Significance
Trading experience has been shown to reduce the endowment effect, a decision-making bias that distorts market prices and reduces trade. Understanding the mechanisms underlying how experience changes this bias will provide important insights for developing interventions to improve market efficiency. Using functional magnetic resonance imaging, we show that market experience causes a reduction in right anterior insula activation during selling, which mediates a decrease in the endowment effect. These findings suggest that trading mitigates negative affective responses in the context of selling.
Keywords: training, neuroeconomics, loss aversion, decision-making
Abstract
People often demand a greater price when selling goods that they own than they would pay to purchase the same goods—a well-known economic bias called the endowment effect. The endowment effect has been found to be muted among experienced traders, but little is known about how trading experience reduces the endowment effect. We show that when selling, experienced traders exhibit lower right anterior insula activity, but no differences in nucleus accumbens or orbitofrontal activation, compared with inexperienced traders. Furthermore, insula activation mediates the effect of experience on the endowment effect. Similar results are obtained for inexperienced traders who are incentivized to gain trading experience. This finding indicates that frequent trading likely mitigates the endowment effect indirectly by modifying negative affective responses in the context of selling.
The most fundamental assumptions in economics revolve around individual preferences. The most basic of these is the independence assumption: that one’s economic valuation does not depend on current entitlements. In a normative sense, this assumption is used in most theoretical and applied economic models to assess the operation of markets. In a positive sense, the assumption underlies benefit–cost analysis, estimates of damages in court, and more generally any interpretation of indifference curves.
However, substantial evidence has mounted that illustrates the importance of entitlements: people ask greater prices for goods that they own than they are willing to pay for identical goods that they do not own, a well-known behavioral anomaly called the endowment effect (1). Importantly, behavioral research demonstrates that trading experience reduces the gap between buying and selling prices (2–4). Nevertheless, little is known about the mechanisms that underlie how experience attenuates the endowment effect. Understanding the mechanisms at work will critically shape how we view the observed violations in inexperienced traders: Are such behavioral patterns errors that violate closely held economic theory, or do they have basic explanations that permit us to retain the standard model with necessary adjustments?
We focus on two possible reasons that frequent trading reduces the endowment effect. First, trading may decrease loss aversion (5), or relatively greater anticipated pain of losing goods than excitement about gaining goods. For example, experienced traders may learn to change their mindset so that they do not view selling an object as a loss (6). Second, owning an object may enhance the object’s attractiveness (7, 8), but experienced traders may learn to valuate goods more consistently.
Distinct neuroanatomical circuits are activated during product preference and loss aversion (9). Specifically, the nucleus accumbens (NAcc) and orbitofrontal cortex (OFC) have been related to product preference and predict buying decisions (10–13). In contrast, right anterior insula activation has been implicated in anticipating and avoiding losses (refs. 14–16, but also see ref. 17). Thus, activation patterns in these regions can provide insight into how trading experience modifies the mechanisms operating during trade.
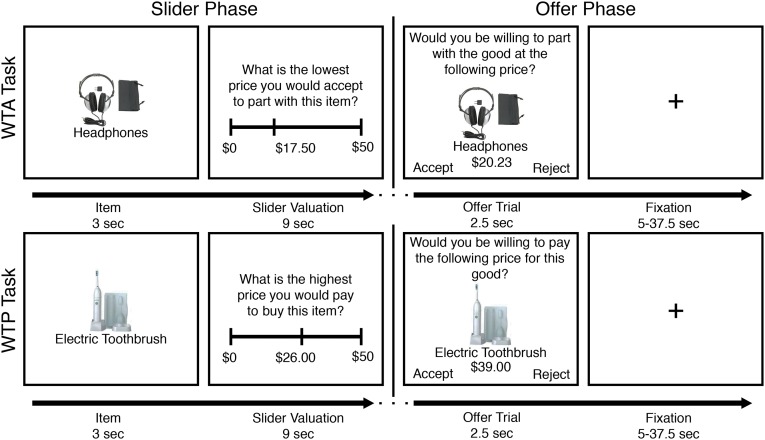
We conducted two functional MRI (fMRI) studies to investigate how experience changes neural correlates of the endowment effect. In Study 1, professional and inexperienced traders indicated the price that they were willing to pay to buy (WTP) and willing to accept to sell (WTA) each of several different products using a slider bar. We scanned these participants while they made decisions about buying and selling these items at different prices (Fig. 1). Importantly, the prices were scaled according to responses on the slider, allowing us to sample neural responses to prospective gains and losses during both tasks. In Study 2, we scanned inexperienced traders on the Study 1 paradigm before and after incentivizing the participants to sell items on eBay over 2 mo. In both studies, the order of buying and selling tasks were counterbalanced. In Study 2, we also varied the set of items presented in each scanning session and counterbalanced the order across participants. To ensure incentive compatibility, participants were paid according to one of their decisions during the slider or the scanner task. See Methods for full details.
Fig. 1.
Sample stimuli from the scanner paradigm. In each task, participants first made slider valuations for the four different products. Afterward, in the offer phase, participants saw 4 blocks (1 per item) of 31 prices at which the participants could choose to buy the item (WTP) or sell the item (WTA). These tasks were counterbalanced between participants and randomized sessions.
Results
Behavioral.
We computed the endowment effect for each consumer good as the difference between WTA and WTP indicated using the slider, normalized by WTP. As in previous studies (1, 16, 18), participants in Study 1 showed a significant positive endowment effect on average [51% of WTP; t (29) = 2.83, P < 0.01]. This finding was particularly pronounced in inexperienced traders [86% of WTP; t (11) = 2.40, P < 0.05] but not experienced traders [28% of WTP; t (17) = 1.62, P = 0.12]. In regression analyses, experience trended toward decreasing the endowment effect, controlling for demographic variables and task order (β = −0.56, P = 0.079; Table S1).
Table S1.
Study 1 endowment effect regression results
| Variables | Endowment effect | |
| Insula activation | 3.996 (1.628)* | |
| Experience | −0.557 (0.305)† | −0.377 (0.286) |
| Task order | −0.571 (0.352) | −0.673 (0.323)* |
| Age | 0.011 (0.023) | 0.018 (0.020) |
| Income | 0.101 (0.090) | 0.061 (0.078) |
| Female | 0.180 (0.330) | 0.397 (0.307) |
| Constant | 0.330 (0.623) | −0.159 (0.558) |
| R2 | 0.15 | 0.24 |
| N | 119 | 119 |
Robust SEs (clustered by subject) are in parentheses. †P < 0.1; *P < 0.05; **P < 0.01 (two-tailed).
Participants in Study 2 had a significant positive endowment effect [21% of WTP; t (14) = 2.50, P < 0.05; averaged over both sessions]. Additionally, being asked to trade over a 2-mo period decreased the endowment effect from 30% [t (14) = 2.59, P < 0.05] to 12% of WTP [t (14) = 1.34, P = 0.20]. This change was significant after controlling for the set of items subjects saw in each session (β = −0.51, P < 0.01; Table S2), suggesting that selling experience, and not endogenous characteristics of professional traders, reduces the endowment effect.
Table S2.
Study 2 endowment effect regression results
| Variables | ΔEndowment effect | ||
| Δ Insula activation | 3.262 (1.518)* | ||
| Item set order | 0.566 (0.194)* | 0.569 (0.194)* | 0.478 (0.190)* |
| Age | −0.001 (0.019) | −0.014 (0.024) | |
| Σ Insula activation | −0.833 (1.006) | ||
| Constant | −0.513 (0.137)** | −0.514 (0.143)** | −0.396 (0.151)* |
| R2 | 0.37 | 0.37 | 0.53 |
| N | 15 | 15 | 15 |
Robust SEs (clustered by subject) are in parentheses. The constant reflects the endowment effect decrease from session 1 to session 2, controlling for other regressors in the model. Because the set of items differed between session 1 and session 2, we calculate change scores by taking the average of the four observations at session 1 subtracted from those at session 2. Age was centered at the median because of skewness. Sum of insula activation was mean centered. *P < 0.05; **P < 0.01 (two-tailed).
Brain Imaging.
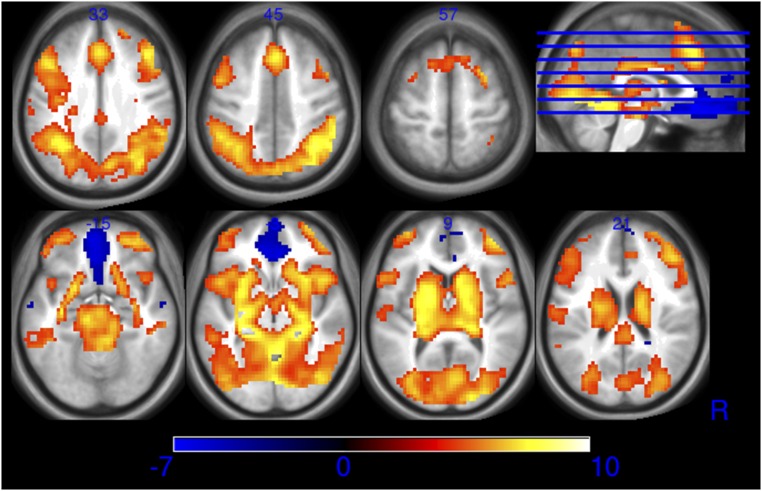
A whole-brain mixed ANOVA was conducted with study (Study 1, Study 2), experience (recruited based on experience in Study 1, pre- vs. post-training in Study 2), task (WTA vs. WTP), and offer (greater vs. less than slider value) as factors, using the sandwich variance estimator to account for repeated-measures covariance (Methods). The task vs. baseline contrast revealed frontoparietal, occipital, and mesolimbic activation but also deactivation in the medial OFC region, bilateral temporal poles, posterior cingulate cortex, precuneus, left middle frontal gyrus, and left postcentral gyrus [false-discovery rate (FDR), P < 0.05; Fig. S1 and Table S3]. OFC activation did not vary as a function of any factor, so we did not investigate this region in subsequent region of interest (ROI) analyses. A significant main effect of study was observed, with greater overall activation for Study 1 in frontal, temporal, and occipital regions (Table S3). However, the effect of study did not interact with any other factor.
Fig. S1.
Task was compared against implicit baseline as a manipulation check. Extensive clusters of activation were observed in visual, frontoparietal, salience network, and limbic regions, whereas reduced activation was observed in the ventromedial prefrontal cortex, commonly included in the default mode network. Voxel-wise threshold P < 0.00001 uncorrected. Units in z scores.
Table S3.
Whole-brain ANOVA results
| Contrast and brain region | Cluster size | Peak χ2 | MNI coordinates | ||
| x | y | z | |||
| Study 1 > Study 2 | |||||
| L IFG | 14 | 23.22 | −51 | 23 | 2 |
| L TP, aSTG, IFG, ventral insula | 47 | 21.82 | −42 | 20 | −12 |
| ACC | 10 | 21.12 | 3 | 20 | 20 |
| L MFG, SFG | 107 | 19.58 | −30 | 29 | 44 |
| L MTG | 49 | 18.75 | −51 | −43 | −4 |
| R aSTG, IFG, ventral insula | 21 | 18.42 | 36 | 14 | −18 |
| R PHC | 2 | 17.69 | 21 | −40 | −4 |
| L MOG | 11 | 17.46 | −36 | −79 | 13 |
| Midbrain | 15 | 17.23 | 3 | −7 | −15 |
| L MTG | 18 | 15.87 | −51 | −67 | 13 |
| R SMG | 7 | 15.42 | 60 | −25 | 24 |
| R PHC | 6 | 15.11 | 30 | −40 | −8 |
| L inferior occipital lobe, ITG | 14 | 14.99 | −51 | −61 | −4 |
| R TP, aSTG | 7 | 14.79 | 48 | 11 | −18 |
| R SMG | 5 | 14.79 | 30 | 41 | 38 |
| Precentral gyrus | 4 | 14.79 | −6 | −19 | 69 |
| L MFG | 1 | 14.61 | −42 | 29 | 44 |
| R SMG | 2 | 14.51 | 63 | −25 | 30 |
| L IFG | 2 | 14.49 | −54 | 17 | 6 |
| R MFG | 2 | 14.43 | 39 | 29 | 34 |
| L MTG | 1 | 14.08 | −57 | −58 | −4 |
| Dorsal MCC | 4 | 13.89 | 9 | −13 | 44 |
| R postcentral gyrus | 3 | 13.83 | 39 | −37 | 52 |
| R posterior insula | 5 | 13.72 | 42 | −13 | −8 |
| L PHC | 5 | 13.59 | −18 | −40 | −4 |
| L PHC | 4 | 13.29 | −24 | −40 | −15 |
| R posterior insula | 5 | 13.19 | 36 | −19 | 2 |
| L IPL, SMG | 8 | 12.86 | −57 | −34 | 30 |
| R posterior cingulate cortex | 3 | 12.62 | 18 | −28 | 41 |
| L temporal pole | 1 | 12.33 | −27 | 8 | −26 |
| L precentral gyrus | 1 | 12.29 | −57 | −4 | 6 |
| R MFG | 3 | 12.18 | 33 | 35 | 38 |
| R MTG | 2 | 12.18 | 60 | −10 | −12 |
| L SMG | 6 | 12.13 | −57 | −49 | 30 |
| L SFG | 5 | 11.78 | −21 | 59 | 10 |
| R precuneus | 3 | 11.7 | 12 | −49 | 62 |
| L MTG | 1 | 11.65 | −66 | −40 | −12 |
| L lateral caudate | 2 | 11.63 | −18 | −4 | 10 |
| R posterior STG | 2 | 11.58 | 57 | −61 | 16 |
| R postcentral gyrus | 1 | 11.56 | 57 | −22 | 27 |
| SFG | 4 | 11.53 | 0 | 50 | 30 |
| R PHC | 1 | 11.34 | 18 | −37 | −1 |
| MCC | 2 | 11.31 | 3 | −7 | 34 |
| R MFG | 1 | 11.3 | 42 | 23 | 34 |
| L precuneus | 2 | 11.21 | −15 | −67 | 27 |
| SFG | 1 | 11.21 | −9 | 53 | 27 |
| L MFG | 1 | 10.99 | −48 | 50 | 10 |
| L SFG | 1 | 10.95 | −15 | 17 | 55 |
| R pSTG | 2 | 10.82 | 54 | −43 | 16 |
| L SFG | 1 | 10.8 | −15 | 53 | 30 |
| L MTG | 1 | 10.8 | −60 | −37 | −12 |
| R ITG | 1 | 10.77 | 57 | −58 | −8 |
| SMA | 1 | 10.6 | 6 | −13 | 69 |
| L MTG | 2 | 10.6 | −45 | −28 | −8 |
| R olfactory bulb | 1 | 10.56 | 24 | 11 | −22 |
| Precuneus | 1 | 10.53 | −9 | −55 | 58 |
| R MTG | 1 | 10.53 | 42 | −34 | −4 |
| SFG | 2 | 10.52 | 12 | 44 | 41 |
| L SFG | 1 | 10.52 | −24 | 53 | 30 |
| R SMG | 2 | 10.47 | 63 | −46 | 24 |
| L SFG | 1 | 10.44 | −21 | 56 | 27 |
| L PHC | 1 | 10.4 | 24 | −37 | −12 |
| R MTG | 1 | 10.27 | 54 | −13 | −8 |
| L SFG | 1 | 10.27 | −12 | 65 | 2 |
| L amygdala | 1 | 10.25 | −18 | −7 | −15 |
| L posterior insula | 1 | 10.2 | −33 | −19 | −1 |
| L postcentral gyrus | 1 | 10.06 | −66 | −19 | 20 |
| MFG | 1 | 10.06 | 6 | 59 | 24 |
| Task × offer | |||||
| R MOG, cuneus | 56 | 29.5 | 30 | −85 | 2 |
| L MOG, cuneus | 40 | 28.05 | −30 | −82 | 13 |
| L lingual gyrus | 17 | 25 | −21 | −67 | −8 |
| SMA | 19 | 17.03 | 0 | 17 | 55 |
| L MTG | 3 | 16.79 | −48 | −25 | −12 |
| R anterior insula | 6 | 16.59 | 33 | 23 | 6 |
| L MTG | 6 | 16.38 | −60 | −46 | −15 |
| L MTG | 5 | 16.05 | −39 | −85 | −4 |
| L IFG | 5 | 15.51 | −39 | 20 | −15 |
| Lingual gyrus | 1 | 15.32 | −3 | −73 | −1 |
| L IPL | 6 | 15.2 | −27 | −55 | 41 |
| L caudate/NAcc | 1 | 15.03 | −12 | 8 | −12 |
| R precuneus | 5 | 14.9 | 18 | −70 | 30 |
| R ITG | 5 | 14.05 | 39 | −70 | −4 |
| R SPL | 10 | 13.75 | 30 | −58 | 55 |
| L thalamus | 1 | 13.56 | −18 | −22 | 2 |
| R precentral gyrus | 2 | 13.16 | 42 | −7 | 44 |
| dACC | 2 | 12.52 | 9 | 14 | 41 |
| L IFG | 1 | 12.33 | −33 | 23 | −15 |
Clusters are significant at FDR-corrected P < 0.05. No additional significant main or interaction effects were observed in any other cluster. aSTG, anterior superior temporal gyrus; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; L, left; MCC, midcingulate cortex; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; PHC, parahippocampal cortex; pSTG, posterior superior temporal gyrus; R, right; SFG, superior frontal cortex; SMA, supplementary motor area; SMG, supramarginal gyrus; SPL, superior parietal lobule; TP, temporal pole.
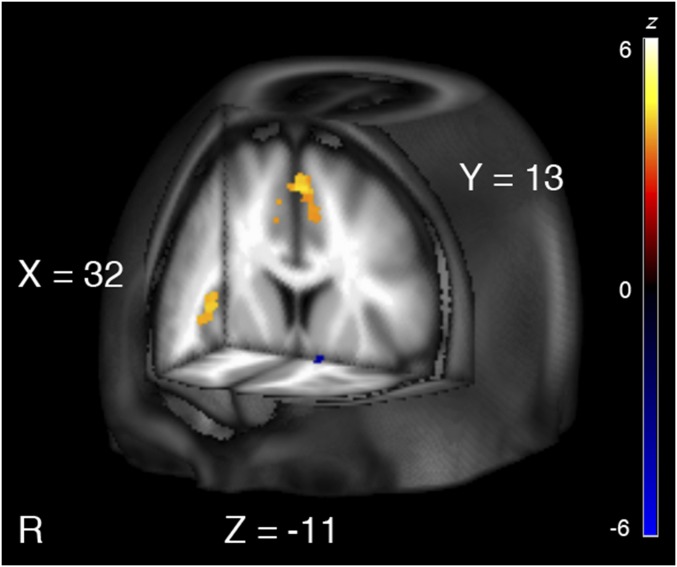
A significant task by offer interaction emerged in bilateral occipital and parietal clusters, including middle occipital gyrus, and left inferior parietal extending into left superior parietal. Additionally, clusters in precentral gyrus and supplementary motor area extending into dorsal anterior cingulate cortex (dACC) were observed bilaterally. Other significant clusters included thalamus, precuneus, bilateral anterior insula, left NAcc, and right putamen (Table S3 and Fig. 2). This effect was driven by a difference between conditions that were favorable (Table S4), including high prices during selling (WTA, above slider value) and low prices during buying (WTP, below slider value), and the unfavorable conditions, such as low selling prices (WTA, below slider value) and high buying prices (WTP, above slider value). No other effects reached significance.
Fig. 2.
Regions responsive to prospective gains (high selling prices, low buying prices) vs. prospective losses (low selling prices, high buying prices) were tested using the task by offer interaction contrast. Differential activation was observed in the right anterior insula (depicted on the sagittal slice; peak z = 4.07; 33, 23, 6) and dACC (depicted on the coronal slice; peak z = 4.13; 0, 17, 55), key nodes of the salience network (22–24), and the left NAcc (depicted on the coronal and axial slices; peak z = −3.88; −12, 8, −12). Salience network activation was greater during losses (warm colors), whereas left NAcc activation was greater during gains (cold colors). Coordinates are in MNI space. Active clusters are FDR-corrected at P < 0.05.
Table S4.
Whole-brain ANOVA task × offer pairwise tests
| Contrast and brain region | Cluster size | Peak z | MNI coordinates | ||
| x | y | z | |||
| Favorable > unfavorable | |||||
| L MTG | 8 | 4.05 | −60 | −46 | −15 |
| L NAcc | 8 | 3.88 | −12 | 8 | −12 |
| L MFG | 5 | 3.38 | −27 | 35 | 44 |
| L SFG | 6 | 3.29 | −15 | 29 | 41 |
| Unfavorable > favorable | |||||
| R MOG, cuneus | 186 | 5.74 | 27 | −88 | 2 |
| L MOG, cuneus | 155 | 5.3 | −30 | −82 | 13 |
| L lingual gyrus | 56 | 5 | −21 | −67 | −8 |
| R precentral gyrus | 16 | 4.41 | 45 | −7 | 48 |
| SMA/dACC | 97 | 4.27 | −3 | 14 | 55 |
| L MTG | 9 | 4.1 | −48 | −25 | −12 |
| R anterior insula | 41 | 4.07 | 33 | 23 | 6 |
| L IFG | 42 | 4.04 | −39 | 17 | −15 |
| R superior occipital lobe, cuneus | 59 | 4 | 18 | −73 | 30 |
| R pSTG | 13 | 3.94 | 51 | −28 | −4 |
| L IPL, SPL | 18 | 3.9 | −27 | −55 | 41 |
| R thalamus | 10 | 3.84 | 18 | −25 | 6 |
| R SPL | 35 | 3.84 | 27 | −52 | 41 |
| R precentral gyrus | 9 | 3.79 | 45 | −1 | 30 |
| L thalamus | 5 | 3.68 | −18 | −22 | 2 |
| R dorsal putamen | 13 | 3.6 | 24 | −1 | 6 |
| R IFG | 18 | 3.53 | 51 | 26 | −12 |
| dACC | 7 | 3.39 | 12 | 26 | 24 |
| L precentral gyrus | 5 | 3.38 | −30 | −7 | 44 |
| dACC | 6 | 3.38 | −9 | 23 | 30 |
| R pSTG | 5 | 3.34 | 54 | −43 | 16 |
Favorable offers indicate trials in which participants stood to gain (either by paying a price lower than the participants’ maximum WTP or by selling at a price higher than their minimum WTA), whereas unfavorable offers indicate trials in which participants stood to lose (by paying a price higher than their maximum WTP, or selling at a price lower than their minimum WTA). Notably, differences emerged between favorable and unfavorable offers, such that the right insula and dACC were more active when viewing unfavorable offers, and left NAcc was more activated in response to favorable offers. Clusters are significant at P < 0.001, with a cluster extent threshold k = 5. IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L, left; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; pSTG, posterior superior temporal gyrus; R, right; SFG, superior frontal gyrus; SMA, supplementary motor area; SMG, supramarginal gyrus; SPL, superior parietal lobule.
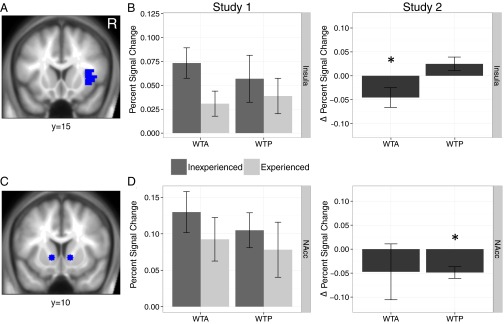
ROI Analysis.
Planned analyses were conducted using NAcc and anterior insula ROIs to test whether positive and negative affective responses (9) mediate the relation between trading experience and the endowment effect. Given the whole-brain differences between our studies, 2 (experience) × 2 (task) ANOVAs were conducted for each study and ROI, controlling for demographics and order variables (Fig. 3). For the insula, an experience main effect was observed in Study 1 [F(1,55) = 4.43, P < 0.05], whereas a significant task by experience interaction emerged in Study 2 [F(1,54) = 7.08, P = 0.01]. For the NAcc, an experience main effect was found in Study 2 [F(1,54) = 4.83, P < 0.05]. No other effects were significant.
Fig. 3.
Right anterior insula and NAcc activation across selling (WTA) and buying (WTP) tasks in the experience groups in Study 1, and the insula and NAcc activation change scores in Study 2. (A) The insula ROI was the Talairach labeled right insula, anterior to y = 0 (16). (B) Selling experience significantly lowered insula signal for WTA in Study 2. The same pattern was observed in Study 1 in a regression analysis, although in the ANOVA, only the main effect of experience emerged (P < 0.05; Results) without interacting with task. (C) The NAcc ROI was defined as 8-mm spheres centered on MNI coordinates ±12, 10, −2 (42). (D) NAcc did not differ with experience in Study 1 but decreased for WTP in Study 2 (P < 0.01; Results). Percentage signal change values were adjusted for demographics and task order in Study 1. Δ Percentage signal change values in Study 2 are computed as Session 1 subtracted from Session 2, adjusted for age and item set. *P < 0.05 (two-tailed).
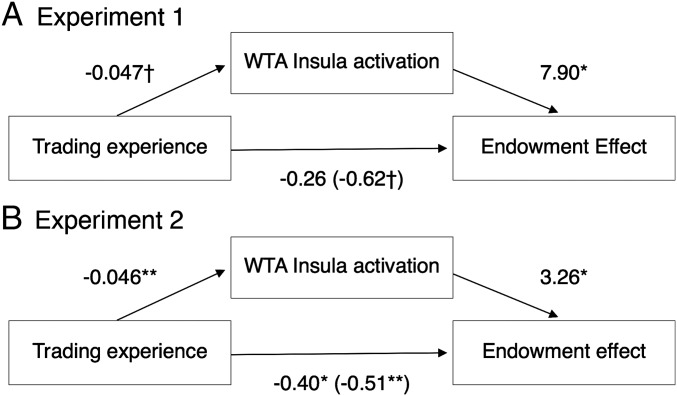
We followed up these ANOVAs with regression analyses. In Study 1, insula activation during the WTA task, but not WTP, was greater in inexperienced compared with experienced participants (β = −0.045, P < 0.05) and was positively related to the endowment effect (β = 4.58, P = 0.005; Fig. 4) controlling for demographics and task order.
Fig. 4.
Insula activation during selling mediated the effect of recruited experience (point estimate: −0.368; 95% CI: −1.287 to −0.016) (A) and experimentally manipulated experience (point estimate: −0.148; 95% CI: −0.359 to −0.025) (B) on the endowment effect. The numbers in parentheses indicate the regression coefficient without the mediating variable. All Study 1 regressions included sex, income, and task order as covariates; coefficients and significance levels differ slightly from those presented in Results because of averaging over measurements within each subject. Study 2 regressions included median centered age and item set order as covariates. Full results are presented in Tables S1 and S2. †P < 0.1; *P < 0.05; **P < 0.01 (two-tailed).
Next, we tested whether insula and NAcc activation changed after participants gained selling experience in Study 2. After controlling for item set order, decreased activation was observed in the right anterior insula during the WTA condition (β = −0.050, P < 0.05) and NAcc during the WTP condition (β = −0.047, P < 0.01).
Additionally, age was marginally negatively associated with WTA insula (β = −0.002, P = 0.058) and positively associated with the WTA insula change score (β = 0.003, P < 0.005), indicating a significant interaction with training. Nevertheless, the WTA insula change remained significant (β = −0.046, P < 0.05) after controlling for median-centered age.
Mediation Analysis.
Given our a priori hypotheses about loss aversion and the specific role of the insula, we tested the indirect effect of experience, through subject-averaged insula activation during the WTA task, on the subject-averaged endowment effect. We used the method of Preacher and Hayes (19), which relaxes the requirement of significant direct effects (20), to estimate a 95% bias-corrected bootstrap confidence interval (CI) based on 5,000 bootstrap samples for the indirect effect. The indirect effect differed significantly from 0 [point estimate: −0.368; 95% CI: −1.287 to −0.016; Fig. 4], controlling for demographics and task order.
For Study 2, we estimated the endowment effect, insula, and NAcc change scores controlling for item set order and median-centered age by taking the residuals and adding the constants. Then, we used the path-analytic method of Montoya and Hayes* to estimate the indirect effects of insula and NAcc with a bias-corrected bootstrap CI based on 5,000 bootstrap samples. Consistent with Study 1, change in insula signal mediated the effect of trading experience on the endowment effect (point estimate: −0.148; 95% CI: −0.359 to −0.025; Fig. 4). The indirect effect of NAcc change did not reach significance (point estimate: −0.102; 95% CI: −0.230 to 0.008).
Discussion
How does economic experience affect economic decision biases that appear to be a characteristic of human decision-making (1, 21)? Previous research (2–4) has suggested that extensive experience in buying and selling goods can reduce the size of these biases. In the present research, we tested specific hypotheses regarding the mechanism by which trading experience modifies consumer decision-making processes.
In two studies, we find convergent evidence that the influence of trading experience on the endowment effect is mediated in part by lower sensitivity of the right anterior insula during selling. This result was robust over both of our studies despite the different populations, procedures, and incentives used, as well as corresponding differences on behavioral effect size and other whole-brain effects. Whereas Study 1 mediation results are susceptible to bias because of their cross-sectional nature (22), Study 2 used experimentally induced trading experience and time-series variation to estimate mediation. It is worth noting that current human in vivo techniques do not permit more continuous measurement or direct manipulation of the insula, which would be ideal for estimating the insula indirect effect.
Activation of the right anterior insula and dACC during consideration of unfavorable offers in the whole-brain analysis coheres with work demonstrating involvement of these regions in processing salience (23–25) and the role of the insula in anticipating and rejecting aversive outcomes in particular (13, 15, 26, 27). The decreased insula activity suggests that trading experience causes agents to experience less loss aversion during selling. This finding is consistent with the observation that most inexperienced traders have substantial buying but not selling experience and dovetails with the intuition that routine exchanges are likely to be evaluated in aggregate (i.e., as a profitable operation overall) instead of independent gains and losses (28).
Because our interpretation of the insula results rely on reverse inference, we queried Neurosynth [version 0.3.4 (29)] using the insula voxels that were most sensitive to variation in experience in our studies (Study 1: 48, 11, −1; Study 2: 36, 14, −1). Neurosynth is a meta-analytic database with a corpus of 11,406 neuroimaging studies, which provides estimates of posterior probabilities that a term will appear in a study, conditional on that study reporting activation in a given voxel, and z scores associated with that voxel in the posterior probability map. For each of the two peak voxels, terms related to pain had the largest reverse inference z scores and posterior probabilities. The peak voxel in Study 1 had a z score of 7.07 for “pain,” and posterior probability P(pain|activation) = 0.75 (the next two nonanatomy terms were “preparation”: z = 4.53, P(preparation|activation) = 0.75, and “noxious”: z = 4.52, P(noxious|activation) = 0.79). Similarly, in Study 2, the first three nonanatomy terms were pain: z = 7.98, P(pain|activation) = 0.75, noxious: z = 7.77, P(noxious|activation) = 0.84, and “heat”: z = 7.45, P(heat|activation) = 0.85. Of course, as evidenced by the large z score and posterior probability for preparation for the Study 1 peak insula voxel, this result is not completely conclusive. However, it is unclear how preparation, which clusters with task switching and response inhibition in Neurosynth (30), might generate variability in the endowment effect in our study. Indeed, experience was not associated with performance on a working memory task [operation span (31)] in Study 1 [t (26) = 0.41; not significant].
In contrast, NAcc activation in the task by offer interaction appeared to be driven by greater activation for high selling offers and low buying offers, consistent with previous results demonstrating increased NAcc signal during anticipation of gain (32). The decreased NAcc signal when buying, together with the decreased insula signal when selling in Study 2, might suggest lower preferences overall posttraining. However, only insula signal significantly mediated the change in endowment effect.
The effect we observe in Study 2 is particularly striking given the briefness of our intervention, suggesting that novice traders may reach criterion levels on the endowment effect quite quickly. These results do not appear to be an artifact of repeating the scanner task, because our results were robust across people with diverse professional trading backgrounds who did not undergo repeated scanning in Study 1. Moreover, other work indicates that whereas fMRI signal in the amygdala habituates over repeated scans in emotion processing tasks, the anterior insula has good test–retest reliability (33, 34). The Study 2 data suggest that the reduction in insula signal and the endowment effect scales in part with the number of items sold as well as items listed (SI Text). The signs of the coefficients indicate that actual selling experience, and not just effort, is required for the results we observe. Nevertheless, more extensive efforts are needed to determine the specific aspects of experience that most effectively reduce decision biases.
The sign of the moderating influence of age on the relation between training and insula activation in Study 2 suggests less treatment-induced reduction in insula signal in older adults. This finding may be explained by lower anterior insula responsiveness to loss anticipation in older adults (15, 34, 35). Indeed, a marginal negative relation was observed between age and insula activation. Thus, market experience may not be as efficacious for older adults to overcome decision biases because of insensitivity of the insula.
Although our results are consistent with the hypothesis that trading experience reduces loss aversion in the context of selling, other alternatives are possible. For one, trading experience may reduce uncertainty about costs or risks associated with selling activities (36). Because anxiety about selling may elicit insula activation, we might observe the same results without any decrease in loss aversion. The selling task in our experiment was designed to be free of possible sources of friction such as risk, transaction costs, and human trading partners. Still, we cannot definitively rule out this explanation without measuring perceived costs.
Another possibility is that trading experience causes agents to reappraise selling activities (6, 37). Specifically, expert traders may learn to frame selling goods as opportunities for monetary gain vs. nongain, rather than from the reference point of loss of ownership vs. nonloss. A related possibility is that experience may induce a wider framing, such that prospects are evaluated in the context of an overall portfolio. According to these explanations, experience facilitates strategies for circumventing loss aversion, rather than reducing sensitivity to loss. Our study did not include measures of how framing changed with experience to test these alternatives, but the fact that only one decision counted suggests against the portfolio strategy. Inductions such as taking the perspective of a trader (38) may be used in future research to directly explore the strategies that experienced traders bring to bear on selling and how these strategies interact with variability on loss aversion to modulate the strength of the endowment effect in inexperienced and experienced traders.
The endowment effect contradicts standard economic theory and distorts market transactions (21). When WTA is generally greater than WTP, buyers and sellers are less likely to agree on a price, resulting in undertrading in real markets (39, 40). Our results suggest that even a modest amount of trading experience may help eliminate such inefficiencies in part through reducing the influence of loss aversion.
SI Text
Self-Reported Pricing Strategies.
As part of the product rating survey, participants were asked to describe the strategy they used when pricing items. For the Study 1 sample, three authors (K.J.Y., S.E., and A.H.) independently rated participant responses; for the Study 2 sample, two authors (K.J.Y. and L.C.P.T.) independently rated the responses. Raters used the following description. “Buy low sell high” is a pricing strategy in which an agent attempts to make a profit by acquiring items at a price below what he perceives to be the market price of the item and selling these items at a price above the perceived market value. This strategy differs from others in that the agent does not view the items in the context of personal consumption and, instead, chooses to extract as much payment as possible from the experiment.
We excluded participants who used a buy-low-sell-high strategy, because the strategy produces a behavioral pattern resembling the endowment effect but invokes mechanisms that cannot explain the endowment effect in other commonly used trade asymmetry paradigms. To identify these participants, we used the intersection of the ratings (responses that all raters agreed as fitting the buy-low-sell-high criteria). No other pricing strategies were excluded.
Study 2 Selling Experience.
Participants in Study 2 could vary on several endogenous variables, such as the effort they put into selling and selling ability. To examine the effect of effort, we use the number of listings participants posted. However, we do not have the resolution to cleanly identify the effect of selling experience independently of selling ability. Despite this caveat, we ran exploratory regression models using items listed and items sold to model what aspects of experience might be contributing to the observed reduction in the endowment effect as well as the insula signal.
Our regression results (Table S7) suggest that the number of items sold decreases both the endowment effect (β = −0.092, P < 0.05) and the insula signal (β = −0.012, P < 0.01), whereas the number of listings tend to have the opposite effects (endowment effect: β = 0.055, P = 0.07; insula: β = 0.014, P < 0.01). These coefficients suggest that simply making an effort to comply with the experiment may not explain our effects, but completing sales may be particularly important. This relationship may be attributable to the mere accrual of selling experience, which could blunt the affective impact of trading outcomes. Alternatively, the relationship may be explained by selling ability, because individuals develop strategies such as cognitive framing that may change affective responses as well as increase sales. Our eBay data do not have high enough resolution to identify which aspects are most important.
Table S7.
Endowment effect and insula vs. individual selling experience regression results in Study 2
| Variables | ΔEndowment effect | ΔInsula |
| Items sold | −0.092 (0.038)* | −0.012 (0.004)** |
| Items listed | 0.055 (0.028)† | 0.014 (0.004)** |
| Constant | −0.287 (0.145)† | −0.094 (0.037) |
| R2 | 0.30 | 0.63 |
| N | 15 | 15 |
P < 0.1; *P < 0.05; **P < 0.01 (two-tailed).
Methods
Study 1.
Participants.
Thirty-nine adult participants were recruited from two different populations; 22 were experienced eBay traders with high seller feedback ratings and other business professionals with financial and selling experience in the Chicago area. Experienced participants were recruited through eBay’s messaging interface, email, phone calls, paper mailings, or in-person using a script. The remaining 17 participants were recruited from among staff members at a Midwestern university with little trading experience. Inexperienced participants were recruited through flyers around campus, as well as participant recruitment mailing lists.
Prospective participants were screened for mood-altering medication and a history of severe head trauma before enrollment. Of the participants who were invited to take part, three (one experienced, two inexperienced) did not complete the scan because of technical difficulties, and six (three experienced, three inexperienced) were excluded for using a “buy-low-sell-high” strategy in the scanner task (SI Text). Because our question is specifically about modeling how experience modifies neural mechanisms underlying the endowment effect, we excluded one inexperienced participant who showed a consistent and idiosyncratic response bias (WTP greater than WTA) for all four items. One inexperienced participant’s responses differed from the mean by more than 2 SDs for a well-defined subset of trials, so those trials were excluded for that participant. The final sample comprised 12 inexperienced and 18 experienced participants, aged 24–58 y, right-handed, native English speakers, with normal or corrected-to-normal vision, and normal hearing. Participants received $100 and additional cash or a consumer good, based on one of their decisions in the scanner task.
As expected, members of the experienced group were more likely to have experience selling to customers (experienced: 83.3%; inexperienced: 0%; P < 0.001), businesses (experienced: 33.3%; inexperienced: 0%; P = 0.01), and in online settings (experienced: 72%; inexperienced: 0%; P < 0.001). Because of the recruitment procedure, the experienced and inexperienced groups had qualitatively different professions. The two groups did not differ on any other demographic variable (Table S5).
Table S5.
Summary statistics for the different samples on trading experience and demographics
| Variables | Study 1 | Study 2 | Test statistic | |
| Inexperienced | Experienced | |||
| Selling experience | ||||
| Sells to customers, proportion | 0 | 0.83 | 0.07 | χ2 (2) = 30.02** |
| Sells to businesses, proportion | 0 | 0.33 | 0.07 | χ2 (2) = 7.44* |
| Online selling, proportion | 0 | 0.72 | 0.07 | χ2 (2) = 23.80** |
| Demographics | ||||
| Sex, proportion female | 0.58 | 0.5 | 0.67 | χ2 (2) = 0.934 |
| Age, y | 34.3 (5.12) | 35.4 (9.08) | 31.0 (7.96) | F(1,43) = 1.33 |
| Income, $20,000 | 2.58 (1.31) | 3.33 (1.37) | 3.80 (1.78) | F(1,43) = 4.37* |
| N | 12 | 18 | 15 | |
Proportions were compared using χ2 tests. Ordinal and continuous variables were tested using ANOVAs. Income was measured using a six-point Likert scale, with bins spanning $20,000 intervals. SDs are in parentheses. *P < 0.05; **P < 0.01 (two-tailed).
Procedure.
Upon arrival at the laboratory, participants reviewed and completed a consent form and MRI screening. Before scanning, participants completed a task measuring working memory capacity [i.e., the operation span task (31)] for which they received $50 for buying products in the scanner task. Next, participants completed the endowment effect task in the scanner. Stimulus presentation and response collection were programmed in MATLAB (MathWorks) using PsychToolBox (41) and presented in the scanner with a back-projection system. Participants completed a buying task and a selling task (Fig. 1., order counterbalanced across participants) while undergoing fMRI.
At the beginning of the buying task, participants saw each of four products (4-gigabyte thumb drive, comfort lap desk, electric toothbrush, or headphones) and indicated the highest price they were willing to pay in exchange for each product using a slider bar with $0.50 increments, ranging from $0 to $50. After the slider phase, participants saw four blocks of trials. In each block, 1 of the 4 products was randomly selected without replacement, and participants saw 15 prices above, 15 prices below, and 1 price equal to their slider value for that product presented in random order (ranging from 25% to 175% of the slider value). Participants accepted or rejected the offer to buy the product at the displayed price. Each trial was 2.5 s, and fixation crosses were presented during intertrial intervals of variable duration determined using optseq2 (42). The selling (WTA) task was symmetric to the WTP task, except that participants were “endowed” with the items in the beginning of the task. Participants held four wooden blocks in their left hand and were asked to imagine holding and using the four products in question (43). Participants were informed that one of their decisions [either one of the sliders using the method of Becker, DeGroot, and Marschak (44) or one of the offer trials] would be randomly selected to count and were encouraged to treat every trial as real.
The WTA (selling) and WTP (buying) tasks each comprised two functional scans, with a short break in between. Each scan included two blocks, such that all four items were presented both in the WTP task and in the WTA task. The WTA scans were separated from the WTP scans by a break during which participants saw images of national parks and the anatomical scan took place. Thus, the endowment effect task was an event-related design, with trials grouped by item and task to avoid cognitive load. After scanning, participants completed demographics, market experience, and product rating surveys. All procedures were approved by the University of Chicago Institutional Review Board.
Study 2.
Participants.
Twenty-four inexperienced participants were recruited from vintage and antique markets in the Chicago area, in-person using a script. Potential participants were asked to complete a brief prescreening survey to assess their eligibility. Visitors who were MRI-eligible and reported minimal professional and personal selling experience (zero items sold in a typical month) were invited to take part in a two-session fMRI study. Participants signed an affidavit promising to take part in an fMRI scan in December and in February.
Of the 24 participants, 1 did not complete the study because of the discovery of an anatomical abnormality, 7 were excluded for using an explicit buy-low-sell-high strategy in at least one of the scanning sessions (SI Text), and 1 was excluded because the individual’s responses differed from the mean of the sample by more than 5 SDs. Additionally, one participant did not complete the WTP portion of the second scan because of technical difficulties. The final sample comprised 15 participants, aged 20–51 y, right-handed, native English speakers, with normal or corrected-to-normal vision, and normal hearing. This sample did not differ from the inexperienced group in Study 1 on selling experience or any demographic variable except for marginally more income [t (25) = −1.97, P = 0.06; Table S5].
Procedure.
Participants took part in two scanning sessions, held 2 mo apart in December 2014 and February 2015, in which they completed the task detailed in Study 1. Each time participants arrived at the laboratory, they completed both a consent form and a MRI screening form. In one session, participants saw the same four items in Study 1, and in another session, participants saw a new set of items. The order of these item sets was counterbalanced across participants. At the end of each session, participants were paid according to one of their decisions in the task. To ensure retention, participants received a $200 show-up fee at the end of the second session.
Between the two sessions, participants were given incentives to gain selling experience by selling items on eBay. At the end of the first session, we gave participants a gift bag containing consumer goods valued at $100 total, with individual items valued at $10–20 each and asked them to make an eBay account. Participants were provided instructions for selling on eBay and were told that they would receive a lottery ticket for each item listed or sold before the second session, up to two tickets per item. The lottery was conducted at the end of all subjects’ participation and awarded four cash prizes of $1,000. In January, a month after the first session, we mailed participants a second gift bag containing consumer goods valued at $100, along with a reminder to sell items on eBay. All procedures were approved by the University of Chicago Institutional Review Board.
A total of 73.3% of participants listed at least one item (average 9.91 conditional on listing) on eBay during the study, and 60% of participants sold at least one item (average 5.22 conditional on selling). No demographic differences were observed between participants who were more or less likely to list or sell items (Table S6). To avoid confounding our results with omitted variables such as selling ability, we use intention-to-treat as a measure of experience in our main results (see SI Text and Table S7 for analyses on individual selling experience).
Table S6.
Study 2 selling experience correlation matrix
| Variables | Items Listed | Items Sold | Age | Female | Income |
| Items listed | — | 0.68** | 0.12 | 0.11 | −0.04 |
| Items Sold | — | −0.16 | 0.19 | −0.07 | |
| Age | — | −0.06 | 0.49† | ||
| Female | — | −0.33 | |||
| Income | — |
Dashes indicate correlation of a variable with itself. †P < 0.1; *P < 0.05; **P < 0.01 (two-tailed).
FMRI acquisition.
MRI was performed using a 3T Philips Achieva Quasar scanner. Whole-brain fMRI data were acquired with a T2*-weighted echoplanar imaging sequence [repetition time: 2.5 s; echo time: 30 ms; field of view (FOV): 192 × 192 mm2; flip angle: 81°; matrix size: 64 × 64; in-plane resolution: 3 × 3 mm2; slice thickness: 3 mm; slice gap: 0.5 mm; 32 slices]. A volume-selective z-shim method was used to reduce susceptibility artifacts in the orbitofrontal region. Four additional slices covering the OFC were acquired in each volume with a compensation gradient applied along the z axis. The final image of the four OFC slices was computed by taking the root sum of squares of the original and z-shimmed slices. High-resolution anatomical images were acquired in the sagittal plane using a Philips T1-weighted SENSE-Ref sequence (171 slices; voxel size: 1 × 1 × 1 mm3; reconstruction matrix size: 240 × 240; FOV: 228 × 240 × 171 mm3).
FMRI analysis.
fMRI data were analyzed using Statistical Parametric Mapping 8 (SPM8) (Wellcome Department of Imaging Neuroscience) and the Sandwich Estimator toolbox (SwE) (45). Raw functional volumes were slice-timing–corrected, followed by the z-shim combination procedure. The resulting volumes were realigned using a six-parameter affine transformation and resliced. The structural image was coregistered to the mean functional image and segmented into gray matter, white matter, and cerebrospinal fluid. The segmentation step produces normalization parameters, which were used to normalize the functional volumes to the Montreal Neurological Institute (MNI) template. Fixed-effects general linear model parameters were estimated using the Variational Bayes algorithm implemented in SPM8 to model the hemodynamic response for each participant, which adaptively smooths the functional volumes (46). Trials of each of four conditions (WTA task, offers above slider; WTA task, offers below slider; WTP task, offers above slider; WTP task, offers below slider) were modeled as 2.5-s boxcars convolved with the canonical hemodynamic response function. Motion parameters were included in the first-level model as regressors of no interest. First-level contrasts for each of the four conditions were smoothed with a 4-mm Gaussian kernel and entered into a second-level model using SwE to implement the sandwich variance estimator, which is robust to the covariance structure present in our repeated-measures data.
To maximize power, we pooled data from the two experiments in a four-way mixed-effects ANOVA with study (Study 1, Study 2), experience (recruited based on experience in Study 1, pre- vs. post-training in Study 2), task (WTA, WTP), and offer (above slider value, below slider value) as factors. Effects surviving FDR correction at P < 0.05 were examined with pairwise t contrasts.
β values from the four stimulus conditions were extracted for use in ROI analyses. Following previous work (15), an anatomical right anterior insula ROI was defined using the voxels in the Talairach-labeled right insula anterior to y = 0 using WFU Pickatlas (47). A bilateral NAcc ROI was created by taking 8mm spheres centered on MNI coordinates ±12, 10, −2 (48). For each participant, percentage signal change in the right anterior insula and NAcc ROIs were calculated with the MarsBaR toolbox† and entered into a series of multiple regressions to probe the role of the insula and the NAcc in the relation between market experience and the endowment effect. All reported regressions use SEs clustered by subject.
Acknowledgments
We thank members of A.H.’s laboratory and H.C.N.’s laboratory for help in data collection and comments on experimental design. We thank E. Weingarten for help with design and A. Lawther for assistance in data analysis. This project was supported by National Science Foundation (NSF) Human and Social Dynamics (HSD) Grant 0729322, a University of Chicago Wisdom Research Grant, and the John Templeton Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Montoya AK, Hayes AF, Estimating and testing indirect effects in within-subject mediation analysis: A path-analytic framework, Annual Convention of the Association for Psychological Science, May 20–24, 2015, New York, X-108.
†Brett M, Anton J-L, Valabregue R, Poline J-B, Region of interest analysis using an SPM toolbox, 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan, available on CD-ROM in NeuroImage, Vol 16, No 2 (abstr).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519853113/-/DCSupplemental.
References
- 1.Kahneman D, Knetsch JL, Thaler RH. Experimental tests of the endowment effect and the Coase theorem. J Polit Econ. 1990;98(6):1325–1348. [Google Scholar]
- 2.List JA. Does market experience eliminate market anomalies? Q J Econ. 2003;118(1):41–71. [Google Scholar]
- 3.List JA. Neoclassical theory vs. prospect theory: Evidence from the marketplace. Econometrica. 2004;72(2):615–625. [Google Scholar]
- 4.List JA. Does market experience eliminate market anomalies? The case of exogenous market experience. Am Econ Rev. 2011;101(3):313–317. [Google Scholar]
- 5.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47(2):263–292. [Google Scholar]
- 6.Kőszegi B, Rabin M. A model of reference-dependent preferences. Q J Econ. 2006;121(4):1133–1165. [Google Scholar]
- 7.Morewedge CK, Shu LL, Gilbert DT, Wilson TD. Bad riddance or good rubbish? Ownership and not loss aversion causes the endowment effect. J Exp Soc Psychol. 2009;45(4):947–951. [Google Scholar]
- 8.Carmon Z, Ariely D. Focusing on the forgone: How value can appear so different to buyers and sellers. J Consum Res. 2000;27(3):360–370. [Google Scholar]
- 9.Knutson B, Katovich K, Suri G. Inferring affect from fMRI data. Trends Cogn Sci. 2014;18(8):422–428. doi: 10.1016/j.tics.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53(1):147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raab G, Elger CE, Neuner M, Weber B. A neurological study of compulsive buying behavior. J Consum Policy. 2011;34(4):401–413. [Google Scholar]
- 12.Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27(37):9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 15.Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19(4):320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson B, et al. Neural antecedents of the endowment effect. Neuron. 2008;58(5):814–822. doi: 10.1016/j.neuron.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 18.Weber B, et al. Neural evidence for Reference-dependence in real-market-transactions. Neuroimage. 2007;35(1):441–447. doi: 10.1016/j.neuroimage.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 20.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. [Google Scholar]
- 21.Jolls C, Sunstein CR, Thaler RH. A behavioral approach to law and economics. Stanford Law Rev. 1998;50(5):1471–1550. [Google Scholar]
- 22.Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 25.Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: An investigation of error processing and effective connectivity. J Neurosci. 2013;33(16):7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 28.Kahneman D, Tversky A. Choices, values, and frames. Am Psychol. 1984;39(4):341–350. [Google Scholar]
- 29.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23(3):739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behav Res Methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- 32.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gee DG, et al. Reliability of an fMRI paradigm for emotional processing in a multisite longitudinal study. Hum Brain Mapp. 2015;36(7):2558–2579. doi: 10.1002/hbm.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage. 2014;84(1):279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samanez-Larkin GR, et al. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10(6):787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelmann D, Hollard G. Reconsidering the effect of market experience on the “endowment effect.”. Econometrica. 2010;78(6):2005–2019. [Google Scholar]
- 37.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokol-Hessner P, et al. Thinking like a trader selectively reduces individuals’ loss aversion. Proc Natl Acad Sci USA. 2009;106(13):5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genesove D, Mayer C. Loss aversion and seller behavior: Evidence from the housing market. Q J Econ. 2001;116(4):1233–1260. [Google Scholar]
- 40.Furche A, Johnstone D. Evidence of the endowment effect in stock market order placement. J Behav Finance. 2006;7(3):145–154. [Google Scholar]
- 41.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 42.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8(2-3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf JR, Arkes HR, Muhanna WA. The power of touch: An examination of the effect of duration of physical contact on the valuation of objects. Judgm Decis Mak. 2008;3(6):476–482. [Google Scholar]
- 44.Becker GM, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behav Sci. 1964;9(3):226–232. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- 45.Guillaume B, Hua X, Thompson PM, Waldorp L, Nichols TE. Alzheimer’s Disease Neuroimaging Initiative Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage. 2014;94(1):287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penny WD, Trujillo-Barreto NJ, Friston KJ. Bayesian fMRI time series analysis with spatial priors. Neuroimage. 2005;24(2):350–362. doi: 10.1016/j.neuroimage.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 48.Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39(1):538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]