obtained from the Consortium for Functional Glycomics) (43), both on C57BL/6 background, were bred in-house. Age- and gender-matched C57BL/6 mice (8–12 wk old) were used as WT controls. All studies involving mice were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Culture of Murine Eosinophils and Preparation of Recombinant Gal-1.
Eosinophils were cultured from naïve BM of WT, Lgals1−/−, and Mgat5−/− mice as described previously (43, 44). Expression and purification of recombinant Gal-1 were accomplished as outlined previously (45). LPS was removed by Detoxi-Gel endotoxin removing gel (Pierce), and samples were tested for LPS content using the gel-clot Limulus test (<0.5 IU/mg).
Cell Lysis and Western Blot Analysis.
Detailed methods for cell lysis and Western blotting are described in SI Materials and Methods.
Gal-1 Expression and Binding to Eosinophils.
Expression of Gal-1 by murine eosinophils was evaluated by flow cytometry and confocal microscopy after IF staining as detailed in SI Materials and Methods using polyclonal antibodies against human Gal-1 prepared as described previously (46).
To determine whether Gal-1 binds to eosinophils, cells were treated with rGal-1 at 0.1 or 0.25 μΜ in PBS or vehicle alone for 15 min at 37 °C. After washing (once with PBS), Gal-1 expression on the cell surface (nonpermeabilized) was examined by flow cytometry using antibodies against Gal-1 (SI Materials and Methods). In some experiments, cells were incubated with rGal-1 in the presence of lactose (10 or 30 mM), a Gal-1–specific disaccharide, or maltose as a control before analysis.
Glycophenotyping and Gal-1–Binding Partners Expressed in Eosinophils.
Biotinylated plant lectins with known specificities were used to identify glycan epitopes on the cell surface of BM-derived eosinophils. Cells were incubated with TL, MAL-II, SNA, PHA-L, or PNA (all at 20 μg/mL; Vector Laboratories) or vehicle (PBS) for 30 min followed by DyLight 488 conjugated to streptavidin (Vector Laboratories). In some studies, eosinophils were preincubated with the above lectins or vehicle alone for 20 min at room temperature and then treated with rGal-1 (0.1 µM). Gal-1 binding to the cell surface was examined by flow cytometry as described earlier. Results were expressed as fold change in geometric mean fluorescence intensity relative to vehicle-treated cells.
To examine the requirement of O-glycans, eosinophils were cultured in medium containing BG (2 mM) or vehicle (DMSO) for 65 h as described (14) and then examined for rGal-1 binding by flow cytometry. To examine the requirement of complex branched N-glycans, binding of rGal-1 to BM eosinophils from Mgat5−/− mice relative to WT mice was evaluated.
Apoptosis Assays.
Eosinophils were treated with 0.1–5 μM rGal-1 in PBS (or PBS only) at 37 °C for up to 60 min and examined for cell viability by trypan blue exclusion. In another set of experiments, eosinophils were treated with rGal-1, washed, and analyzed by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit (eBioscience). Briefly, eosinophils were collected after treatment with Gal-1, resuspended in binding buffer, and stained with FITC-conjugated annexin V for 15 min followed by propidium iodide (PI). To additionally examine the effect of Gal-1 on peripheral blood eosinophils, total leukocytes (after lysis of red blood cells) obtained from allergen-challenged mice were exposed to PBS or rGal-1 (1 μM for 15 min at 37 °C). Cells were dual-stained with annexin V-FITC and phycoerythrin (PE)-conjugated rat anti-mouse Siglec-F (BD Biosciences). PE-conjugated rat IgG was used as isotype control. Changes in annexin V positivity in the Siglec-F–positive population (eosinophils) were determined by flow cytometry. Involvement of O- versus complex N-glycans in Gal-1–mediated apoptosis was examined by exposing eosinophils cultured in medium containing BG or BM eosinophils from Mgat5−/− mice to rGal-1 (5 µM) and analyzed for annexin V staining. To examine the role of specific signaling molecules in Gal-1–mediated eosinophil apoptosis, cells were incubated with Y-27632 (inhibitor of ROCK1; Cayman Chemical), U0126 (inhibitor of MEK; Cayman Chemical), LY294002 (inhibitor of PI3K; Sigma-Aldrich), Bay 61-3606 (synonym Syk inhibitor IV, inhibitor of Syk tyrosine kinase; EMD Millipore), or pan-caspase inhibitor [synonym Z-VAD (OMe)-FMK; Santa Cruz Biotechnology] at 10, 50, or 100 μΜ for 20 min in DMEM at 37 °C and then exposed to rGal-1 (1–5 µM) or PBS alone as described above. Cells treated with staurosporine (20 µM; Santa Cruz Biotechnology), a known inducer of caspase-mediated apoptosis (47), served as a positive control. Cells preincubated with DMSO (Sigma-Aldrich) or water alone in media served as the vehicle control. Additionally, cells treated with rGal-1 or staurosporine (20 μΜ) for 60 min were applied to poly-l-lysine–coated slides and evaluated by TUNEL assay (DeadEnd Colorimetric TUNEL System; Promega).
Adhesion and Migration Assays.
The effects of Gal-1 on eosinophil adhesion and migration were evaluated as described previously (48, 49) and are detailed in SI Materials and Methods.
Cell-Surface Receptor Expression and Actin Polymerization.
Changes in the expression of cell-surface adhesion molecules and the actin cytoskeleton (based on phalloidin binding) after treatment with rGal-1 were examined by flow cytometry and confocal microscopy as detailed in SI Materials and Methods.
Evaluation of Gal-1–α4-Integrin Interaction.
BM eosinophils were treated with rGal-1 (0.25 μM) in the presence or absence of 30 mM lactose (or maltose) or with buffer alone in suspension and then cytocentrifuged (75 × g) onto glass slides. Cells were dual-stained with anti–Gal-1 polyclonal antibodies (30 μg/mL) and anti-CD49d mAb (clone R1-2, 10 μg/mL; BD Biosciences) followed by Rhodamine Red-X–conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-rat IgG, respectively (both from Jackson ImmunoResearch Laboratories), and examined by confocal microscopy. Rabbit IgG (for Gal-1) and rat IgG (for CD49d) were used as negative controls. In some studies, α4-transfected CHO-K1, kindly provided by Yoshikazu Takada, Scripps Research Institute, La Jolla, CA (50), or nontransfected control CHO-K1 cells cultured to 70% confluence were treated with PBS or rGal-1 and examined for Gal-1 binding by flow cytometry with antibodies against Gal-1 (see SI Materials and Methods for details).
Models of Allergic Airway Inflammation.
For acute allergen-induced airway inflammation, WT or Lgals1−/− mice (C57BL/6; 8–12 wk old) were sensitized and challenged with OVA (grade V; Sigma-Aldrich) as described (51). Control mice were administered saline instead of OVA for sensitization and challenge. Additionally, lungs from C57BL/6 WT mice exposed to chronic challenge with OVA (52), acute challenge with CRA (Hollister-Stier Laboratories) (48), or an extract of A. alternata (Greer) (53) were harvested. Mice were killed 24 h after the last allergen challenge in all models.
Measurement of Airway Responsiveness.
Pulmonary function in control and acute OVA-challenged WT and Lgals1−/− mice was assessed by invasive plethysmography (FinePointe RC System; Buxco) (51). Changes in pulmonary resistance were monitored continuously in response to saline followed by increasing concentrations of inhaled methacholine (3–50 mg/mL) nebulized for 18–20 s and expressed as mean RL values following each dose of methacholine.
Sample Collection and Analysis.
Total cell counts in the BALF were determined using a hemocytometer. Differential cell counts in the BALF as well as blood and BM were determined based on morphologic and histologic criteria after staining cytocentrifuged samples with the Hema 3 System (Thermo Fisher Scientific). BALF supernatants were stored at −80 °C for later analysis. Right lungs were snap-frozen and left lungs were perfused and fixed in 4% (vol/vol) paraformaldehyde before paraffin embedding. Lgals1 expression in lung tissue was examined by quantitative PCR as described in SI Materials and Methods.
Lung Histology.
Paraffin-embedded tissue sections from OVA-challenged WT and Lgals1−/− mice were evaluated for cellular infiltration [total cells, eosinophils (MBP-positive), and lymphocytes (CD3-positive)], Gal-1 expression, smooth muscle hyperplasia, and airway mucus secretion by histology as described in SI Materials and Methods. To examine Gal-1 expression by lung tissue eosinophils, sections were dual-stained with antibodies against Gal-1 (4 μg/mL) and mAb against EPX (10 μg/mL) (54) and examined by confocal microscopy.
Measurement of Lung Cytokines and Chemokines.
Th1 (IL-2, IFN-γ)/Th2 (IL-4, IL-5, IL-13) cytokine and TNF-α levels in supernatants of lung tissue lysates and/or BALF were determined by flow cytometry using CBA Flex Set Kits (BD Biosciences) and expressed as pg cytokine per 100 mg lung tissue protein or pg/mL BALF. Eotaxin-1, eotaxin-2, and TGF-β1 in the BALF were measured using ELISA kits (R&D Systems).
In Vivo Migration Assay.
Migration/recruitment of infused CFSE-labeled BM-derived eosinophils from WT versus Lgals1−/− mice was evaluated in a model of TG-induced peritoneal inflammation (43). Total and differential cell counts in the peritoneal fluid were determined and cells were analyzed by flow cytometry to determine the number of CFSE-labeled eosinophils.
Statistical Analysis.
Results are expressed as mean ± SEM (or mean ± SD where noted) of combined data from multiple experiments. Statistical comparison between two groups was determined by an unpaired Student’s t test. Comparisons between control and multiple treatment conditions were carried out by ANOVA followed by Dunnett’s posttest. A P value < 0.05 was considered to be significant.
SI Materials and Methods
Cell Lysis and Western Blot Analysis.
Bone marrow (BM) eosinophils or lung tissue was lysed in radioimmunoprecipitation assay (RIPA) buffer containing proteinase inhibitors, and total protein in the supernatants was measured using the BCA Protein Assay Kit (Thermo Fisher Scientific). Expression of Gal-1 in cell (30 µg per lane) or lung (25 µg per lane) lysates was evaluated by SDS/PAGE (15%) under reduced conditions followed by incubation with rabbit polyclonal anti-human Gal-1 (0.25 µg/mL for cell lysates or 0.4 µg/mL for lung tissue lysates, overnight at 4 °C) obtained as previously described (46, 55). Expression levels of actin were monitored as an internal control using a monoclonal antibody (mAb) against mouse muscle actin (0.05 μg/mL; BD Biosciences). Goat anti-rabbit IRDye 800CW (for BM eosinophils) and goat anti-mouse IRDye 680LT (both at 1:8,000, 1 h at room temperature; LI-COR Biosciences) were used as secondary antibodies for Gal-1 and actin, respectively. Bound antibodies were detected using the Odyssey Infrared Imaging System (LI-COR Biosciences). For lung lysates, HRP-conjugated goat anti-rabbit IgG (1:5,000; Jackson ImmunoResearch Laboratories) was used as the secondary antibody. HRP-conjugated mouse mAb against actin (1:5,000; Santa Cruz Biotechnology) was used to monitor expression of β-actin. Bound antibodies were detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore), and bands were visualized on X-ray films. The intensity of bands was quantified using ImageJ image analysis software (56), and the expression level of Gal-1 was normalized against that of actin.
In some experiments, eosinophils were treated with rGal-1 at 0.25 or 1–5 µM for 15 min at 37 °C before lysis with RIPA buffer and then analyzed for activation of ERK(1/2) by Western blot analysis. For detection of total and phospho-ERK(1/2), rabbit mAb against total ERK(1/2) (1:1,000) and rabbit polyclonal antibodies against phospho-ERK(1/2) (1:1,000) (both from Cell Signaling Technology), respectively, were used followed by HRP-conjugated goat anti-rabbit IgG (1:3,000) with 40 μg eosinophil lysate per lane. HRP-conjugated mouse mAb against actin was used to monitor expression of β-actin. The intensity of detected bands was quantified using ImageJ image analysis software, and the expression level of phospho-ERK(1/2) was normalized against that of total ERK(1/2) or actin. In certain experiments, cells were treated with MG132 (EMD Millipore), a proteasome inhibitor (25 μM) (33), or DMSO (vehicle) for 30 min before exposure to rGal-1 (1–5 µM) and then examined for ERK(1/2) activation as described above. For detection of cleaved caspase-3, cells were treated with rGal-1 (1–5 µM) or staurosporine (20 μM) for 60 min at 37 °C. Lysates in RIPA buffer (50 µg per lane) were examined by immunoblotting with a polyclonal antibody against cleaved caspase-3 (1:100; Cell Signaling Technology) followed by HRP-conjugated goat anti-rabbit IgG. Cells treated with PBS or DMSO served as controls.
The presence of soluble Gal-1 or Gal-3 in the BALF (20 µL per lane) from saline- and OVA-challenged WT mice was evaluated by immunoblotting with anti–Gal-1 as described above or for anti–Gal-3 as described previously (49). Lung tissue from an OVA-challenged mouse was used as a positive control in each case.
Gal-1 Expression by Murine Eosinophils.
Murine eosinophils were fixed and permeabilized in 4% paraformaldehyde containing 0.1% saponin for 20 min on ice or used without fixing and permeabilizing. Cells were then treated with antibodies against Gal-1 (5 µg/mL) or rabbit IgG (control), followed by FITC-conjugated donkey anti-rabbit IgG (10 μg/mL; Jackson ImmunoResearch Laboratories) after washing (once with PBS) and evaluated by flow cytometry with a FACScan flow cytometer (BD Biosciences) equipped with FlowJo software for analysis (version 8.8.2; Tree Star). Eosinophils were also examined for Gal-1 expression by immunofluorescence (IF) staining. Cells cytocentrifuged onto glass slides were fixed and permeabilized (4% paraformaldehyde followed by 0.2% Triton X-100). After blocking (1.5% goat serum in Tris-buffered saline; blocking buffer), cells were incubated overnight at 4 °C with antibodies against Gal-1 (4 μg/mL) or rabbit IgG (control) in blocking buffer. Bound antibodies were detected using Rhodamine Red-X–conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories). Cells were counterstained with DAPI to visualize nuclei and examined using a confocal laser scanning biological microscope (FV1000/BX61; Olympus). FV10-ASW 3.1 software was used for image acquisition (Olympus).
In some experiments, eosinophils were cultured for 24 h in medium alone or medium containing murine eotaxin-1 (CCL11; 100 nM), TNF-α (100 ng/mL), or SCF-1 (200 ng/mL) before evaluating the expression of Gal-1. The presence of Gal-1 in culture supernatants (secreted Gal-1) was evaluated by Western blot after concentration of samples (∼10-fold). The effect of cytokines on Lgals1 expression was examined by quantitative (q)PCR.
Adhesion and Migration Assays.
Eosinophils treated with rGal-1 (0.1 and 0.25 μM for 15 min at 37 °C) or PBS alone were allowed to adhere to recombinant mouse vascular cell adhesion molecule-1 (VCAM-1; 10 μg/mL; Leinco Technologies)–coated coverslips (5 × 105 cells per coverslip) for 30 min. After washing, adherent cells were fixed with 4% paraformaldehyde containing saponin and stained with Alexa Fluor 488 phalloidin (Life Technologies) as well as DAPI to visualize nuclei and examined by confocal microscopy (600× magnification). Adherent cells in five randomly selected fields of each coverslip were counted and expressed as percent adhesion relative to untreated cells.
Cells treated with rGal-1 (or buffer alone) as described above were added to the upper wells of HTS Transwell-96 well plates (Corning), and migration toward murine eotaxin-1 (100 nM; PeproTech) in the lower wells of the chambers was assessed after 3–4 h at 37 °C. The number of migrating cells was evaluated using an Olympus CK2 inverted microscope (200× magnification). Cells in a fixed number of randomly selected nonoverlapping fields were counted for each well and expressed as percent migration relative to untreated cells.
Cell-Surface Receptor Expression and Actin Polymerization.
Eosinophils were treated with rGal-1 (0.1, 0.25, or 1 μM) and examined by flow cytometry for changes in expression of cell-surface adhesion molecules or for actin polymerization using mAb against CD49d (α4; clone PS/2), CD11a (αL; eBioscience), CD11b (αM; eBioscience), L-selectin (clone MEL-14), or CD18 (β2; clone 2E6) followed by FITC-conjugated goat anti-rat IgG- or FITC-conjugated goat anti-hamster IgG. Rat IgG2b (for CD49d and CD11b), rat IgG2a (for CD11a and L-selectin), or hamster IgG (for CD18) was used as isotype control. All antibodies were used at 10 µg/mL. For expression of CCR3, the receptor for eotaxin-1, cells were incubated with FITC-conjugated rat anti-mouse CCR3 (2.5 μg/mL; R&D Systems) with FITC-conjugated rat IgG2a as isotype control.
For detection of actin polymerization, Gal-1–treated cells were fixed and permeabilized using 4% paraformaldehyde and 0.1% saponin in PBS for 20 min on ice. Cellular F-actin was stained with Alexa Fluor 488 phalloidin (Thermo Fisher Scientific) and analyzed by flow cytometry. Additionally, Gal-1–treated (1 µM) cells were cytocentrifuged onto glass slides, fixed with 4% paraformaldehyde, and incubated with Alexa Fluor 488 phalloidin (1:100 in 0.05% saponin in PBS) for 30 min at room temperature. Cells were counterstained with DAPI to visualize nuclei and examined by confocal microscopy.
Evaluation of Gal-1–α4-Integrin Interaction.
α4-transfected (50) and control (nontransfected) CHO-K1 cells were grown to 70% confluence in DMEM/F12 with 10% FBS. Cells were detached with a cell scraper (not with trypsin-EDTA), passed through a cell strainer to disrupt aggregates, and then treated with PBS or rGal-1 at 0.25 µM (15 min at 37 °C) in the presence or absence of lactose or maltose. Cells were then examined for surface Gal-1 binding by flow cytometry with antibodies against Gal-1 followed by FITC-conjugated donkey anti-rabbit IgG as detailed in an earlier section. Baseline α4 expression in α4-transfected and control CHO-K1 cells was determined by flow cytometry with mAb against CD49d as described above.
Lung Histology.
Paraffin-embedded tissue sections (4-μm) were stained with H&E (Thermo Fisher Scientific) to determine cellular infiltration. For all IHC analysis, tissue sections were subjected to antigen retrieval followed by quenching of endogenous peroxidase activity before staining with specific antibodies. VECTASTAIN ABC Kits containing biotinylated secondary antibodies (anti-rat, anti-rabbit, or anti-mouse) and avidin–biotin horseradish peroxidase complex (Vector Laboratories) were used along with the AEC Peroxidase (3-amino-9-ethylcarbazole) Substrate Kit (Vector Laboratories) for detection. Stained slides were analyzed using a Nikon Microphot Epi-Fl microscope, and images were captured with an Olympus DP71 camera. Gal-1 expression in lung tissue from mice exposed to allergens was detected using rabbit polyclonal antibodies against Gal-1 (2 μg/mL). Evaluation and quantitation of lung tissue eosinophil infiltration in acute OVA-challenged WT and Lgals1−/− mice was performed by IHC staining for eosinophil-specific major basic protein (MBP) with rat mAb against murine MBP (2 μg/mL) as described earlier (57). MBP-positive cells (stained reddish brown) in randomly selected nonoverlapping microscopic fields (18 ± 4) were counted at 400× magnification and expressed as the average number of cells per field. In addition, the total number of blood vessels in nonoverlapping microscopic fields from one end of the section to the other as well as the number of vessels with MBP-positive cells adherent on the vascular endothelium was determined. T cells in lung tissue were evaluated by IHC staining using goat polyclonal antibodies against mouse CD3ε (4 μg/mL; Santa Cruz Biotechnology) (58) and quantitated as described for MBP. Expression of α-smooth muscle actin (α-SMA) was evaluated using a mAb against α-SMA (2 μg/mL; Sigma-Aldrich), and the area of the peribronchial smooth muscle layer was quantitated from captured images (four to five airways per mouse) using ImageJ image analysis software (56) as described (49). Results were expressed as α-SMA–positive area (μm2) per mm basement membrane length (BML). Rabbit IgG (for Gal-1), rat IgG (for MBP), goat IgG (for CD3ε), and mouse IgG (for α-SMA) were used as control antibodies. Airway mucus accumulation was assessed by staining lung sections with periodic acid–Schiff (PAS) reagent (Sigma-Aldrich). The PAS-positive area in airways of relatively similar size (four to six airways per mouse) was quantitated by ImageJ analysis and expressed as PAS-positive area (μm2) per 100 μm BML (51).
Quantitative Real-Time PCR for Gal-1 Expression in Eosinophils and Lung Tissue.
Total RNA was extracted from eosinophils or lung tissue with TRIzol (Life Technologies) and reverse-transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Gene expression was examined by qPCR with primers specific for mouse Gal-1 (59), β-actin (for eosinophils), or GAPDH (for lung tissue) (Integrated DNA Technologies). qPCR was performed using iTaq Universal SYBR Green Supermix 200 (Bio-Rad) with an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) under the following conditions: denaturation at 95 °C for 10 min, 30 s at 95 °C, followed by 30 s at 60 °C for 50 cycles. The relative amount of target mRNA was calculated based on its threshold cycle, Ct, suggested by the software (iQ5 Optical System Software) in comparison with the Ct of the housekeeping gene β-actin (for eosinophils) or GAPDH (for lung tissue). Results were expressed as relative fold change using the ddCt method (2−ΔΔCT) (60).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.G. is a guest editor invited by the Editorial Board.
See Commentary on page 9139.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601958113/-/DCSupplemental.
References
- 1.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy. 2009;39(9):1314–1323. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. Pathophysiology of asthma: What has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011;128(3):495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9(5):338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 5.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: A small protein with major functions. Glycobiology. 2006;16(11):137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36(3):322–335. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Liu F-T, Yang R-Y, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci. 2012;1253:80–91. doi: 10.1111/j.1749-6632.2011.06386.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper D, Norling LV, Perretti M. Novel insights into the inhibitory effects of galectin-1 on neutrophil recruitment under flow. J Leukoc Biol. 2008;83(6):1459–1466. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30(5):1331–1339. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Garín MI, et al. Galectin-1: A key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109(5):2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 11.Rostoker R, et al. Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat. 2013;107:85–94. doi: 10.1016/j.prostaglandins.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Cuellar S, et al. Reduced expression of galectin-1 and galectin-9 by leucocytes in asthma patients. Clin Exp Immunol. 2012;170(3):365–374. doi: 10.1111/j.1365-2249.2012.04665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinovich GA, et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190(3):385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 15.Deák M, et al. Novel role for galectin-1 in T-cells under physiological and pathological conditions. Immunobiology. 2015;220(4):483–489. doi: 10.1016/j.imbio.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Stowell SR, et al. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109(1):219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinovich GA, et al. Specific inhibition of lymphocyte proliferation and induction of apoptosis by CLL-I, a β-galactoside-binding lectin. J Biochem. 1997;122(2):365–373. doi: 10.1093/oxfordjournals.jbchem.a021762. [DOI] [PubMed] [Google Scholar]
- 18.Matarrese P, et al. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J Biol Chem. 2005;280(8):6969–6985. doi: 10.1074/jbc.M409752200. [DOI] [PubMed] [Google Scholar]
- 19.Kita H. Eosinophils: Multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242(1):161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Baum LG. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab Invest. 2006;86(6):578–590. doi: 10.1038/labinvest.3700420. [DOI] [PubMed] [Google Scholar]
- 21.He J, Baum LG. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 2006;417:247–256. doi: 10.1016/S0076-6879(06)17017-2. [DOI] [PubMed] [Google Scholar]
- 22.Fouillit M, et al. Regulation of CD45-induced signaling by galectin-1 in Burkitt lymphoma B cells. Glycobiology. 2000;10(4):413–419. doi: 10.1093/glycob/10.4.413. [DOI] [PubMed] [Google Scholar]
- 23.Muglia C, et al. The glycan-binding protein galectin-1 controls survival of epithelial cells along the crypt-villus axis of small intestine. Cell Death Dis. 2011;2:e163. doi: 10.1038/cddis.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouve N, et al. The involvement of CD146 and its novel ligand galectin-1 in apoptotic regulation of endothelial cells. J Biol Chem. 2013;288(4):2571–2579. doi: 10.1074/jbc.M112.418848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu FT, Rabinovich GA. Galectins: Regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–182. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 26.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delbrouck C, et al. Galectin-1 is overexpressed in nasal polyps under budesonide and inhibits eosinophil migration. Lab Invest. 2002;82(2):147–158. doi: 10.1038/labinvest.3780407. [DOI] [PubMed] [Google Scholar]
- 28.Ilan N, Mohsenin A, Cheung L, Madri JA. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB J. 2001;15(2):362–372. doi: 10.1096/fj.00-0372com. [DOI] [PubMed] [Google Scholar]
- 29.Koh HS, et al. CD7 expression and galectin-1-induced apoptosis of immature thymocytes are directly regulated by NF-kappaB upon T-cell activation. Biochem Biophys Res Commun. 2008;370(1):149–153. doi: 10.1016/j.bbrc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: Past, present and future. Clin Immunol. 2012;142(2):107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 32.Cagnol S, Chambard J-C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 33.Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell. 2002;9(5):945–956. doi: 10.1016/s1097-2765(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 34.La M, et al. A novel biological activity for galectin-1: Inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am J Pathol. 2003;163(4):1505–1515. doi: 10.1016/s0002-9440(10)63507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulcher JA, et al. Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and protein kinase C signaling. J Biol Chem. 2009;284(39):26860–26870. doi: 10.1074/jbc.M109.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinovich GA, et al. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology. 1999;97(1):100–106. doi: 10.1046/j.1365-2567.1999.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moiseeva EP, Williams B, Goodall AH, Samani NJ. Galectin-1 interacts with β-1 subunit of integrin. Biochem Biophys Res Commun. 2003;310(3):1010–1016. doi: 10.1016/j.bbrc.2003.09.112. [DOI] [PubMed] [Google Scholar]
- 38.Boehme SA, et al. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol. 1999;163(3):1611–1618. [PubMed] [Google Scholar]
- 39.Kiss J, et al. A novel anti-inflammatory function of human galectin-1: Inhibition of hematopoietic progenitor cell mobilization. Exp Hematol. 2007;35(2):305–313. doi: 10.1016/j.exphem.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Choi I-W, et al. TNF-α induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol. 2005;116(3):537–543. doi: 10.1016/j.jaci.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25high T-regulatory cells in allergic asthma is subjected to TNF-α modulation. Allergy. 2008;63(1):67–74. doi: 10.1111/j.1398-9995.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 42.Mello CB, et al. Immunomodulatory effects of galectin-1 on an IgE-mediated allergic conjunctivitis model. Invest Ophthalmol Vis Sci. 2015;56(2):693–704. doi: 10.1167/iovs.14-15100. [DOI] [PubMed] [Google Scholar]
- 43.Bahaie NS, et al. N-glycans differentially regulate eosinophil and neutrophil recruitment during allergic airway inflammation. J Biol Chem. 2011;286(44):38231–38241. doi: 10.1074/jbc.M111.279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyer KD, et al. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181(6):4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrionuevo P, et al. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: Galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol. 2007;178(1):436–445. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- 46.Rabinovich GA, et al. Activated rat macrophages produce a galectin-1-like protein that induces apoptosis of T cells: Biochemical and functional characterization. J Immunol. 1998;160(10):4831–4840. [PubMed] [Google Scholar]
- 47.Bertrand R, Solary E, O’Connor P, Kohn KW, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211(2):314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 48.Kang BN, et al. The p110δ subunit of PI3K regulates bone marrow-derived eosinophil trafficking and airway eosinophilia in allergen-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012;302(11):L1179–L1191. doi: 10.1152/ajplung.00005.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge XN, Ha SG, Liu FT, Rao SP, Sriramarao P. Eosinophil-expressed galectin-3 regulates cell trafficking and migration. Front Pharmacol. 2013;4:37. doi: 10.3389/fphar.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuura N, et al. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol. 1996;148(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 51.Bahaie NS, et al. Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation. J Immunol. 2012;188(3):1479–1490. doi: 10.4049/jimmunol.1102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge XN, et al. Allergen-induced airway remodeling is impaired in galectin-3-deficient mice. J Immunol. 2010;185(2):1205–1214. doi: 10.4049/jimmunol.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ha SG, et al. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat Commun. 2013;4:2479. doi: 10.1038/ncomms3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ochkur SI, et al. The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: Assessment of eosinophil degranulation ex vivo and in models of human disease. J Immunol Methods. 2012;375(1–2):138–147. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirabayashi J, Ayaki H, Soma G, Kasai K. Production and purification of a recombinant human 14 kDa β-galactoside-binding lectin. FEBS Lett. 1989;250(2):161–165. doi: 10.1016/0014-5793(89)80711-2. [DOI] [PubMed] [Google Scholar]
- 56.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 57.Zuberi RI, et al. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol. 2009;183(6):3971–3979. doi: 10.4049/jimmunol.0901604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirot N, et al. Lung endothelial barrier disruption in Lyl1-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2014;306(8):L775–L785. doi: 10.1152/ajplung.00200.2013. [DOI] [PubMed] [Google Scholar]
- 59.Chung L-Y, et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18(15):4037–4047. doi: 10.1158/1078-0432.CCR-11-3348. [DOI] [PubMed] [Google Scholar]
- 60.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]