Although many people would agree with the proposition that factors in early life can have profound consequences for a person’s health and well-being in later life, the extent of this relationship and the mechanisms that underlie it are debatable. This is especially true when considering how childhood and even gestation might affect outcomes seven or eight decades later. We know that aging is associated with great variability in cognitive and physical health, yet attempts to explain this variability have largely been limited to a few genetic factors and a host of exposures measured in mid- and late-life. Increasing attention, however, is being drawn to the ways in which development and aging may be linked. In PNAS, Walhovd et al. (1) provide novel observations on how early-life events are related to pervasive lifelong effects on brain and cognition.
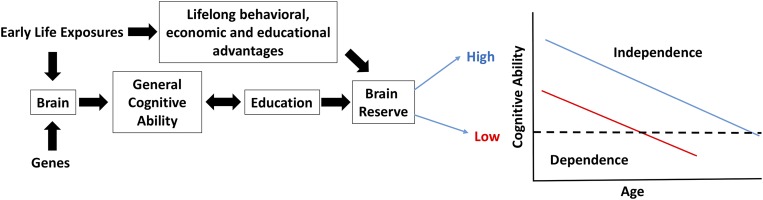
Cognitive abilities in older people are highly variable between individuals but tend to be consistent across cognitive domains within individuals (2). Put simply, some people do better than others in general; those with preserved memory also tend to show preservation in language, perceptual speed, and visuospatial ability. This finding is consistent with studies of intelligence through the lifespan, with a general intelligence factor, or g, explaining considerable variance across many different cognitive abilities. The source of this age-related variability is often ascribed to clinical and preclinical age-related brain disease along with “brain aging,” a poorly understood process that affects multiple neural systems (3). Both genes and the environment drive these processes, and their effects on behavior and cognition may be mediated by another factor, defined as “brain reserve.” Brain reserve is a construct that cannot be directly measured; its presence is inferred through the observation that individuals show different behavioral responses to similar levels of brain pathology (4). One of the proxy variables used to assess brain reserve has been educational attainment, with the supposition that those with more education have been endowed with greater neural resources to withstand the effects of neurological disease (Fig. 1). Although reserve may encompass dynamic or plastic responses to aging and disease, here we are discussing what are presumably static processes present throughout much of life.
Fig. 1.
Conceptual model linking brain development, cognition, brain reserve, and late-life cognitive decline. Early life exposures and genes affect brain development, which in turn is related to GCA. GCA and education are related to one another, and provide brain reserve with advancing age. The graph demonstrates two individuals with high (blue) and low (red) brain reserve. Although the rate of their age-related cognitive decline is identical, the person with higher reserve crosses the threshold for dependence at an older age, thus experiencing a longer independent life. Early-life exposures, however, also confer indirect beneficial effects in addition to brain development, and these are likely to be salutary over the lifespan.
This model has been tested by measuring the effects of Alzheimer’s disease (AD) brain pathology. Convincing data across different cohorts have shown that higher levels of education are not associated with differential neuropathology, but are associated with less dementia risk and less-severe cognitive change in the face of such pathology (5). These data have been widely interpreted as the effects of brain reserve, measured as educational attainment, limiting the behavioral effects of age-related neuropathology. However, this model is unsatisfying because it is devoid of underlying neural mechanisms with limited understanding of the environmental and genetic factors that might underlie reserve.
The results presented by Walhovd et al. (1) expand our ideas about brain reserve and brain aging. In their study, the authors began by determining relationships between general cognitive ability (GCA, measured with a battery of cognitive assessments) and brain cortical surface area, measured with MRI scanning in a group of children between the ages of 4 and 12 y. The authors found that a substantial portion of the cortical surface was associated with GCA; they went on to measure this GCA region in a large sample of individuals ranging in age from 4 to 88 y, some of whom had longitudinal as well as cross-sectional measurements. Because Walhovd et al. also had information about GCA in this sample, they were able to characterize these individuals across the lifespan as having “high” vs. “low” GCA. The authors found that for all subjects, the cortical area associated with GCA declined with age and that subjects in the high-GCA group had larger surface area than those in the low-GCA group. However, the trajectories of change over the lifespan in the GCA regions were essentially identical in the two groups, indicating that cortical differences between high- and low-GCA people present in childhood were maintained throughout life. Walhovd et al. then went on to examine the early-life factors that might underlie this relationship and found that among a number of potential explanatory variables, birthweight and parental education were most strongly associated with the GCA cortical surface area. Evidence for a strong genetic component underlying the GCA surface area came from a separate analysis in a sample of middle-aged twins.
The observations in this work (1) build upon and extend existing data. For example, the association between genes and brain structure is well established, although the nature of this relationship seems to differ for cortical surface area, thickness, and brain volume (6). Studies from the Scottish Birth Cohort indicate a strong relationship between tests of intelligence in early and late life, which can be ascribed to genetic factors (7). Additional results from that cohort have shown that the associations between cortical thickness and intelligence in old age are strongly related to intelligence in childhood, suggesting that relationships found in aging reflect lifelong patterns (8). Finally, birth size is related to total brain size (adjusting for head size) and to cognitive function, particularly in those with lower education, in late life (9), and childhood socioeconomic status has been linked to the volume of the hippocampus in older people (10). Thus, there are clear trends in the literature to suggest that both genes and early-life variables are related to late-life brain structure and cognitive aging. The Walhovd et al. (1) study expands upon these findings by defining some of the early-life measures that appear to drive cortical surface area (birthweight and parental education) and by showing how these factors in conjunction with genetics produce consistent patterns across the lifespan. This consistency is crucial: although cortical surface area (and probably GCA as well) appears to decline over the lifespan, between-individual relationships are generally stable such that those with larger surface area tend to maintain this presumed advantage as they age.
These findings have crucial bearing on how we interpret relationships between brain and cognition over the lifespan. Abundant data have shown that older age is associated with smaller and shrinking brains and declines in GCA (11), along with some evidence that these are related (12). Although brain atrophy and declining cognition could reflect preclinical or early AD, these phenomena occur even in those unlikely to have AD (13, 14). The lifespan data reported by Walhovd et al. (1) begin to deconstruct the static and dynamic aspects of these relationships. Thus, although differences in brain structure and cognition between older people may result from processes related to aging and age-related disease, this is superimposed on lifelong differences in brain structure and cognitive ability, which are established early in life and maintained over time. Because these individual differences are generally related to cognition, this means that a person’s cognitive ability at a given time point is a function of how much they have changed and where they started.
Is a larger cortical surface area, associated with greater cognitive ability, a neural substrate of brain reserve? Theoretical models of reserve endorse this idea (4) but the actual data are limited. A number of studies have reported that larger head circumference, a proxy variable for early-life brain size, reduces the risk of AD (15), and other data show that individuals with smaller regional brain volumes are at increased risk of AD (16). Measures of brain size in late life, however, may be affected by degenerative processes, making causal associations difficult to infer. Education is associated with reduced risk of AD, an association taken as evidence of brain reserve, and is strongly related to GCA, with which it shares genetic associations (17). The picture that emerges is one in which genes and early-life exposures result in larger cortical surface area and greater cognitive ability, which in turn is associated with higher educational attainment and, over the long run, protection from late-life conditions that reduce neural resources (Fig. 1). This model requires further elaboration and testing.
The implications of this work go well beyond the topic of cognitive aging and brain reserve, however. Although couched in terms of brain health, the idea that GCA and its neural determinants are related to environmental factors as well as genes has enormous import for public health. Extensive data show that many aspects of health and even longevity are linked to measures of general intelligence (18). In other words, people with larger cortical surface area and higher GCA are likely to experience better outcomes in multiple measures of their quality of life. Conceptualizing these effects as simply advantages in neural resources based on a larger brain or greater education probably represents just a portion of the benefits that are conferred upon some people throughout life. Understanding the complex relationships between brain health and many other aspects of health, including healthcare disparities, educational and economic opportunities, harmful environmental exposures, and health behaviors that interact to produce better late-life cognitive outcomes is a major challenge. A still greater one is assuring the best early-life conditions for as many people as possible to support a long, healthy, and productive life.
Footnotes
The author declares no conflict of interest.
See companion article on page 9357.
References
- 1.Walhovd KB, et al. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc Natl Acad Sci USA. 2016;113:9357–9362. doi: 10.1073/pnas.1524259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson RS, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 3.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77(2):219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 5.Bennett DA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, et al. Genetic topography of brain morphology. Proc Natl Acad Sci USA. 2013;110(42):17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deary IJ, et al. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 2012;482(7384):212–215. doi: 10.1038/nature10781. [DOI] [PubMed] [Google Scholar]
- 8.Karama S, et al. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Mol Psychiatry. 2014;19(5):555–559. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller M, et al. Birth size and brain function 75 years later. Pediatrics. 2014;134(4):761–770. doi: 10.1542/peds.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staff RT, et al. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- 11.Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32(6) doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson N, et al. Regional brain shrinkage and change in cognitive performance over two years: The bidirectional influences of the brain and cognitive reserve factors. Neuroimage. 2016;126:15–26. doi: 10.1016/j.neuroimage.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Alzheimer’s Disease Neuroimaging Initiative Brain changes in older adults at very low risk for Alzheimer’s disease. J Neurosci. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh H, Madison C, Haight TJ, Markley C, Jagust WJ. Effects of age and β-amyloid on cognitive changes in normal elderly people. Neurobiol Aging. 2012;33(12):2746–2755. doi: 10.1016/j.neurobiolaging.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer’s disease in a population-based study of aging and dementia. Neurology. 1997;49(1):30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- 16.Dickerson BC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trampush JW, et al. Independent evidence for an association between general cognitive ability and a genetic locus for educational attainment. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(5):363–373. doi: 10.1002/ajmg.b.32319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death: How researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol Sci Public Interest. 2010;11(2):53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]