Significance
St. Paul Island, Alaska, is famous for its late-surviving population of woolly mammoth. The puzzle of mid-Holocene extinction is solved via multiple independent paleoenvironmental proxies that tightly constrain the timing of extinction to 5,600 ± 100 y ago and strongly point to the effects of sea-level rise and drier climates on freshwater scarcity as the primary extinction driver. Likely ecosystem effects of the mega-herbivore extinction include reduced rates of watershed erosion by elimination of crowding around water holes and a vegetation shift toward increased abundances of herbaceous taxa. Freshwater availability may be an underappreciated driver of island extinction. This study reinforces 21st-century concerns about the vulnerability of island populations, including humans, to future warming, freshwater availability, and sea level rise.
Keywords: mammoth, extinction, Holocene, St. Paul Island, ancient DNA
Abstract
Relict woolly mammoth (Mammuthus primigenius) populations survived on several small Beringian islands for thousands of years after mainland populations went extinct. Here we present multiproxy paleoenvironmental records to investigate the timing, causes, and consequences of mammoth disappearance from St. Paul Island, Alaska. Five independent indicators of extinction show that mammoths survived on St. Paul until 5,600 ± 100 y ago. Vegetation composition remained stable during the extinction window, and there is no evidence of human presence on the island before 1787 CE, suggesting that these factors were not extinction drivers. Instead, the extinction coincided with declining freshwater resources and drier climates between 7,850 and 5,600 y ago, as inferred from sedimentary magnetic susceptibility, oxygen isotopes, and diatom and cladoceran assemblages in a sediment core from a freshwater lake on the island, and stable nitrogen isotopes from mammoth remains. Contrary to other extinction models for the St. Paul mammoth population, this evidence indicates that this mammoth population died out because of the synergistic effects of shrinking island area and freshwater scarcity caused by rising sea levels and regional climate change. Degradation of water quality by intensified mammoth activity around the lake likely exacerbated the situation. The St. Paul mammoth demise is now one of the best-dated prehistoric extinctions, highlighting freshwater limitation as an overlooked extinction driver and underscoring the vulnerability of small island populations to environmental change, even in the absence of human influence.
During the wave of late Quaternary extinctions, nearly two-thirds of megafaunal genera disappeared worldwide (1, 2). Proposed extinction drivers include environmental change, human impacts, or some combination, with the relative contributions of these varying by species and region (2). Woolly mammoths, an iconic Ice Age species, vanished from mainland Asia and North America between 14,000 and 13,200 y ago, with some mainland populations perhaps persisting until approximately 10,500 y ago (1, 3, 4); however, relict populations survived on two newly formed Beringian islands into the middle Holocene (4–7) (Fig. 1A).
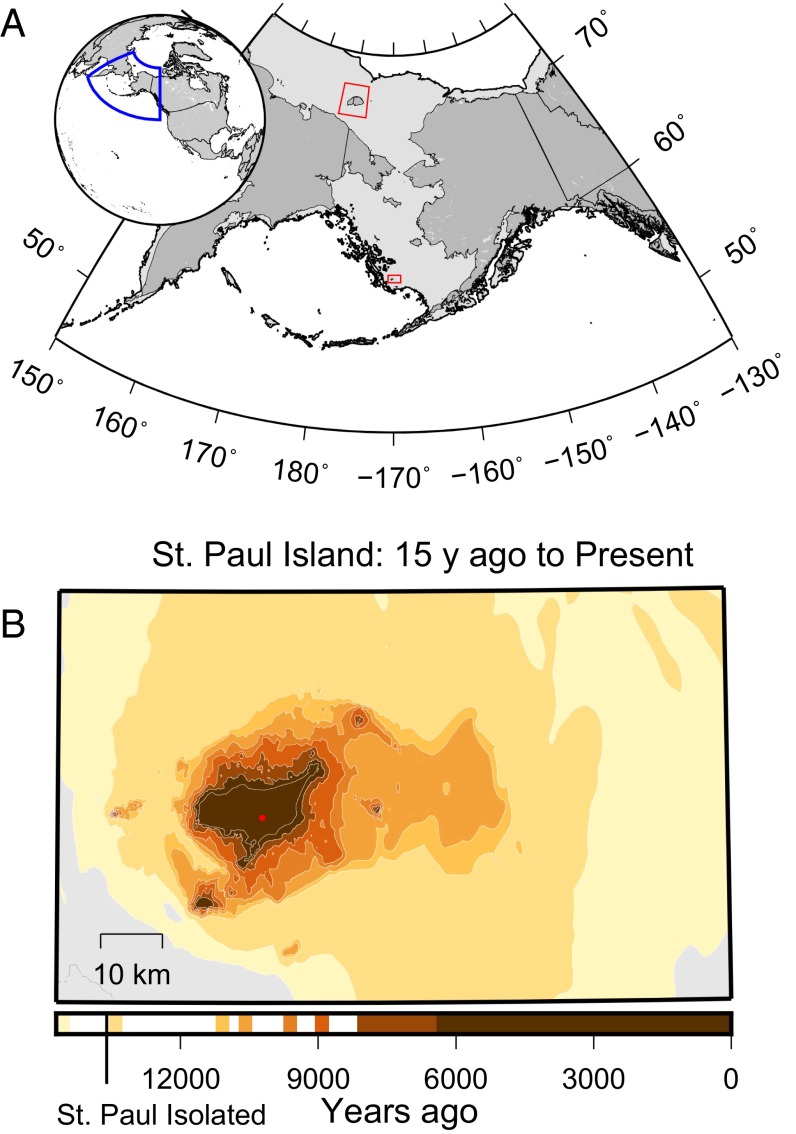
Fig. 1.
Location of St. Paul Island, Alaska and areal losses due to rising sea level. (A) Map of current continents (dark gray) and the past position of the Bering Land Bridge (light gray) with red boxes indicating Wrangel Island (Upper) and St. Paul Island (Lower). (B) The shrinking area of St. Paul Island from 15,000 y ago to the present due to rising sea level during the late Pleistocene through the middle Holocene. The red dot indicates the location of Lake Hill.
Wrangel Island is relatively large (7,600 km2), and the late survival of mammoths until 4,020 y ago (5) is consistent with a retreat to refugia in northeastern Siberia as temperatures rose (6). Conversely, the remarkable persistence of mammoths on the much smaller St. Paul Island (110 km2) until the mid-Holocene suggests that isolated megafaunal populations can survive thousands of years after habitat fragmentation. Because there is no evidence of humans on St. Paul Island before the arrival of Russian whalers in 1787 CE (7), the island affords a unique opportunity to study the processes governing the persistence and extinction of small megafaunal populations in the absence of humans.
St. Paul Island is a remnant of the Bering Land Bridge that became isolated between 14,700 and 13,500 y ago due to sea level rise during the last deglaciation. The island rapidly shrank in area until 9,000 y ago, and then slowly shrank until 6,000 y ago (Fig. 1B). Today, St. Paul is highly isolated (>450 km from Alaska and Aleutians) and is characterized by low relief (maximum elevation 203 m above sea level), a few freshwater lakes, no springs or streams, no permafrost, and moderately productive moss-herbaceous tundra vegetation (8, 9). Apart from a small population of reindeer (Rangifer tarandus) introduced to the island in 1911 (10), the largest terrestrial mammal on St. Paul today is the Arctic fox (Alopex lagopus). Previous radiocarbon dates on five mammoth remains from St. Paul indicated a last appearance date (LAD) of 6,480 y ago (11); however, LADs rarely represent the last surviving individual, creating uncertainty in the actual extinction timing. Previously proposed hypotheses for the cause of extinction of the St. Paul population include changes in vegetation (8) or increasing snowpack (12), but to date there is little direct evidence to support any proposed hypothesis.
In this study, we seek to better understand the timing, causes, and consequences of the mid-Holocene extinction of this late-surviving mammoth population. To achieve this goal, we collected in 2013 a series of sediment cores from Lake Hill, a small freshwater lake near the center of St. Paul (Fig. 1C and SI Appendix, Fig. S1). Lake Hill was cored previously in the 1960s to test hypotheses about whether the coastal Bering Land Bridge was a glacial refugium for Picea and other plant species (9). From our new sediment cores, we analyzed four independent proxies known to correlate with megafaunal presence (13–16): sedimentary ancient DNA (sedaDNA) and three coprophilous fungal spore types—Sporormiella, Sordaria, and Podospora (Fig. 2 and SI Appendix, SI Text) (16). We also reconstructed past environments using multiple proxies from the cores, including cladocerans, diatoms, pollen, plant macroremains, and stable isotopes, and dated the cores using radiocarbon dates from terrestrial plant macroremains and tephrochronology (SI Appendix, SI Text). In addition, we collected and radiocarbon-dated 14 newly identified St. Paul mammoth remains using ultrafiltered collagen extracts and measured their stable isotopic composition (δ13C and δ15N values) (SI Appendix, SI Text and Table S1).
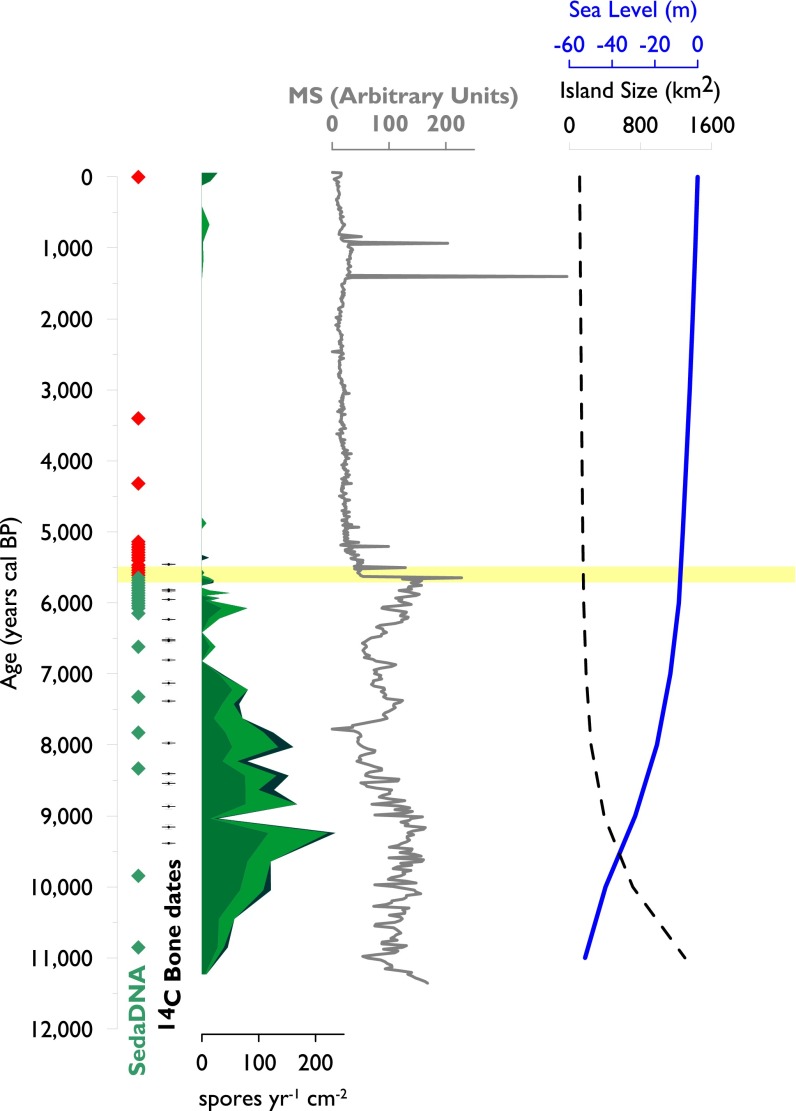
Fig. 2.
Timing of woolly mammoth extinction on St. Paul. Five indicators are plotted against time (calendar years before 1950 CE): sedimentary ancient DNA (sedaDNA; green and red circles indicate inferred presence and absence, respectively), direct AMS radiocarbon dates on mammoth remains (black crosses), and spore accumulation rates for the coprophilous fungi Sporormiella (medium green, bottom of stack), Sordaria (light green, middle), and Podospora (dark green, top) (spores yr-1 cm-2). Magnetic susceptibility, in arbitrary units (AU) of Lake Hill sediments, sea level (m), and St. Paul Island area (km2) are shown for comparison. Note the truncation by plot of highest peak of magnetic susceptibility at 1,500 AU. The yellow band indicates inferred timing of extinction at 5,600 ± 100 y ago. Magnetic susceptibility is a measure of sediment characteristics; its rapid decline indicates a rapid reduction in watershed erosion at the same time as the extinction.
Results and Discussion
Timing of Extinction.
The five independent indicators of extinction timing are in close agreement and together indicate that the St. Paul mammoths went extinct 5,600 ± 100 y ago (Fig. 2), which is 900 y later than the previous LAD value for St. Paul mammoths (11) and 1,580 y before the final extinction of mammoths on Wrangel Island (4, 7). The youngest of the newly dated mammoth remains from St. Paul provided a median calibrated radiocarbon date of 5,530 y ago (95% confidence interval, 5,585–5,330 y ago), which overlaps the extinction timing inferred from sedaDNA and fungal spores of 5,650 ± 80 y ago (SI Appendix, Fig. S3). Mammoth sedaDNA is present in all tested sediment samples between 10,850 ± 150 y ago (the oldest sample tested) and 5,650 ± 80 y ago (Fig. 2 and SI Appendix, Fig. S5), and absent in samples younger than 5,610 ± 80 y ago (Fig. 2). Sporormiella and Sordaria terminate at 5,680 ± 80 y ago and 5,650 ± 80 y ago, respectively, the latter exactly coinciding with the last appearance of mammoth sedaDNA. Declining Sporormiella and Sordaria abundances from 9,000–5,650 y ago (Fig. 2) might indicate declining mammoth population size, but coprophilous spore abundances may be influenced by sedimentary processes (14). Sporormiella abundances rise again at 1890 ± 50 CE, presumably reflecting the introduction of caribou (Rangifer tarandus) in 1911 CE (10). Podospora spores are less abundant than Sporormiella and Sordaria and disappear earlier, 7,020 ± 170 y ago. Overall, the close agreement among these five independent proxies makes 5,600 ± 100 y ago one of the most robust and precise estimates of timing ever recorded for a prehistoric species extinction.
Drivers of Extinction.
The disappearance of mammoths from St. Paul Island was accompanied by pronounced lake shallowing and increased water turbidity between 7,850 and 5,600 y ago, indicated by significant shifts from pelagic to littoral cladocerans, decreases in planktonic diatoms, and increases in tychoplanktonic diatoms (Fig. 3 and SI Appendix, SI Text). A concurrent rise in conductivity is indicated by an increased abundance of diatoms and cladocerans that are tolerant of high electrolyte concentrations, notably the cladoceran Alona circumfibriata (Fig. 3), which is resilient to fluctuating salinity and can be an indicator of enhanced salinity in lakes (17) (SI Appendix, SI Text).
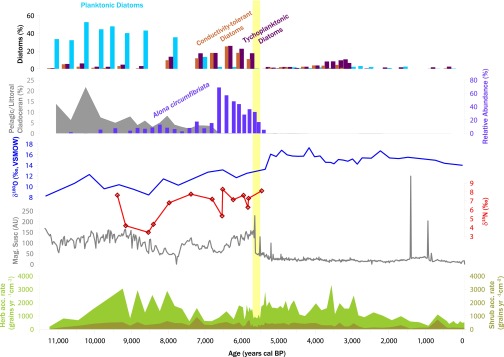
Fig. 3.
Paleoenvironmental proxies at Lake Hill, plotted against time. Shown are relative abundances of planktonic (blue bars), tychoplanktonic (maroon bars), and high-conductivity–tolerant diatoms (orange bars); ratio of pelagic and littoral cladocerans (gray curve); relative abundance (%) of cladoceran Alona circumfibriata, which is tolerant of high lake water conductivity (purple bars); δ18O values from aquatic invertebrate chitin (blue curve); δ15N values from mammoth collagen (red curve); sediment magnetic susceptibility (in AU); and accumulation rates for herb and shrub pollen types (light green and tan curves, respectively). The estimated timing of extinction (yellow vertical bar) and the magnetic susceptibility curve are included to help establish the stratigraphic correlation between Figs. 2 and 3.
Bayesian change-point analysis indicates that the most probable change points for A. circumfibriata occurs at 6,430 y ago, with its rapid rise, and a secondary change point occurs at 5,430, when it declines. Lake shallowing and enhanced evaporative loss is also supported by a trend of increasing stable oxygen isotope values (expressed as δ18O values) in Lake Hill between 8,500 and 5,300 y ago (Fig. 3), with δ18O values significantly higher after 5,600 y ago than before (P = 9.4 × 10−15, two-sample t test). Stable nitrogen isotope values (expressed as δ15N values) from mammoth remains seem to increase between 9,500 and 5,500 y ago (Fig. 3), consistent with decreasing precipitation and soil moisture (18, 19). However, the slope of a linear regression fitted to the δ15N time series does not significantly differ from 0 at a two-sigma threshold (P = 0.07) because of the single enriched value at 9,500 y ago, and thus the δ15N data are suggestive rather than definitive. Finally, an abrupt decrease in magnetic susceptibility at 5,650 ± 80 y ago coincides exactly with the Sordaria and sedaDNA disappearances and indicates a rapid shift in depositional regime to one characterized by decreased inputs of terrestrial sediments, likely due to reduced rates of watershed erosion after the extinction. Taken together, these proxies strongly indicate lake shallowing, enhanced evaporation, increased turbidity, and increased solute concentrations at Lake Hill during the mid-Holocene extinction window of mammoth on St. Paul Island.
Freshwater availability on oceanic islands is an underappreciated, but important, driver of vertebrate mortality. On Mauritius, a mass mortality event 4,150 y ago coincided with a severe drought that concentrated animals near increasingly toxic and saline lakes (20). Modern elephants (Loxodonta and Elephas), the closest living equivalents to mammoths, consume 70–200 L/day (21), and their survival depends on water availability (22). Elephants prefer clear water and will dig “wells” near sediment-choked water sources to filter and clarify drinking water (23). St. Paul mammoths may have needed even more water than modern elephants for evaporative cooling during the Holocene interglacial (24), because many of the morphological and physiological adaptations for M. primigenius were for retaining heat (25, 26). As island size decreased, freshwater resources on St. Paul became more limited. Surface availability of fresh water is presently—and was probably at the time of extinction—restricted to shallow groundwater perched on a seawater base and several freshwater coastal lagoons and three closed-basin crater lakes (27). Of these, the freshwater lake at Lake Hill is currently the deepest (1.3 m) (9) and thus was the primary (or only) freshwater source for mammoths during dry periods (20). Thus, freshwater availability on St. Paul is highly limited, and diminished freshwater availability is a plausible agent of extinction on St. Paul.
Several previously hypothesized extinction drivers for St. Paul can be discarded. Human colonization during the mid-Holocene is unlikely, given the extreme isolation of St. Paul in the Bering Sea and the lack of any archaeological record (7). Polar bear predation is unlikely, because all St. Paul polar bear remains postdate the mammoth extinction by >1,000 y (11). Volcanic activity (11, 28) is unlikely, given the absence of visible tephra in Lake Hill sediments during the extinction window. Extinction due to increasing winter snowpack (12, 29) is inconsistent with the increase in δ18O values at Lake Hill during the Holocene (Fig. 3). Extinction due to vegetation shifts (8) is unlikely, given the stable vegetation composition on St. Paul during the Holocene (Fig. 3), although declining pollen accumulation rates in the early Holocene might indicate declining tundra productivity.
Reduction in island size per se (8) is not a direct driver, because the modern size of the island (110 km2) was reached by 9,000 y ago, more than 3,000 y before extinction. However, decreasing island size may have indirectly caused extinction, by reducing mammoth population sizes and reducing the number and volume of freshwater resources. If so, the St. Paul mammoth extinction may be a natural example of extinction debt (30), and suggests that the delay between habitat loss and final extinction can last for millennia.
Causes of Freshwater Scarcity.
The causes of reduced freshwater availability on St. Paul are less certain, with likely factors including sea level rise, climate change, and resource overuse. Rising sea levels gradually decreased land area and availability of freshwater resources (Fig. 1), but a freshwater crisis might have been triggered by mid-Holocene regional climate change and variability. The Lake Hill findings and δ15N values from mammoth bones indicate increased aridity between 8,000 and 5,300 y ago on St. Paul (Fig. 3). Other lake records from mainland Alaska show decreased effective moisture and increased hydrological variability between 6,800 and 5,000 y ago (31–34), suggesting that water scarcity of St. Paul might have been caused by regionally enhanced hydrological variability during the mid-Holocene.
Mammoth activity around Lake Hill probably contributed to the degradation of freshwater quality. As ecosystem engineers, elephants strongly modify their local environments, especially at water holes and other areas of heavy use (35). As plant cover is destroyed, environmental deterioration rapidly radiates outward (12). The abrupt decrease in magnetic susceptibility, shift from terrigenous to lacustrine sediments (Fig. 3), disappearance of pelagic cladocerans, near-disappearance of planktonic diatoms, and presence of tychoplanktonic diatoms all coincide with the time of mammoth extinction. Thus, mammoth activity likely denuded the lake margin, accelerated rates of erosion, and contributed to lake infilling and decreased water quality. The increase in magnetic susceptibility before extinction (Fig. 3) is consistent with intensified water use and rates of watershed erosion, perhaps as other water sources were depleted.
Conclusions and Implications.
Multiple reinforcing lines of evidence indicate that reduced freshwater availability triggered the extinction of St. Paul mammoths at 5,600 ± 100 y ago. Sea level rise and declining island area set the stage for extinction (8) by reducing island carrying capacity, decreasing population size, and increasing mammoth dependency on diminishing freshwater resources, leaving mammoths vulnerable to environmental fluctuations. Regional climate variability and aridification then may have applied the coup de grâce, possibly exacerbated by resource degradation by the mammoths. Thus, the St. Paul history demonstrates that in the absence of human pressures, small megafaunal populations can persist for thousands of years, while highlighting the susceptibility of island populations to environmental fluctuations, including freshwater availability, particularly during periods of rising sea level. Island biogeographic theory predicts that rates of extinction should be elevated on small islands with limited resources; these findings support that theory while pointing to freshwater scarcity as an underappreciated driver. Our results suggest that as sea levels rise and hydroclimatic variability increases over the coming centuries due to anthropogenic warming (36), freshwater scarcity may become an increasingly critical limiting resource for island vertebrate populations.
Methods
Specific details of all methods are provided in SI Appendix, SI Text. Island size through time was reconstructed using a GIS bathymetric map of the St. Paul Island area and the sea level rise curve from the Bering Strait (37). Three overlapping cores were collected at the primary Lake Hill core in 2013 CE (57.17809N, 170.24828W) and used to construct a composite core using clearly identifiable marker layers, such as visible tephras and lithologic transitions, in the overlapping sections (SI Appendix, Fig. S2). Samples for all paleoecological and paleoenvironmental proxies were taken at National Lacustrine Core Facility (LacCore) mostly at identical depths, to eliminate problems with proxy correlation. A Bayesian age model for the upper 740 cm of the composite core (SI Appendix, Fig. S3) was constructed using OxCal from accelerator mass spectrometry (AMS) radiocarbon (14C) dating of six terrestrial macrobotanical remains and a tephra layer representing the Aniakchak CFE II tephra, with a known age of 3,595 ± 4 cal yr BP (SI Appendix, Table S3). Tephra extraction was done following standard methods (38). Radiocarbon dating and isotopic analyses of mammoth teeth were derived from collagen extracted and purified following the modified Longin method with ultrafiltration (39). All AMS 14C results were corrected for isotopic fractionation according to standard conventions (40). Conventional 14C ages were calibrated with OxCal 4.2.3 (41) using the IntCal13 curve (42).
SedaDNA from 38 samples throughout the upper 740 cm of the core was extracted, sequenced, and bioinformatically processed at the Paleogenomics Laboratory, University of California Santa Cruz (SI Appendix, Fig. S5 and Table S5). Sequencing data are available from the National Center for Biotechnology Information’s Short Read Archive (BioProject accession no. PRJNA320875). Extraction and processing of spores and pollen followed a modified version of the LacCore protocol (43). Plant macrofossil samples were taken every 10 cm, but shifted to finer resolution (sampling every 2–5 cm) for the lower portions of the core. All macrofossil samples were disaggregated in a glass Petri dish with distilled water and were manually collected. Samples were not sieved.
Cladocera, diatom, and stable isotopic analyses were all conducted at the Alaska Stable Isotope Facility, University of Alaska Fairbanks. Preparation of diatom samples was done following a modified previously published protocol (44). Cladocera were processed with an adapted version of a method described previously (45). The δ18O values of chironomid head capsules and other aquatic insect chitin were processed and analyzed following previously published protocols (46, 47). Bulk sediment samples were collected from the core at 16-cm intervals for isotopic and other geochemical analyses and processed following previously published protocols (48).
Supplementary Material
Acknowledgments
We thank all of the inhabitants of St. Paul Island and in particular Jason Bourdukofski, Bill Briggs, Aquilina Lestenkof, Gary Stanley, Mac Mandregan, Phillip Zavadil, Brenda Jones, Connie Newman, and the National Oceanic and Atmospheric Administration for their hospitality, assistance with site access, and field logistics. Simeon Swetzof, Laura Lestenkof, Nick Kozloff, Rob Owens, and Logan Tetov kindly provided specimens of woolly mammoth fossils from personal collections for radiocarbon dating. Voucher specimens have been preserved in the Earth and Mineral Sciences Museum collections at the Pennsylvania State University. The Limnological Research Center provided facilities for core splitting, imaging, description, and sampling. Elle Palkopoulou and Love Dalén provided prepublication access to the Wrangel Island woolly mammoth genome. We thank Nick Holschuh for assistance with figure drafting, Nancy Bigelow and Greg Smith for assistance with fieldwork, and two anonymous reviewers for their careful review and comments. This work was supported by National Science Foundation Grants PLR-1204233, PLR-1203772, and PLR-1203990.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M.L. is a guest editor invited by the Editorial Board.
Data deposition: Data have been deposited at the Neotoma Paleoecological Database (www.neotomadb.org/).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604903113/-/DCSupplemental.
References
- 1.Guthrie RD. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature. 2006;441(7090):207–209. doi: 10.1038/nature04604. [DOI] [PubMed] [Google Scholar]
- 2.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306(5693):70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 3.Haile J, et al. Ancient DNA reveals late survival of mammoth and horse in interior Alaska. Proc Natl Acad Sci USA. 2009;106(52):22352–22357. doi: 10.1073/pnas.0912510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikoskiy PA, Sulerzhitsky LD, Pitulko VV. Last straw versus Blitzkrieg overkill: Climate-driven changes in the Arctic Siberian mammoth population and the Late Pleistocene extinction problem. Quat Sci Rev. 2011;30:2309–2328. [Google Scholar]
- 5.Vartanyan SL, Arslanov KA, Karhu JA, Possnert G, Sulerzhitsky LD. Collection of radiocarbon dates on the mammoths (Mammuthus primigenius) and other genera of Wrangel Island, northeast Siberia, Russia. Quat Res. 2008;70(1):51–59. [Google Scholar]
- 6.Lorenzen ED, et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 2011;479(7373):359–364. doi: 10.1038/nature10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veltre DW, Veltre MJ. 1981. A preliminary baseline study of subsistence resource utilization in the Pribilof Islands. Alaska Department of Fish and Game, Division of Subsistence, Technical Paper no. 57. Available at: www.subsistence.adfg.state.ak.us/TechPap/tp057.pdf. Accessed June 22, 2016.
- 8.Guthrie RD. Radiocarbon evidence of mid-Holocene mammoths stranded on an Alaskan Bering Sea island. Nature. 2004;429(6993):746–749. doi: 10.1038/nature02612. [DOI] [PubMed] [Google Scholar]
- 9.Colinvaux P. Historical ecology in Beringia: The south land bridge coast at St. Paul Island. Quat Res. 1981;16(1):18–36. [Google Scholar]
- 10.Hanna GD. The reindeer herds of the Pribilof Islands. Sci Mon. 1922;15:181–186. [Google Scholar]
- 11.Veltre DW, Yesner DR, Crossen KJ, Graham RW, Coltrain JB. Patterns of faunal extinction and paleoclimatic change from mid-Holocene mammoth and polar bear remains, Pribilof Islands, Alaska. Quat Res. 2008;70(1):40–50. [Google Scholar]
- 12.Bryson RA, Agenbroad LD, McEnaney DeWall K. Paleoclimate modeling and paleoenvironmental interpretations for three instances of island dwelling mammoths. Quat Int. 2010;217(1–2):6–9. [Google Scholar]
- 13.Willerslev E, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300(5620):791–795. doi: 10.1126/science.1084114. [DOI] [PubMed] [Google Scholar]
- 14.Baker AG, Bhagwat SA, Willis KJ. Do dung fungal spores make a good proxy for past distribution of large herbivores? Quat Sci Rev. 2013;62:21–31. [Google Scholar]
- 15.Rawlence NJ, et al. Using palaeoenvironmental DNA to reconstruct past environments: Progress and prospects. J Quat Sci. 2014;29(7):610–626. [Google Scholar]
- 16.van Geel B, Zazula GD, Schweger CE. Spores of coprophilous fungi from under the Dawson tephra (25,300 14C years BP), Yukon Territory, northwestern Canada. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;252:481–485. [Google Scholar]
- 17.Thienpont JR, et al. Exploratory hydrocarbon drilling impacts to Arctic lake ecosystems. PLoS One. 2013;8(11):e78875. doi: 10.1371/journal.pone.0078875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens RE, Hedges REM. Carbon and nitrogen stable isotope analysis of northwest European horse bone and tooth collagen, 40,000 BP–present: Palaeoclimatic interpretations. Quat Sci Rev. 2004;23(7–8):977–991. [Google Scholar]
- 19.Newsome SD, Miller GH, Magee JW, Fogel ML. Quaternary record of aridity and mean annual precipitation based on δ15N in ratite and dromornithid eggshells from Lake Eyre, Australia. Oecologia. 2011;167(4):1151–1162. doi: 10.1007/s00442-011-2046-5. [DOI] [PubMed] [Google Scholar]
- 20.de Boer EJ, et al. A deadly cocktail: How a drought around 4200 cal yr BP caused mass mortality events at the infamous “dodo swamp” in Mauritius. Holocene. 2015;25(5):758–771. [Google Scholar]
- 21.Benedict FG. The Physiology of the Elephant. The Carnegie Institution of Washington; Washington, DC: 1936. [Google Scholar]
- 22.Western D. Water availability and its influence on the structure and dynamics of a savanna large mammal community. E Afr Wildl J. 1975;13:265–286. [Google Scholar]
- 23.Ramey EM, Ramey RR, Brown LM, Kelley ST. Desert-dwelling African elephants (Loxodonta africana) in Namibia dig wells to purify drinking water. Pachyderm. 2013;53:66–72. [Google Scholar]
- 24.Dunkin RC, Wilson D, Way N, Johnson K, Williams TM. Climate influences thermal balance and water use in African and Asian elephants: Physiology can predict drivers of elephant distribution. J Exp Biol. 2013;216(Pt 15):2939–2952. doi: 10.1242/jeb.080218. [DOI] [PubMed] [Google Scholar]
- 25.Kubiak H. Morphological characters of the mammoth: an adaptation to the arctic-steppe environment. In: Hopkins DM, Matthews JV, Schweger CE, editors. Paleoecology of Beringia. Academic; New York: 1982. pp. 281–289. [Google Scholar]
- 26.Tridico SR, Rigby P, Kirkbride KP, Haile J, Bunce M. Megafaunal split ends: Microscopical characterisation of hair structure and function in extinct woolly mammoth and woolly rhino. Quat Sci Rev. 2014;83:68–75. [Google Scholar]
- 27.Feulner AJ. 1980. Water resources reconnaissance of the southeastern part of St. Paul Island. USGS Water-Resources Investigations Report 80-61 (US Geological Survey, Reston, VA)
- 28.Clegg BF, Hu FS. An oxygen-isotope record of Holocene climate change in the south-central Brooks Range, Alaska. Quat Sci Rev. 2010;29(7–8):928–939. [Google Scholar]
- 29.Finney BP, Bigelow NH, Barber VA, Edwards ME. Holocene climate change and carbon cycling in a groundwater-fed, boreal forest lake: Dune Lake, Alaska. J Paleolimnol. 2012;48:43–54. [Google Scholar]
- 30.Kuussaari M, et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol Evol. 2009;24(10):564–571. doi: 10.1016/j.tree.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Jones MC, Wooller M, Peteet DM. A deglacial and Holocene record of climate variability in south-central Alaska from stable oxygen isotopes and plant macrofossils in peat. Quat Sci Rev. 2014;87:1–11. [Google Scholar]
- 32.Laws RM. Elephants as agents of habitat and landscape change in East Africa. Oikos. 1970;21:1–15. [Google Scholar]
- 33.Van Wyk P, Fairall N. The influence of the African elephant on the vegetation of the Kruger National Park. Koedoe. 1969;12:57–89. [Google Scholar]
- 34.Mayewski PA, et al. Holocene climate variability. Quat Res. 2004;62(3):243–255. [Google Scholar]
- 35.Feeley TC, Winer GS. Volcano hazards and potential risks on St. Paul Island, Pribilof Islands, Bering Sea, Alaska. J Volcanol Geotherm Res. 2009;182(1–2):57–66. [Google Scholar]
- 36.Clark PU, et al. Consequences of twenty-first-century policy for multi-millennial climate and sea-level change. Nat Clim Change. 2016;6:360–369. [Google Scholar]
- 37.Hu A, et al. Influence of Bering Strait flow and North Atlantic circulation on glacial sea-level changes. Nat Geosci. 2010;3(2):118–121. [Google Scholar]
- 38.Blockley SPE, et al. A new and less destructive laboratory procedure for the physical separation of distal glass tephra shards from sediments. Quat Sci Rev. 2005;24(16-17):1952–1960. [Google Scholar]
- 39.Brown TA, Nelson DE, Vogel JS, Southon JR. Improved collagen extraction by modified Longin method. Radiocarbon. 1988;30(2):171–177. [Google Scholar]
- 40.Stuiver M, Polach HA. Discussion: Reporting of 14C data. Radiocarbon. 1977;19(3):355–363. [Google Scholar]
- 41.Bronk RC. Bayesian analysis of radiocarbon dates. Radiocarbon. 2009;51:337–360. [Google Scholar]
- 42.Reimer PJ, et al. Intcal13 and Marine13 radiocarbon age calibration curves 0-50,000 years cal BP. Radiocarbon. 2013;55(4):1869–1887. [Google Scholar]
- 43.Faegri K, Kaland PE, Krzywinski K. Textbook of Pollen Analysis. 4th Ed Wiley; New York: 1989. [Google Scholar]
- 44.Battarbee RW, et al. 2001. Diatoms. Tracking Environmental Change Using Lake Sediments, Vol. 3: Terrestrial, Algal and Siliceous Indicators, eds Smol JP, Birks HJB, Last WM (Kluwer, Dordrecht), pp 155–202.
- 45.Korhola A, Rautio M. 2001. Cladocera and other branchiopod crustaceans. Tracking Environmental Change Using Lake Sediments, Vol. 4: Zoological Indicators, eds Smol JP, Birks HJB, Last WM (Kluwer, Dordrecht), pp 5–41.
- 46.Wooller MJ, et al. Quantitative paleotemperature estimates from δ18O of chironomid head capsules preserved in arctic lake sediments. J Paleolimnol. 2004;31(3):267–274. [Google Scholar]
- 47.Wooller MJ, et al. Reconstruction of past methane availability in an Arctic Alaska wetland indicates climate influenced methane release during the past similar to 12,000 years. J Paleolimnol. 2012;48(1):27–42. [Google Scholar]
- 48.Wooller MJ, et al. An ∼11,200-year paleolimnological perspective for emerging archaeological findings at Quartz Lake, Alaska. J Paleolimnol. 2012;48(1):83–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.