Significance
The hippocampus and basolateral amygdala—modulated by β-noradrenergic, D1/D5 dopaminergic, and H2-histaminergic receptors—control memory processing of many memories, but their role in social recognition memory (SRM) has been little studied. SRM is fundamental for the establishment of social relationships and, consequently, for the formation and stability of social groups. The social deficits of psychiatric disorders, such as autism and schizophrenia, are believed to be caused by alterations in SRM processing by the hippocampus and amygdala. Here we examine the involvement of the hippocampus and basolateral amygdala—and β-noradrenergic, D1/D5 dopaminergic, and H2-histaminergic receptors therein—in SRM consolidation. The results suggest an important and complex modulation of this process, which may help to elucidate the basis of inappropriate social behavior in psychiatric patients.
Keywords: social memory, basolateral amygdala, hippocampus, norepinephrine, dopamine
Abstract
Social recognition memory (SRM) is crucial for reproduction, forming social groups, and species survival. Despite its importance, SRM is still relatively little studied. Here we examine the participation of the CA1 region of the dorsal hippocampus (CA1) and the basolateral amygdala (BLA) and that of dopaminergic, noradrenergic, and histaminergic systems in both structures in the consolidation of SRM. Male Wistar rats received intra-CA1 or intra-BLA infusions of different drugs immediately after the sample phase of a social discrimination task and 24-h later were subjected to a 5-min retention test. Animals treated with the protein synthesis inhibitor, anisomycin, into either the CA1 or BLA were unable to recognize the previously exposed juvenile (familiar) during the retention test. When infused into the CA1, the β-adrenoreceptor agonist, isoproterenol, the D1/D5 dopaminergic receptor antagonist, SCH23390, and the H2 histaminergic receptor antagonist, ranitidine, also hindered the recognition of the familiar juvenile 24-h later. The latter drug effects were more intense in the CA1 than in the BLA. When infused into the BLA, the β-adrenoreceptor antagonist, timolol, the D1/D5 dopamine receptor agonist, SKF38393, and the H2 histaminergic receptor agonist, ranitidine, also hindered recognition of the familiar juvenile 24-h later. In all cases, the impairment to recognize the familiar juvenile was abolished by the coinfusion of agonist plus antagonist. Clearly, both the CA1 and BLA, probably in that order, play major roles in the consolidation of SRM, but these roles are different in each structure vis-à-vis the involvement of the β-noradrenergic, D1/D5-dopaminergic, and H2-histaminergic receptors therein.
Social recognition memory (SRM) is essential for social interaction, adaptive social behavior, reproduction, and survival (1–6). In rodents, SRM is assessed by using their natural tendency to investigate unfamiliar conspecifics more persistently than familiar ones in what has become known as the social-discrimination paradigm (3, 5, 7–9). This discrimination relies largely on odor recognition by rodents, called by many “social odor” (10). Other sensory are deemed much less important for social recognition (10, 11).
To establish whether a given brain structure or cellular ensemble is important for a given type of behavior, the effect of protein synthesis inhibition in that structure on the particular behavior is often measured; if a deleterious effect is found, then the structure is assumed to play a role in that behavior (for example, see refs. 3 and 12). After this process, some key biochemical processes related to protein synthesis would be studied (13, 14). In the case of SRM, Kogan et al. (3) showed that, in mice, SRM is dependent upon protein synthesis and cyclic adenosine monophosphate responsive-element-binding protein (CREB) function in the hippocampus. Given the fact that the hippocampus and the basolateral amygdala (BLA) work in close association in the making and the regulation of many types of memory (13–17), we decided to study the participation of both structures in SRM. Historically, the next step to ascertain a putative brain mechanism in a behavior is to examine the eventual role of known neurotransmitters in those structures within the mechanism (13, 15).
Classic neurotransmitters, such as norepinephrine, dopamine, and histamine, have been suggested to play a role on SRM; the pharmacological elevation or depletion of norepinephrine in the central nervous system, respectively, enhances or disrupts social recognition in rats (18). The direct infusion of the norepinephrine reuptake inhibitor, nisoxetine, into the olfactory bulb improves the ability of rats to identify conspecifics (19) and dopamine β-hydroxylase knockout mice that are unable to synthetize norepinephrine show a SRM deficit (20). These findings suggest that norepinephrine plays an important role in the formation of SRM but there is a notable lack of knowledge about the role of the norepinephrine receptors involved, especially the β-adrenoreceptors, which have been shown to be important for other types of recognition memory: for example, object recognition (21–24) and odor recognition (25–29).
There is also evidence that SRM can be modulated by dopamine. During social interaction dopaminergic neurotransmission in the nucleus accumbens is enhanced (30, 31). Systemic administration and direct infusion into the nucleus accumbens or into the frontal cortex of D1 dopamine receptor agonist improves SRM (32, 33) but systemic injection of D1 dopamine receptor antagonist disrupts social learning (32, 34). Evidence shows that the dopamine type 1 receptor is important for social interaction (35) and object-recognition memory (21). H1, H2, and H3 histamine receptors are important for object-recognition memory (36). Furthermore, the intracerebroventricular infusion of histamine, histidine, and H3 histamine receptor agonist improves SRM, whereas the central inhibition of neuronal synthesis of histamine and H3 histamine receptor antagonist hinders this memory (37).
In view of the above considerations, the present study addresses two issues: the first concerns the role of the CA1 region of the dorsal hippocampus and the BLA, and the second addresses the role of the major norepinephrine, dopamine, and histamine receptors in those structures in the consolidation of SRM. We investigated first the effect of the protein synthesis inhibitor, anisomycin (Ani), on SRM when infused into the CA1 or the BLA. Subsequently, we studied the participation of β-adrenergic, D1/D5 dopamine, and H2 histamine receptors on the consolidation of SRM by infusing their agonists and antagonists into the CA1 or BLA.
Results
Effect of Ani Given into the CA1 or the BLA on the Consolidation of SRM.
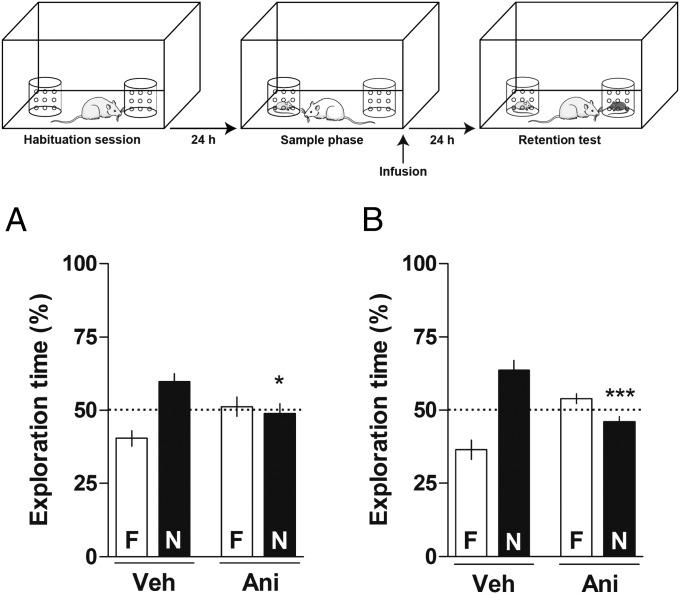
Animals received intra-CA1 or intra-BLA microinfusions of vehicle (Veh) or Ani (100 µg per side) immediately after the sample phase of a social discrimination task and 24-h later were subjected to a 5-min retention test. As shown in Fig. 1, animals that received Veh into the CA1 (Fig. 1A) or the BLA (Fig. 1B) immediately after the sample phase were able to recognize the familiar juvenile 24-h later [one-sample t test: (Fig. 1A) Veh t(9) = 3.577, P < 0.01; (Fig. 1B) Veh t(8) = 4.124, P < 0.01]. In contrast, animals that received intra-CA1 infusions of Ani spent about the same time exploring the novel (N) and the familiar (F) juveniles [one-sample t test: (Fig. 1A) Ani t(9) = 0.3542, P > 0.1] during the retention test. Additionally, animals that received Ani into the BLA spent more time exploring the familiar juvenile than the novel one [one-sample t test: (Fig. 1B) Ani t(7) = 2.306, P > 0.05] during the retention test. These results suggest that animals treated with Ani in either structure were unable to recognize the familiar juvenile 24-h after the sample phase. One-way ANOVA showed significant differences among groups [CA1: F(3, 36) = 6.952, P < 0.001; BLA: F(3, 30) = 18.95, P < 0.0001]. Bonferroni posttest revealed significant differences between Veh-N and Ani-N groups on the retention test when infused with intra-CA1 (P < 0.05) or intra-BLA (P < 0.001) immediately after the sample phase. The results obtained using Ani infusions suggest that the CA1 and BLA are both involved in the consolidation of SRM.
Fig. 1.
Effect of Ani given into the CA1 or BLA on the consolidation of SRM. The schematic representation on the top of the figure shows the behavioral protocol used in this and in the following experiments. Animals were subjected to a social discrimination task and, immediately after the sample phase, received intra-CA1 (A; 1 µL per side) or intra-BLA (B; 0.5 µL per side) infusions of vehicle (Veh) or Ani (100 µg per side). Twenty-four hours later, animals were subjected to a 5-min retention test in the presence of the familiar juvenile (F) and a novel one (N). Dots indicate the theoretical mean of 50%. Data are expressed as mean ± SEM (n = 8–10 animals per group) and presented as percentage of total exploration time. *P < 0.05 and ***P < 0.001 Veh-N vs. Ani-N, Bonferroni’s Multiple Comparison test after one-way ANOVA.
Effect of β-Adrenoreceptor Antagonist and Agonist Given into the CA1 or the BLA on the Consolidation of SRM.
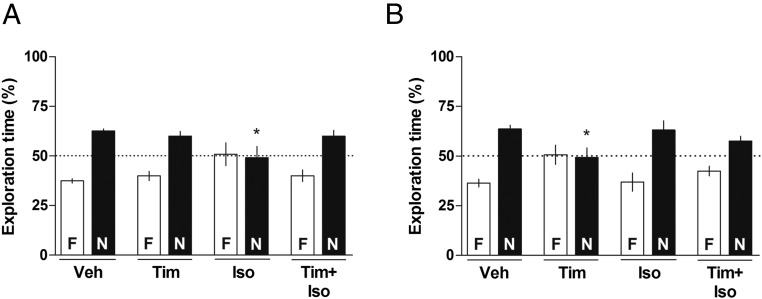
Immediately after the sample phase of the social discrimination task, animals received infusions of Veh, Timolol (Tim, 1.0 µg per side), or Isoproterenol (Iso, 10.0 µg per side), β-adrenoreceptor antagonist, and agonist, respectively, intra-CA1 or intra-BLA. Twenty-four hours later, the animals were subjected to a 5-min retention test. As shown in Fig. 2, animals that received intra-CA1 or intra-BLA infusion of Veh or infusions of Tim intra-CA1 [one-sample t test: (Fig. 2A) Tim t(11) = 4.329, P < 0.01] or infusions of Iso intra-BLA [one-sample t test: (Fig. 2B) t(7) = 2.873, P < 0.05] were able to recognize the familiar juvenile [one-sample t test: (Fig. 2A) Veh t(10) = 11.56, P < 0.0001; (Fig. 2B) Veh t(8) = 7.195, P < 0.0001] during the retention test. On the other hand, animals that received infusions of Iso intra-CA1 [one-sample t test: (Fig. 2A) Iso t(9) = 0.1482, P > 0.05] or Tim intra-BLA [one-sample t test: (Fig. 2B) Tim t(7) = 0.1401, P > 0.05] were unable to recognize the previously exposed juvenile during the retention test. This impairment in the ability to discriminate between familiar and novel juveniles was abolished by the coinfusion of Tim plus Iso into the CA1 [one-sample t test: (Fig. 2A) Tim+Iso t(7) = 3.416, P < 0.05] or into the BLA (one-sample t test: (Fig. 2B) Tim+Iso t(8) = 3.142, P < 0.05]. One-way ANOVA showed significant differences between groups [CA1: F(3, 37) = 3.145, P < 0.05; BLA: F(3, 30) = 3.459, P < 0.05]. Bonferroni posttest revealed significant differences between Veh-N and Iso-N groups in CA1 (P < 0.05) and between Veh-N and Tim-N in the BLA (P < 0.05) on the retention test. The effects of the drugs in the CA1 were larger than those in the BLA, suggesting a higher importance of the former in the regulation of this behavior.
Fig. 2.
Effect of β-adrenoreceptor antagonist and agonist infused into the CA1 or into the BLA on the consolidation of social recognition memory. Animals were subjected to a social discrimination task and immediately after the sample phase received intra-CA1 (A; 1 µL per side) or intra-BLA (B; 0.5 µL per side) infusions of vehicle (Veh), Tim (1.0 µg per side), or Iso (10.0 µg per side) or coinfusion of Timolol plus Isoproterenol (Tim+Iso). Twenty-four hours later, animals were subjected to a 5-min retention test in the presence of the familiar juvenile (F) and a novel one (N). Dots indicate the theoretical mean of 50%. Data are expressed as mean ± SEM (n = 8–12 animals per group) and presented as percentage of total exploration time. *P < 0.05 Veh-N vs. Iso-N (CA1), Veh-N vs. Tim-N (BLA), Bonferroni’s Multiple Comparison test after one-way ANOVA. In this and in Fig. 3, note that the drug effects were more intense in the CA1 than in BLA, probably indicating a larger importance of the former in modulation of this behavior both by noradrenergic receptors (this figure) and dopaminergic receptors (Fig. 3).
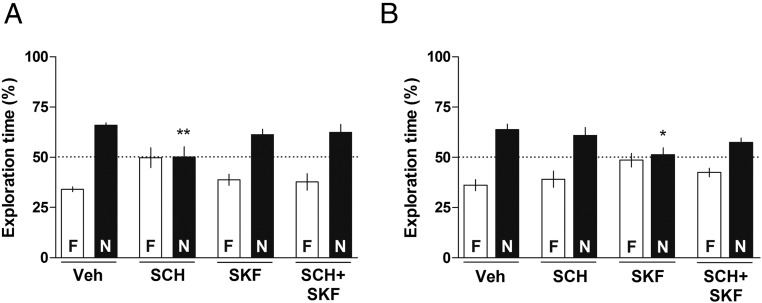
Effect of D1/D5 Dopamine Receptor Antagonist and Agonist Given into the CA1 or the BLA on the Consolidation of SRM.
Animals received intra-CA1 or intra-BLA infusions of Veh, D1/D5 dopamine receptor antagonist SCH23390 (SCH, 1.50 µg per side), or D1/D5 dopamine receptors agonist SKF38393 (SKF, 12.5 µg per side) or coinfusion of SCH23390 plus SKF38393 (SCH+SKF) immediately after the sample phase of a social discrimination task and were subjected to a retention test 24-h later. As shown in Fig. 3, animals that received intra-CA1 or intra-BLA infusions of Veh [one-sample t test: (Fig. 3A) Veh t(11) = 13.18, P < 0.0001; (Fig. 3B) Veh t(11) = 4.955, P < 0.001], intra-CA1 infusion of SKF38393 [one-sample t test: (Fig. 3A) SKF t(10) = 4.037, P < 0.01], or intra-BLA infusion of SCH23390 [one-sample t test: (Fig. 3B) SCH t(11) = 2.696, P < 0.05] were able to recognize the previously exposed juvenile 24-h after the sample phase. However, animals that received infusion of SCH23390 intra-CA1 [one-sample t test: (Fig. 3A) SCH t(8) = 0.04324, P > 0.05] or SKF38393 intra-BLA [one-sample t test: (Fig. 3B) SKF t(11) = 0.4073, P > 0.05] spent a similar amount of time exploring the novel and the familiar juveniles during the retention test. This inability to discriminate between familiar and novel juvenile was abolished by the combined infusion of SCH23390 plus SKF38393 into the CA1 [one-sample t test: (Fig. 3A) SCH+SKF t(11) = 3.026, P < 0.05] or into the BLA [one-sample t test: (Fig. 3B) SCH+SKF t(11) = 3.556, P < 0.01]. One-way ANOVA indicated difference between groups [CA1: F(3, 40) = 3.592, P < 0.05; BLA: F(3, 45) = 3.155, P < 0.05]. Bonferroni’s posttest revealed differences between Veh-N and SCH-N in the CA1 (P < 0.01) and between Veh-N and SKF-N in the BLA (P < 0.05) on the retention test. Again, drug effects in the CA1 were larger than those in the BLA, suggesting that the former is probably more important than the latter in the regulation of this behavior.
Fig. 3.
Effect of D1/D5 dopamine receptors antagonist and agonist infused into the CA1 or into the BLA on the consolidation of social recognition memory. Animals were subjected to a social discrimination task and immediately after the sample phase received intra-CA1 (A; 1 µL per side) or intra-BLA (B; 0.5 µL per side) infusions of vehicle (Veh), SCH23390 (SCH; 1.50 µg per side) or SKF38393 (SKF; 12.5 µg per side), or coinfusion of SCH23390 plus SKF38393 (SCH+SKF). Twenty-four hours later, animals were subjected to a 5-min retention test in the presence of the familiar juvenile (F) and a novel one (N). Dots indicate the theoretical mean of 50%. Data are expressed as mean ± SEM (n = 9–12 animals per group) and presented as percentage of total exploration time. *P < 0.05 Veh-N vs. SKF-N (BLA); **P < 0.01 Veh-N vs. SCH-N, Bonferroni’s Multiple Comparison test after one-way ANOVA.
Effect of H2 Histamine Receptor Antagonist and Agonist Given into the CA1 or the BLA on the Consolidation of SRM.
Animals received intra-CA1 or intra-BLA infusions of Veh or of an antagonist and an agonist of the H2 histamine receptor, Ranitidine (Rani, 17.5 µg per side) and Dimaprit (Dima, 2.3 µg per side), respectively, immediately after the sample phase of a social discrimination task. Twenty-four hours later animals were subjected to a 5-min retention test. As shown in Fig. 4, animals that received infusions intra-CA1 or intra-BLA of Veh [one-sample t-test: (Fig. 4A) Veh t(10) = 9.382, P < 0.0001; (Fig. 4B) Veh t(11) = 7.303, P < 0.0001] or Dima [one-sample t test: (Fig. 4A) Dima t(9) = 2.549, P < 0.05; (Fig. 4B) Dima t(11) = 2.708, P < 0.05] spent significantly more time exploring the novel juvenile than the familiar one during the retention test. However, animals that received infusion of Rani [one-sample t test: (Fig. 4A) Rani t(9) = 0.06443, P > 0.05; (Fig. 4B) Rani t(7) = 0.2364, P > 0.05] into the CA1 or into the BLA were unable to recognize the familiar juvenile 24-h after the sample phase. However, when animals received coinfusion of Rani plus Dima [one-sample t test: (Fig. 4A) Rani+Dima t(10) = 2.524, P < 0.05; (Fig. 4B) Rani+Dima t(7) = 3.218, P < 0.05] intra-CA1 or intra-BLA explored the novel juvenile for longer than the familiar one during the retention test. One-way ANOVA showed significant differences among groups [CA1: F(3, 38) = 2.872, P < 0.05; BLA: F(3, 36) = 3.075, P < 0.05]. Bonferroni’s posttest revealed differences between groups Veh-N and Rani-N in CA1 and BLA on the retention test (P < 0.05). These results suggest that H2 histaminergic receptors in both the CA1 and BLA are involved in the consolidation of SRM.
Fig. 4.
Effect of H2 histamine receptors antagonist and agonist infused into the CA1 or into the BLA on the consolidation of social recognition memory. Animals were subjected to a social discrimination task and immediately after the sample phase received intra-CA1 (A; 1 µL per side) or intra-BLA (B; 0.5 µL per side) infusions of vehicle (Veh), Rani (17.5 µg per side), or Dima (2.3 µg per side), or coinfusion of Rani plus Dima (Rani+Dima). Twenty-four hours later, animals were subjected to a 5-min retention test in the presence of the familiar juvenile (F) and a novel one (N). Dots indicate the theoretical mean of 50%. Data are expressed as mean ± SEM (n = 8–12 animals per group) and presented as percentage of total exploration time. *P < 0.05 Veh-N vs. Rani-N, Bonferroni’s Multiple Comparison test after one-way ANOVA.
Importantly, no differences were found between the CA1 groups [F(10, 136) = 1.740, P = 0.0778] and BLA groups [F(10, 130) = 1.837, P = 0.0603] in total exploration time during the retention test (Table 1). These results suggest that the doses of the drugs used did not affect motor skills or basal motivation to explore the juveniles.
Table 1.
Total exploration time during the 5-min retention test of the social discrimination task
| Treatment | Total exploration time (s) | |
| CA1 | BLA | |
| Vehicle | 95.5 ± 4.0 | 90.8 ± 4.1 |
| Anisomycin | 78.2 ± 7.2 | 95.7 ± 6.2 |
| Isoproterenol | 94.5 ± 12.1 | 92.8 ± 7.0 |
| Timolol | 101.3 ± 7.4 | 71.1 ± 5.2 |
| Timolol+Isoproterenol | 77.5 ± 11.5 | 83.8 ± 8.1 |
| SKF38393 | 82.4 ± 7.2 | 91.3 ± 7.0 |
| SCH23390 | 94.9 ± 5.7 | 87.0 ± 8.0 |
| SCH23390+SKF38393 | 79.45 ± 4.1 | 74.0 ± 5.7 |
| Dimaprit | 96.5 ± 9.6 | 103.1 ± 9.8 |
| Ranitidine | 89.1 ± 6.0 | 69.9 ± 11.5 |
| Ranitidine+Dimaprit | 72.2 ± 8.1 | 76.5 ± 8.8 |
Means ± SEM are shown; one-way ANOVA analysis.
Discussion
Concerning whether the CA1 and BLA play a role in SRM, our findings show that Ani given into the CA1 region of the dorsal hippocampus or into the BLA impairs the consolidation of SRM. Given the connections between the various amygdala subnuclei (38), our data fit with those of Gur et al. (39), showing that Ani given into the medial amygdala disrupts SRM, and with those of Wang et al. (40), who showed that lesion of the medial or basolateral amygdala impairs social but not other types of recognition in mice.
The data of Figs. 2–4 lead to several conclusions on the role of β-adrenoreceptors, D1/D5 dopamine receptors, and H2 histamine receptors in the modulation of SRM consolidation. In the CA1, Iso inhibits SRM, but because Tim has no effect of its own in that structure, the modulation of SRM by β-adrenoreceptors probably does not occur physiologically. In contrast, in the BLA, the data suggest that β-adrenoreceptors are probably useful to sustain SRM physiologically, because Tim on its own is depressant, even though Iso given alone seems without effect (Fig. 2). Concerning dopamine D1/D5 modulation of SRM in the CA1, the inhibitory effect of SCH suggests that this modulation may take place physiologically, but SKF has no action on its own in that structure (Fig. 3). At any rate, the effects of drugs acting on β- and on D1/D5 receptors show that concerning these receptors, the CA1 and BLA do not act in unison but instead play differential modulatory functions. Such differences between structures are not uncommon with β-adrenoreceptors (see ref. 41) as well as with D1/D2 receptor actions (see refs. 32 and 33). The data on H2 histamine receptor regulation of SRM suggest that it exists physiologically in both the CA1 and BLA (Fig. 4), as is the case with the modulation by H2 histamine receptors of several other types of memory in these and in other brain structures (42).
Wang et al. (40) demonstrated the participation of the dorsal hippocampus and the BLA in SRM. As will be seen, although our results generally agree with this finding, they suggest different roles for each of these structures. Despite the fact that some studies have failed to show any contribution of the hippocampus to social memory (43–45), a recent review of optogenetic studies showed that a connection between the BLA and hippocampus is strongly involved in SRM and social behaviors (46). Indeed, a recent optogenetic study in mice showed that activation of the BLA–medial prefrontal cortex pathway reduced social interaction in the resident-intruder test, whereas inhibition facilitated social interaction. These results establish a causal relationship between activity in the BLA–medial prefrontal cortex pathway in the bidirectional modulation of anxiety-related and social behaviors (47).
Stevenson and Caldwell (48) and Hitti and Siegelbaum (49) demonstrated that the CA2 region of the hippocampus is important for SRM. The CA2 region serves as a link between CA1 and CA3 (50) and it would seem likely that perhaps the whole hippocampus may be necessary for the formation of social memory; further research is required. Thirty years ago Corkin et al. (51) suggested a role for the hippocampus in social recognition in humans.
Findings on animal social behavior are often taken to bear on the study of human autism and other disorders involving social behavior (see, for example, ref. 46). Our results may bear on studies on autism reporting a link of impaired facial recognition (52), which has been attributed to abnormalities in the volume of the amygdala and so very probably in its microscopic organization, leading to dysregulated activity (53–59). Some have shown that autistic individuals may present a reduction of the volume of the amygdala during development but not in adulthood, in contrast with the increased hippocampus volume in autistic individuals at all ages compared with nonautistic individuals (59).
Other studies relying on localized brain lesions instead of the regional infusion of Ani point to roles for other brain structures in SRM: the olfactory bulb (which is to be expected because SRM, as studied by most authors, involves obviously mainly olfactory cues) and the septal nuclei (60, 61).
Other neurotransmitter or neuromodulator systems have also been studied: infusion of the selective 5-HT1A receptor agonist, 8-OH-DPAT, into the BLA decreased levels of social investigation (62). Oxytocin and vasopressin, which in the brain purportedly act as neuromodulators and are found in the hippocampus and amygdala, among other places (6), have been well and amply studied (60, 63–67). Oxytocin has been linked to social recognition in the medial region of the amygdala (68) and seems to play a role only in the acquisition of SRM, whereas vasopressin acts in the acquisition and consolidation of SRM (6). Estrogens promote the synthesis of oxytocin and its receptor (69). Interestingly, in female rats and mice, social memory has a peak during the proestrous phase, which is when estrogens are in higher concentration (70). In addition to their role in social behavior, oxytocin stimulates the release of dopamine, and the interaction of both neuromodulators might promote social interactions (2, 71).
Further research will no doubt clear up the relation of the present findings with those in the literature. Meanwhile, it can be concluded from the present data that the CA1 region of the dorsal hippocampus and the BLA are key participants in the regulation of SRM in rats, and that norepinephrine acting on β-receptors, dopamine acting on D1/D5 receptors, and histamine acting on H2 receptors in these two brain regions play a pivotal role in social recognition measured in a standard social discrimination paradigm. The roles of the catecholamines (14, 18, 38), and to an extent that of histamine (16, 36), appear peculiarly interesting because they probably affect this (3) and other forms of memory (14) by actions in the hippocampus and BLA, mediated by the enhanced synthesis of the nuclear CREB (3, 16, 38), which is related to protein synthesis, here shown to be necessary for SRM, as previously posited by Kogan et al. (3).
Materials and Methods
Animals.
Adult (3-mo-old, 300–330 g) and juvenile (22–30 postnatal days) male Wistar rats purchased from Centro de Modelos Biologicos Experimentais of the Pontifical Catholic University of Rio Grande do Sul (our regular provider) were used. Adults were housed four to a cage and juveniles were housed two to a cage, with free access to food and water and under a 12/12-h light/dark cycle (lights on at 7:00 AM). The behavioral experiments were conducted during the light phase of the cycle. All procedures were approved by the Animal Committee on Ethics in the Care and Use of Laboratory Animals of the Pontifical Catholic University of Rio Grande do Sul, in compliance with National Institutes of Health guidelines for the care and use of laboratory animals.
Surgery.
Adult animals were implanted bilaterally with stainless steel 22-gauge guide cannulae under deep anesthesia (75 mg/kg ketamine plus 10 mg/kg xylazine, i.p.) by stereotaxic procedures. The tips of the cannulae were aimed 1-mm above the CA1 region of the dorsal hippocampus (anterior, −4.2 mm; lateral, ±3.0 mm; ventral, −1.8 mm; from Bregma) or the basolateral complex of the amygdala (anterior, −2.4 mm; lateral, ±5.1 mm; ventral −7.5 mm; from Bregma) according to the atlas by Paxinos and Watson (72). The guide cannulae were fixed to the skull with dental acrylic cement. All animals were allowed 7 d for recovery from surgery before behavioral procedures. All animals were handled daily for 3 d before the behavioral experiments.
Pharmacological Treatments.
The drugs were purchased from Sigma–Aldrich. The doses used were derived from previous data in the literature in which their effect on memory variables was established (41, 73–75). The doses were: for the protein synthesis inhibitor, Ani, 100 µg per side; for β-adrenoreceptor agonist, Iso, 10.0 µg per side; for the β-blocker, Tim, 1.0 µg per side; for the D1/D5 dopamine receptor agonist, SKF, 12.5 µg per side; for the D1/D5 dopamine receptors antagonist, SCH, 1.5 µg per side; for the agonist of the H2 histamine receptor, Dima, 2.3 µg per side; and for the H2 blocker, Rani, 17.5 µg per side. All drugs were dissolved in sterile saline 0.9% (Veh), which was administered to the control groups. Infusion volume used was 0.5 μL per side into the BLA and 1.0 μL per side into the CA1 region of the dorsal hippocampus.
At the time of the microinfusion, a tight-fitting 30-gauge infusion cannula connected to a Hamilton microsyringe by polyethylene tubing was introduced into the guide cannula. The infusion cannula extended 1.0-mm beyond the cannula tip. Infusion was carried out over 60 s and the infusion cannula was left in place for an additional 60 s to maximize diffusion and to prevent backflow of the drug, and then carefully withdrawn and placed on the other side, after which the procedure was repeated.
Social Discrimination Paradigm.
The apparatus used was a white wooden open-field arena (60 cm × 40 cm × 50 cm) placed in a dimly illuminated room. Two identical transparent Plexiglas cylindrical cages (9-cm diameter × 13-cm high) were kept inside the arena near to the corners. The cylinder cages had small holes (1-cm diameter spaced by 1 cm diameter) on the wall, allowing the passage of odors (olfactory cues) while preventing the direct interaction between adults and juveniles. A clear cup filled with water (500 mL) was placed on top of each cylinder to prevent adults from moving or climbing it. The arena and the cylinder cages were cleaned with 70% (vol/vol) ethanol before and after each use.
Adult animals were individually habituated to the open-field arena for 20 min per day for 4 consecutive days. The empty cylinder cages were kept inside the arena during the habituation session. Juveniles were habituated to being placed in the cylinder cages for 20 min 24-h before the sample phase. The sample phase took place 24-h after the last habituation session. The adults were individually placed in the center of the arena and allowed, for 1 h, to freely explore an unfamiliar juvenile placed in one of the cylinder cages (randomly selected and counterbalanced for each group) and an empty cylinder. The retention test occurred 24-h later, the adults were placed again in the same arena with the previously presented juvenile (familiar) and a second juvenile (novel) that had no prior contact with the adult, placed in the cylinder that had been empty during the sample phase, and allowed to freely explore the set-up for 5 min, after which they were returned to their home cages. The two juveniles were from different home cages to prevent the redundancy of olfactory cues. During the retention test the exploration time of each juvenile (familiar and novel) was measured. Social exploratory behavior was defined as sniffing and touching the cylinder cages. The microinjections into the CA1 or BLA were carried out immediately after the sample phase.
Histology.
As usual in our laboratory (12, 41, 75, 76), cannulae placements were determined by infusing 4% (vol/vol) methylene blue into the cannulae 2 d after the last behavioral procedure. The spread of the dye was taken as an estimate of the drug infusions in the same animal. Placements were considered correct when the spread was 1 mm3 or less from the intended infusion sites. Only data from animals with correct cannulae implants were analyzed.
Statistical Analysis.
Data obtained in social discrimination task were converted in percentage of total exploration time of the juveniles, expressed as means and SE, and analyzed using one-sample Student's t test, considering the theoretical mean equal to 50% to compare the difference between familiar and novel juvenile exploration time in the same group. One-way ANOVA followed by Bonferroni’s Multiple Comparison Test was performed to assess differences between percentages of exploration time of novel juveniles on the retention test. The one-way ANOVA was used to verify differences in total time of exploration between the different treatments. GraphPad Prism software was used to analyze the data. P < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by research grants from the National Council of Research of Brazil, Conselho Nacional de Desenvolvimento Científico e Tecnologico and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lai W-S, Ramiro L-LR, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: A new method and functional neuroanatomy. J Neurosci. 2005;25(49):11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cacioppo JT, Decety J. Social neuroscience: Challenges and opportunities in the study of complex behavior. Ann N Y Acad Sci. 2011;1224(1):162–173. doi: 10.1111/j.1749-6632.2010.05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10(1):47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Norman GJ, Hawkley LC, Cole SW, Berntson GG, Cacioppo JT. Social neuroscience: The social brain, oxytocin, and health. Soc Neurosci. 2012;7(1):18–29. doi: 10.1080/17470919.2011.568702. [DOI] [PubMed] [Google Scholar]
- 5.Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96(6):1000–1006. [Google Scholar]
- 6.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126(1):97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 7.Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58(2):315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- 8.Gheusi G, Bluthé RM, Goodall G, Dantzer R. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behav Processes. 1994;33(1-2):59–87. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 9.Moura PJ, Meirelles ST, Xavier GF. Long-term social recognition memory in adult male rats: Factor analysis of the social and non-social behaviors. Braz J Med Biol Res. 2010;43(7):663–676. doi: 10.1590/s0100-879x2010007500047. [DOI] [PubMed] [Google Scholar]
- 10.Wacker DW, Engelmann M, Tobin VA, Meddle SL, Ludwig M. Vasopressin and social odor processing in the olfactory bulb and anterior olfactory nucleus. Ann N Y Acad Sci. 2011;1220:106–116. doi: 10.1111/j.1749-6632.2010.05885.x. [DOI] [PubMed] [Google Scholar]
- 11.Wacker DW, Ludwig M. Vasopressin, oxytocin, and social odor recognition. Horm Behav. 2012;61(3):259–265. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho Myskiw J, Furini CRG, Benetti F, Izquierdo I. Hippocampal molecular mechanisms involved in the enhancement of fear extinction caused by exposure to novelty. Proc Natl Acad Sci USA. 2014;111(12):4572–4577. doi: 10.1073/pnas.1400423111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29(9):496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68(3):285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo I, et al. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58(1):16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- 16.Benetti F, et al. Histamine in the basolateral amygdala promotes inhibitory avoidance learning independently of hippocampus. Proc Natl Acad Sci USA. 2015;112(19):E2536–E2542. doi: 10.1073/pnas.1506109112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izquierdo LA, et al. A link between role of two prefrontal areas in immediate memory and in long-term memory consolidation. Neurobiol Learn Mem. 2007;88(2):160–166. doi: 10.1016/j.nlm.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Griffin MG, Taylor GT. Norepinephrine modulation of social memory: Evidence for a time-dependent functional recovery of behavior. Behav Neurosci. 1995;109(3):466–473. doi: 10.1037//0735-7044.109.3.466. [DOI] [PubMed] [Google Scholar]
- 19.Shang Y, Dluzen DE. Nisoxetine infusion into the olfactory bulb enhances the capacity for male rats to identify conspecifics. Neuroscience. 2001;104(4):957–964. doi: 10.1016/s0306-4522(01)00120-8. [DOI] [PubMed] [Google Scholar]
- 20.Marino MD, Bourdélat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161(2):197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Furini CRG, Myskiw JC, Schmidt BE, Marcondes LA, Izquierdo I. D1 and D5 dopamine receptors participate on the consolidation of two different memories. Behav Brain Res. 2014;271:212–217. doi: 10.1016/j.bbr.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006;103(17):6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90(3):576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dornelles A, et al. Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem. 2007;88(1):137–142. doi: 10.1016/j.nlm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Dluzen DE, Muraoka S, Engelmann M, Ebner K, Landgraf R. Oxytocin induces preservation of social recognition in male rats by activating alpha-adrenoceptors of the olfactory bulb. Eur J Neurosci. 2000;12(2):760–766. doi: 10.1046/j.1460-9568.2000.00952.x. [DOI] [PubMed] [Google Scholar]
- 26.Guérin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci. 2008;122(4):816–826. doi: 10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lévy F. Neurobiological mechanisms involved in recognition of olfactory signature of the young in sheep. J Soc Biol. 2002;196(1):77–83. doi: 10.1051/jbio/2002196010077. [DOI] [PubMed] [Google Scholar]
- 28.Miranda MI, Ortiz-Godina F, García D. Differential involvement of cholinergic and beta-adrenergic systems during acquisition, consolidation, and retrieval of long-term memory of social and neutral odors. Behav Brain Res. 2009;202(1):19–25. doi: 10.1016/j.bbr.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Veyrac A, Nguyen V, Marien M, Didier A, Jourdan F. Noradrenergic control of odor recognition in a nonassociative olfactory learning task in the mouse. Learn Mem. 2007;14(12):847–854. doi: 10.1101/lm.708807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez LE, et al. Medial prefrontal transection enhances social interaction. I: Behavioral studies. Brain Res. 2000;887(1):7–15. doi: 10.1016/s0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- 31.Tucci S, et al. Medial prefrontal transection enhances social interaction. II: Neurochemical studies. Brain Res. 2000;887(2):259–265. doi: 10.1016/s0006-8993(00)02932-2. [DOI] [PubMed] [Google Scholar]
- 32.Di Cara B, et al. Activation of dopamine D1 receptors enhances cholinergic transmission and social cognition: A parallel dialysis and behavioural study in rats. Int J Neuropsychopharmacol. 2007;10(3):383–399. doi: 10.1017/S1461145706007103. [DOI] [PubMed] [Google Scholar]
- 33.Loiseau F, Millan MJ. Blockade of dopamine D(3) receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: Similar actions of D(1) receptor agonists, but not of D(2) antagonists. Eur Neuropsychopharmacol. 2009;19(1):23–33. doi: 10.1016/j.euroneuro.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Choleris E, Clipperton-Allen AE, Gray DG, Diaz-Gonzalez S, Welsman RG. Differential effects of dopamine receptor D1-type and D2-type antagonists and phase of the estrous cycle on social learning of food preferences, feeding, and social interactions in mice. Neuropsychopharmacology. 2011;36(8):1689–1702. doi: 10.1038/npp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada R, et al. Involvement of dopaminergic and cholinergic systems in social isolation-induced deficits in social affiliation and conditional fear memory in mice. Neuroscience. 2015;299:134–145. doi: 10.1016/j.neuroscience.2015.04.064. [DOI] [PubMed] [Google Scholar]
- 36.da Silveira CKB, Furini CRG, Benetti F, Monteiro SdaC, Izquierdo I. The role of histamine receptors in the consolidation of object recognition memory. Neurobiol Learn Mem. 2013;103:64–71. doi: 10.1016/j.nlm.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Prast H, Argyriou A, Philippu A. Histaminergic neurons facilitate social memory in rats. Brain Res. 1996;734(1-2):316–318. [PubMed] [Google Scholar]
- 38.Izquierdo I, Furini CRG, Myskiw JC. Fear memory. Physiol Rev. 2016;96(2):695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- 39.Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry. 2014;76(5):377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Zhao S, Liu X, Fu Q. Effects of the medial or basolateral amygdala upon social anxiety and social recognition in mice. Turk J Med Sci. 2014;44(3):353–359. doi: 10.3906/sag-1301-2. [DOI] [PubMed] [Google Scholar]
- 41.Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232(1):210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Myskiw JC, Furini CRG, Izquierdo I. 2016. Modulation of memory consolidation by brain histamine. Histamine Receptors: Preclinical and Clinical Aspects, eds Blandina P, Passani MB (Springer, New York)
- 43.Bannerman DM, Lemaire M, Beggs S, Rawlins JNP, Iversen SD. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res. 2001;138(1):100–109. doi: 10.1007/s002210100687. [DOI] [PubMed] [Google Scholar]
- 44.Feinberg LM, Allen TA, Ly D, Fortin NJ. Recognition memory for social and non-social odors: Differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem. 2012;97(1):7–16. doi: 10.1016/j.nlm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Squires AS, Peddle R, Milway SJ, Harley CW. Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol Learn Mem. 2006;85(1):95–101. doi: 10.1016/j.nlm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40(9):3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508(7494):88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekino Y, Obata K, Tanifuji M, Mizuno M, Murayama J. Delayed signal propagation via CA2 in rat hippocampal slices revealed by optical recording. J Neurophysiol. 1997;78(3):1662–1668. doi: 10.1152/jn.1997.78.3.1662. [DOI] [PubMed] [Google Scholar]
- 51.Corkin S, Sullivan EV, Carr FA. Prognostic factors for life expectancy after penetrating head injury. Arch Neurol. 1984;41(9):975–977. doi: 10.1001/archneur.1984.04050200081022. [DOI] [PubMed] [Google Scholar]
- 52.Weigelt S, Koldewyn K, Kanwisher N. Face recognition deficits in autism spectrum disorders are both domain specific and process specific. PLoS One. 2013;8(9):e74541. doi: 10.1371/journal.pone.0074541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abell F, et al. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10(8):1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- 54.Aylward EH, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 55.Baron-Cohen S, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11(6):1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 56.Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11(1):175–187. [PubMed] [Google Scholar]
- 57.Morgan JT, Barger N, Amaral DG, Schumann CM. Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PLoS One. 2014;9(10):e110356. doi: 10.1371/journal.pone.0110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radeloff D, et al. Structural alterations of the social brain: A comparison between schizophrenia and autism. PLoS One. 2014;9(9):e106539. doi: 10.1371/journal.pone.0106539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulkin J. Autism and the amygdala: An endocrine hypothesis. Brain Cogn. 2007;65(1):87–99. doi: 10.1016/j.bandc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Bluthé RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18(4):323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 61.Maaswinkel H, Baars AM, Gispen WH, Spruijt BM. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol Behav. 1996;60(1):55–63. doi: 10.1016/0031-9384(95)02233-3. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732(1-2):145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- 63.Bychowski ME, Mena JD, Auger CJ. Vasopressin infusion into the lateral septum of adult male rats rescues progesterone-induced impairment in social recognition. Neuroscience. 2013;246:52–58. doi: 10.1016/j.neuroscience.2013.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Everts HG, Koolhaas JM. Lateral septal vasopressin in rats: Role in social and object recognition? Brain Res. 1997;760(1-2):1–7. doi: 10.1016/s0006-8993(97)00269-2. [DOI] [PubMed] [Google Scholar]
- 65.Popik P, van Ree JM. Neurohypophyseal peptides and social recognition in rats. Prog Brain Res. 1998;119:415–436. doi: 10.1016/s0079-6123(08)61585-x. [DOI] [PubMed] [Google Scholar]
- 66.Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 1992;106(1):71–74. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- 67.van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713(1-2):153–159. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- 68.Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: Potential rodent models of autism. Int J Dev Neurosci. 2005;23(2-3):235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Dellovade TL, Zhu YS, Pfaff DW. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J Neuroendocrinol. 1999;11(1):1–10. doi: 10.1046/j.1365-2826.1999.00250.x. [DOI] [PubMed] [Google Scholar]
- 70.Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90(1):89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 71.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 72.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 73.Qi X-L, Zhu B, Zhang X-H, Li B-M. Are beta-adrenergic receptors in the hippocampal CA1 region required for retrieval of contextual fear memory? Biochem Biophys Res Commun. 2008;368(2):186–191. doi: 10.1016/j.bbrc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Zhang HT, Frith SA, Wilkins J, O’Donnell JM. Comparison of the effects of isoproterenol administered into the hippocampus, frontal cortex, or amygdala on behavior of rats maintained by differential reinforcement of low response rate. Psychopharmacology (Berl) 2001;159(1):89–97. doi: 10.1007/s002130100889. [DOI] [PubMed] [Google Scholar]
- 75.Mello-Carpes PB, Izquierdo I. The Nucleus of the Solitary Tract → Nucleus Paragigantocellularis → Locus Coeruleus → CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem. 2013;100:56–63. doi: 10.1016/j.nlm.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Furini CRG, et al. The relationship between protein synthesis and protein degradation in object recognition memory. Behav Brain Res. 2015;294:17–24. doi: 10.1016/j.bbr.2015.07.038. [DOI] [PubMed] [Google Scholar]