Significance
Brain and cognition change with age, with early gains and later declines. Attempts have been made to identify age-specific mechanisms, focusing on when and how declines begin in adults. However, even though general cognitive ability declines with age, there is a high stability in individuals’ cognitive ability relative to their same-age peers. Here we show that the relation between brain and cognition appears remarkably stable through the human lifespan. The cortical area change trajectories of higher and lower cognitive ability groups were parallel through life. Birth weight and parental education were identified as predictors, which provides novel evidence for stability in brain–cognition relationships throughout life, and indicates that early life factors impact brain and cognition for the entire life course.
Keywords: development, aging, cortical change
Abstract
Neurodevelopmental origins of functional variation in older age are increasingly being acknowledged, but identification of how early factors impact human brain and cognition throughout life has remained challenging. Much focus has been on age-specific mechanisms affecting neural foundations of cognition and their change. In contrast to this approach, we tested whether cerebral correlates of general cognitive ability (GCA) in development could be extended to the rest of the lifespan, and whether early factors traceable to prenatal stages, such as birth weight and parental education, may exert continuous influences. We measured the area of the cerebral cortex in a longitudinal sample of 974 individuals aged 4–88 y (1,633 observations). An extensive cortical region was identified wherein area related positively to GCA in development. By tracking area of the cortical region identified in the child sample throughout the lifespan, we showed that the cortical change trajectories of higher and lower GCA groups were parallel through life, suggesting continued influences of early life factors. Birth weight and parental education obtained from the Norwegian Mother–Child Cohort study were identified as such early factors of possible life-long influence. Support for a genetic component was obtained in a separate twin sample (Vietnam Era Twin Study of Aging), but birth weight in the child sample had an effect on cortical area also when controlling for possible genetic differences in terms of parental height. Our results provide novel evidence for stability in brain–cognition relationships throughout life, and indicate that early life factors impact brain and cognition for the entire life course.
It is well-established that both brain and cognition change with age, and that although there are early gains, older age brings with it decrements in aspects of both (1, 2). Much focus has been on age-specific mechanisms of neural foundations of cognition and their change (3, 4). In contrast, neurodevelopmental origins of functional variation in older age are now increasingly being acknowledged (5–8), but identification of how early factors may impact human brain and cognition throughout the lifespan has remained challenging.
General cognitive ability (GCA) is essential to human beings, relates to a multitude of health and social outcomes (9), and necessarily originates in characteristics of the central nervous system at all ages. Paradoxically, even though GCA is highly vulnerable to the influence of aging, there is a remarkable stability in individuals’ GCA relative to their same-age peers (10, 11). It has even been shown that childhood GCA can account for GCA-cortical thickness associations in old age (12). Cortical thickness is known to decrease with age monotonously from relatively early childhood through the entire lifespan (6, 13, 14). This thinning, albeit continuous, signifies different neurobiological events at different stages of life (15, 16), and does not have a stable functional correlate at different ages; opposite relationships between cognitive ability and cortical thickness have been identified in development and aging (17, 18). We recently showed that genetic factors contribute to apparent cortical thickness changes through life, calling for a lifespan perspective in research aimed at identifying the genetic and environmental determinants of cortical development and aging (6). Cortical surface area, which is the other component of cortical volume, is genetically (19), phylogenetically, and ontogentically (20) distinct from cortical thickness. In childhood, cortical surface area increases into adolescence, with decreases in older age (13, 14). Whereas both apparent cortical thickness and cortical area decrease in older age (14), cortical volumetric changes appear to be differentially driven by the two components in development and aging: in development, increase appears most driven by expansion of cortical area (21), whereas in older age, decrease appears most driven by cortical thinning (14). Although no study has directly tested the lifespan relationship between cortical surface area and cognitive function, the observed age differences in cortical surface area (13, 14) correspond more than those of thickness to the observed age differences in general cognitive functions, such as fluid ability (22). Indeed, anatomically extensive relationships have been observed for cortical area and GCA (23, 24). However, the brain characteristics underlying the stability of GCA through life have remained elusive. Early life factors have been studied epidemiologically, linking broad variables of health and disease in large samples (25), but it is unknown how the substantial variation in brain and cognition across the lifespan relates to these early factors.
There is an apparent bias in cognitive neuroscience toward the quest of finding age-specific mechanisms of change. Studies of select age groups over restricted time ranges have focused on changing neural foundations of cognition with age, both in childhood and in older adults (3, 4). The present study takes an alternate approach, the starting point being that in some respects “aging starts in the womb.” Here we investigated the relationship between cortical area and GCA in development, and tested whether this relationship remained stable in a lifespan sample covering almost 85 y. We targeted early candidate factors that could potentially impact cortical area and general cognitive function, and hypothesized that such influences on brain and cognition in development would have continuous impacts. The targeted factors included pre- and neonatal biomedical health variables (26, 27), specifically length of gestation (28), birth weight (26, 27), and Apgar score obtained 5 min after birth (a measure of newborn vital signs) (29), as well as socioeconomic variables (30) [i.e., parental education, income, and single parenthood (31)]. An independent sample of twins was used to estimate the heritability of the surface area of the identified cortical regions, and how much of the phenotypic correlations of cortical area and GCA that could be accounted for by genetic factors. An overview of all samples used in this study is given in Table 1. Cortical area rather than thickness was targeted because of the apparent correspondence of age trajectories of cortical area and GCA through life (13, 14, 22), and because of the previously identified relationships between cortical area and GCA (23, 24).
Table 1.
Overview of samples, subsample characteristics, and how samples are used in the study
| Sample | n | Sex (M) | Obs | 2 Tps | 3 Tps | Age (y) | Interval (y) | Income | Edu | IQ | Sample use in study; identify/assess: |
| Subsample 1 MoBa MRI | 472 | 241 | 773 | 301 | 0 | 7.3 (4.1–12.0) | 1.5 (1.0–2.2) | 3.0 (1.1) | 3.2 (0.7) | 108.5 (12.8) | Cortical area–GCA relation in development; pre- and neonatal factors |
| Subsample 2 ND/CPLS | 502 | 225 | 860 | 334 | 24 | 42.4 (8.2–88.5) | 3.1 (0.2–6.6) | 3.8 (1.2) | 3.2 (0.7) | 112.8 (12.8) | Cortical change trajectories through the lifespan |
| Summed Full lifespan sample (1+2) | 974 | 466 | 1633 | 635 | 24 | 25.8 (4.1–88.5) | 2.3 (0.2–6.6) | 3.3* | 3.2* | Stability of brain–cognition relationships through the lifespan | |
| Sample 3 VETSA | 515 | 515 | 515 | 0 | 0 | 56.1 (51.1–60.2) | — | $53.904 (29.556) | 3.2 | 108.4 (12.5) | Genetic component of brain–cognition relationship |
Edu, education n, number of participants; Obs, number of observations; Tp, timepoint n. Values are for age and interval means and ranges, for income, education and IQ, means and SDs. Income is recoded for subsamples 1 and 2 on a 5-point scale in Norwegian krone (NOK): 1 ≤ 200,000, 2 = 200–299,000, 3 = 300–399,000, 4 = 400–499,000, 5 = 500,000 + Education on a 4-point scale: 1 ≤ 9 y, 2 = 10–12 y, 3 = 13–16 y, 4 > 16 y; see Materials and Methods and Supporting Information. If participants < 20 y at time point 1, parental education and income are used. Sample 1: education available for 438, income for 439; for IQ, values are for n = 211 participants above 6.5 y at Tp1, for younger children, mean subtest WPPSI scaled scores was 11.5, SD = 1.9, roughly corresponding to the IQ as observed for the older part of subsample 1 (i.e., IQ of about M = 108, SD about 10). Sample 2: Education available for 487, income for 336. Sample 3: Education originally coded in years (Supporting Information). Income, in American dollars (USD), originally coded on a 13-point scale (Supporting Information).
Weighted average.
Participants in the full-lifespan sample on which analyses were conducted (n = 974) were community-dwelling volunteers. About half were recruited through the Norwegian Mother–Child Cohort study (MoBa; subsample 1) (32), which contains information about biomedical and socioeconomical variables from pre- and neonatal stages. The majority of individuals in the full-lifespan sample had repeated brain scans, yielding a total of 1,633 observations, enabling modeling of both cross-sectional and longitudinal brain changes. Brain imaging data were acquired at two sites, both with 1.5-Tesla Siemens Avanto scanners. T1-weighted anatomical scans were processed and analyzed with FreeSurfer 5.3 (33, 34), yielding a measure of cortical area for each person at each point (vertex) on the reconstructed surface. Details are given in Table 1 and Materials and Methods.
Results
Cortical Surface Area Is Positively Related to GCA.
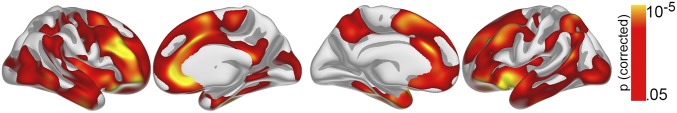
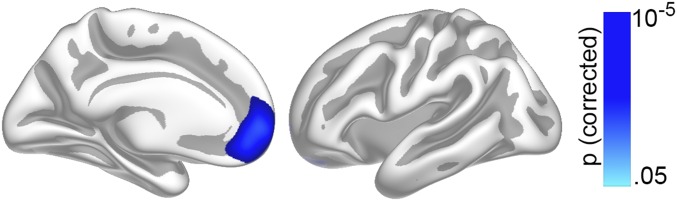
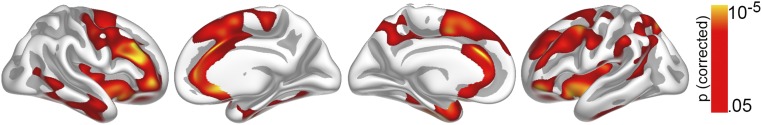
First, the relationship between cortical surface area (cortical areal expansion factor) (Materials and Methods) and GCA was tested in the MoBa participants (subsample 1; 4–12 y) with valid baseline data (n = 449) vertex-wise across the cortex by use of general linear models implemented in FreeSurfer. This child sample, rather than the full-lifespan sample, was used because we wanted to test the stability of a relationship observed in childhood through the lifespan. Only cross-sectional data were used in this first analysis because the purpose was to establish a time-invariant brain–cognition relationship that would later be tested with the longitudinal design in the full age-range. Sex was included as covariate. Results were tested against an empirical null-distribution of maximum cluster size across 10,000 iterations using z Monte Carlo simulations, synthesized with a cluster-forming threshold of P < 0.05 (two-tailed), yielding clusters corrected for multiple comparisons across the surface. The cortical regions wherein area significantly related to GCA are shown in Fig. 1 (cluster P < 0.001). The correlation of GCA and cortical area in the region of interest (ROI) was r = 0.28 controlling for sex, age, and site. Extensive effects were observed bilaterally, covering 63.0% of the total cortical surface, with the strongest relationships seen in lateral and medial prefrontal cortex. As noted above, our rationale in this paper was to target cortical area, but for comparison, results of the same analysis with cortical thickness and volume are included in Supporting Information (Figs. S1 and S2, respectively). As expected, a relationship of GCA and cortical thickness was only seen in a much smaller area, here confined mainly to the right medial prefrontal cortex, whereas volume effects were seen in much of the same areas as those for cortical area, albeit more limited, as they appeared driven primarily by area.
Fig. 1.
GCA relates to regional cortical area. GCA was related to cortical area vertex-wise across the surface in 449 children below 12 y, controlling for age and sex. Results corrected for multiple comparisons are shown from left to right: right hemisphere lateral and medial view, left hemisphere medial and lateral view.
Fig. S1.
The relationship of GCA and regional cortical thickness. GCA was related to cortical thickness vertex-wise across the surface in 449 children below 12 y, controlling for age and sex. Results corrected for multiple comparisons are shown for the left hemisphere only, as effects were found only here, in an area primarily of the medial prefrontal cortex, where thinner cortex in children is related to higher GCA. Left hemisphere medial view is shown Left, and lateral view is shown Right.
Fig. S2.
The relationship of GCA and regional cortical volume. GCA was related to cortical volume vertex-wise across the surface in 449 children below 12 y, controlling for age and sex. Results corrected for multiple comparisons are shown for (from left to right) the left hemisphere lateral and medial view, and right hemisphere medial and lateral view. Effects are largely confined to the same areas where effects are found for cortical area (Fig. 1), but are more restricted, as the relationship between GCA and cortical volume appears driven by cortical area (Fig. 1) rather than cortical thickness (Fig. S1).
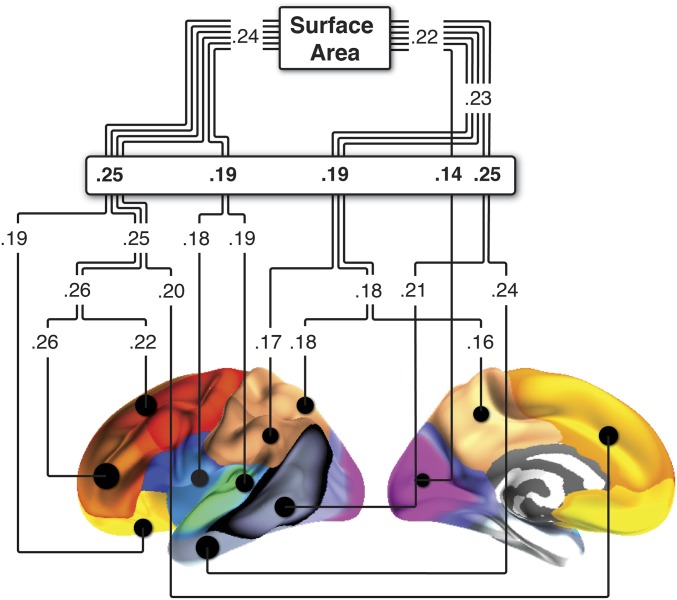
Because it appears from this analysis that area of most of the cortex was related to GCA, we think caution is warranted in drawing inferences about functional specificity of this relationship. However, when directly contrasted, the GCA–cortical area relationship was significantly (P < 0.00001) stronger for the GCA region than for the rest of the cortex or the total cortical surface area (Supporting Information). To further explore regional differences in surface area–GCA relationships, we tested whether the GCA–area relationship in subsample 1 differed between regions of the cortex established to be influenced by genetic differences (35). Previous research has shown that the cortex can be divided into regions of maximum shared genetic variance, and these regions can further be organized into superordinate clusters based on genetic similarity. As described above, we have recently shown that developmental and adult age-related changes in cortical thickness follow this genetic organization of the cerebral cortex (6). The most fundamental genetic influence on cortical surface area goes along an anterior–posterior axis (35). However, analyses in the current subsample 1 showed that the relationship between GCA and cortical area did not differ between the anterior (r = 0.24) vs. posterior (r = 0.22) genetic cluster (Fig. S3). At the next level of genetic division, we found significant differences in relationship to GCA across five genetic clusters. A prefrontal (r = 0.25) and a medial and posterolateral temporal (r = 0.25) cluster correlated significantly higher (all Ps < 0.05) with GCA than the remaining three clusters (pars opercularis/superior temporal cluster r = 0.19; parietal cluster r = 0.19; occipital cluster r = 0.14) (Supporting Information). Hence, there was some evidence for the specificity of identified regions with higher area of, for example, the prefrontal cortex being more related to GCA than the area of occipital cortices, as also observed elsewhere (see, for example, ref. 36). However, cognitive ability appears to have a highly polyregional substrate (36). In line with this finding, regardless of age, in the present study children with higher GCA had larger cortical area in relatively broad regions.
Fig. S3.
Surface area–GCA relationships according to cortical regions influenced by different genes. We tested whether the GCA–area relationship in MoBa differed between regions of the cortex influenced by different genes. Previous research on VETSA has shown that the cortex can be divided into regions of maximum shared genetic variance (21), and these regions can further be organized into superordinate clusters based on genetic similarity. The relationship between GCA and cortical area did not differ between the anterior (r = 0.24) vs. posterior (r = 0.22) genetic cluster. At the next level of genetic division, we found significant differences in relationship to GCA across the five genetic clusters. A prefrontal (r = 0.25) and a medial and posterolateral temporal (r = 0.25) cluster correlated significantly higher (all Ps < 0.05) with GCA than the remaining three clusters (pars opercularis/superior temporal cluster r = 0.19; parietal cluster r = 0.19; occipital cluster r = 0.14).
The Cortical Surface Area Age Trajectories of Different GCA Groups Remain Parallel Throughout the Life Course.
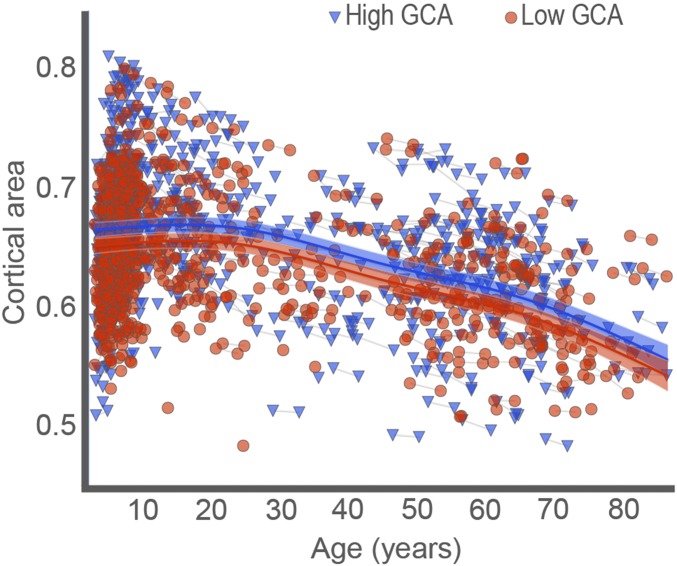
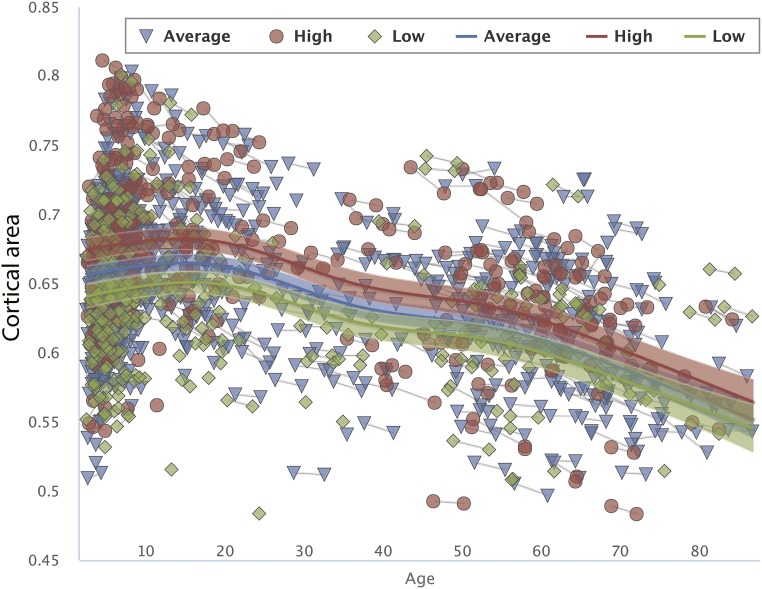
Next, to investigate whether these relationships identified in the child sample (subsample 1) could be extended to the broader age range and longitudinal change, the full-lifespan sample was split into two parts based on their age-standardized GCA scores (mean = 0, SD = 1, “low” GCA ≤ 0, 772 observations; “high” GCA > 0, 861 observations). Surface area from regions significantly related to GCA from the analysis in the child sample (subsample 1) shown in Fig. 1 was extracted for each participant in the full-lifespan sample. These regions, collectively referred to as the “GCA region,” were fitted to age using generalized additive mixed modeling (GAMM), implemented with the package “mgcv” in R (37, 38) through the PING data portal (39). GAMM yields a nonlinear but smooth effect of age for both longitudinal and cross-sectional data, including all 1,633 data points in the analyses. The Bayesian Information Criterion (BIC) was used to prevent overfitting (40). A linear function was used to relate the GCA region to age, partialling out effects of sex, and it yielded a significant effect of age (P < 0.0001), with BIC = −7,270. The GCA group had a significant effect on cortical area (P < 0.0001, t =2.93) but did not interact with age (P = 0.83, t = 0.21), indicating that the intercepts but not trajectories associated with the GCA group were different. Allowing nonlinear smooth effects substantially reduced BIC (−7,345), indicating a much better fit than the linear model. GCA still had a highly significant main effect on cortical area (t = 3.46, P < 0.0001), and as can be seen from the scatterplot in Fig. 2, the age-trajectories of the two groups were close to identical. Because the younger part of the lifespan is very densely sampled over a narrow age range, and hence the individual data may be hard to see in Fig. 2, a separate fitting was also done for the age range 4–12 y, included in Fig. S4, also showing parallel age trajectories for the GCA groups in this age range. Splitting the whole sample into three groups instead of two yielded basically the same results (Fig. S5), with three almost parallel trajectories, confirming the robustness of the approach. Analysis with GCA as a continuous variable, rather than split groups, confirmed that there was no interaction of age × GCA on surface area (F = 2.108, P = 0.336). Power calculations performed by simulations, using parameter estimates from the actual data, showed that there was 80% power to detect an effect size of D = 0.10. D here is the regression coefficient divided by its SD, analogous to Cohen’s d, with the usual interpretation that 0.8 is large, 0.5 is medium, and 0.2 is small (41). Based on the findings of this approach, there was good power in the sample to detect an interaction of small effect.
Fig. 2.
The relationships of GCA-cortical area across age. Cortical area for the regions related to GCA in children is mapped across the full age range by GAMM, using both cross-sectional and longitudinal information (1,633 observations), for participants with “higher” vs. “lower” GCA. The GCA–cortical area relationship is invariant across age, with parallel change trajectories. The width of the curve represents the 95% CI.
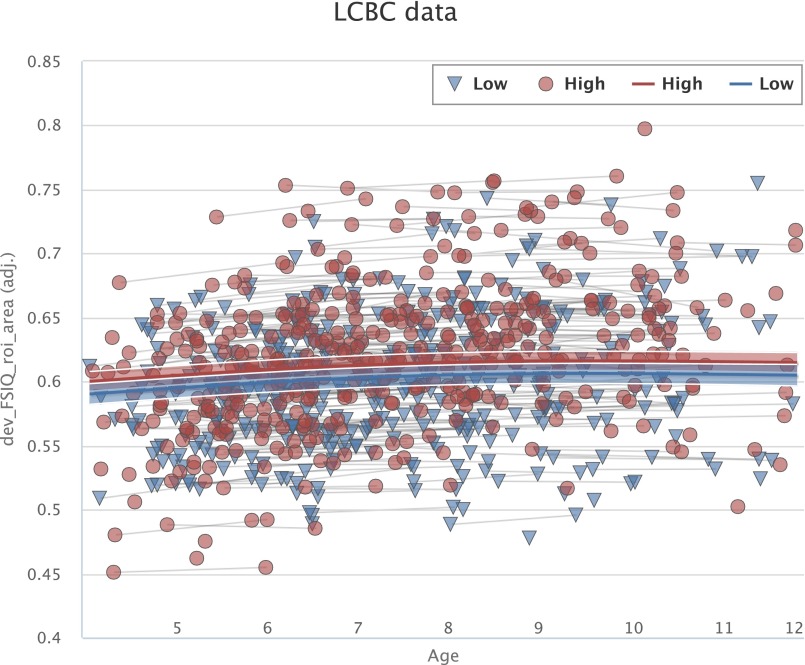
Fig. S4.
The relationships of GCA–cortical area across ages 4–12 y. Cortical area for the regions related to GCA in children is mapped across the age range 4–12 y by GAMM, using both cross-sectional and longitudinal information, for participants with “higher” versus “lower” GCA. The GCA–cortical area relationship is invariant across age, with parallel change trajectories. The width of the curve represents the 95% CI. LCBC, Lifespan Changes in Brain and Cognition.
Fig. S5.
The relationships of GCA–cortical area across age. Cortical area for the regions related to GCA in children is mapped across the full age range by GAMM, using both cross-sectional and longitudinal information (1,633 observations), for participants with “higher” vs. “intermediate” versus “lower” GCA. The GCA–cortical area relationship is invariant across age, with parallel change trajectories. The width of the curve represents the 95% CI.
The Influence of Early Life Factors on Cortical Surface Area and GCA.
After having established parallel brain change trajectories for the different GCA groups, with no interaction of age × GCA on surface area, we next turn to the issue of whether early life factors determine these trajectories. To test this theory, we selected a number of potentially important early life factors from the MoBa database (32), collected at pre- and neonatal stages, and investigated their relationship to cortical area and GCA in subsample 1. We focused on the most widely used neonatal health variables worldwide: gestational age, birth weight (BW) (26, 27), and the Apgar score (29). Socioeconomic status (SES) variables, including parental education and income, as well as single parenthood (30, 31), are factors that may relate to neurocognitive development. For descriptives on these variables in subsample 1, see Supporting Information and Table S1. Some of these variables may be interrelated, and correlation analyses showed significant positive relationships between BW and gestational age, gestational age and Apgar score, and parental education and income, as well as a negative relationship between parental education and single parenthood (Table S2). Multicollinearity was assessed with collinearity diagnostics running linear regressions with these variables iteratively as dependent and independent variables. These analyses showed no indication of multicollinarity for these variables, with variance inflation factors for all analyses being <2. We thus selected these variables (Materials and Methods, Supporting Information, and Tables S1 and S2) for testing early life determinants of brain and cognition. Partial correlations were run, controlling for sex and age in all analyses. Bonferroni corrections were performed for 12 comparisons: that is, significance level was set at P < 0.05 corrected, equivalent of P < 0.004 uncorrected. Only corrected P values are reported in the following.
Table S1.
Descriptive characteristics for variables obtained pre- and neonatally
| Variable | Mean | SD | Range | n |
| Gestational age (d) | 279 | 13 | 179–298 | 443 |
| Birth weight (g)a | 3,596 | 551 | 864–5,138 | 443 |
| Apgar score 5 min (0–10) | 9.42 | 0.88 | 2–10 | 443 |
| Parental educationa | 5.11 | 0.92 | 1–6 | 438 |
| Parental incomeb | 4.85 | 1.29 | 1–7 | 439 |
| Single parenthoodc | 0.03 | 0.18 | 0 or 1 | 432 |
Because there are some cases of missing data with varying numbers across variables in the MoBa-database, the exact number of cases entered into analyses varies slightly, and exact n along with descriptives are given for all variables of interest (a, b, c). a and b: Where possible, mean values of parental education were used, and these values refer to the original scales used, described in Additional Information on Pre- and Neonatal Variables Used in Subsample 1 Analyses. For single parenthood (c), 1 = absence and 0 = presence of the other parent in the household (Materials and Methods).
Table S2.
Intercorrelations among pre- and neonatal variables
| Variable | Gestational age | Apgar score | Parental education | Parental income | Single parenthood |
| Birth weight | 0.64 | 0.11 | −0.04 | −0.09 | 0.05 |
| Gestational age | 0.15 | −0.02 | −0.04 | 0.06 | |
| Apgar score | −0.01 | −0.09 | 0.05 | ||
| Parental education | 0.43 | −0.22 | |||
| Parental income | −0.10 |
Correlations significant at P < 0.05, when corrected for 15 comparisons are marked in bold font.
Among the neonatal health variables, BW showed a significant relationship with cortical area within the GCA region (r = 0.16, P = 0.0091). This relationship remained after controlling for parental height (r = 0.15, P = 0.0262), and increased in strength when excluding 11 children with low BW < 2,500 g (r = 0.21, P = 0.0002) and when also controlling for parental height in the sample without the low BW children (r = 0.20, P = 0.0006). Among the SES variables, parental education predicted GCA (r = 0.18, P = 0.0024), a relationship that was also seen when excluding low BW children (r = 0.16, P = 0.0096). Single parenthood was weakly negatively related to GCA, but this did not survive correction for multiple comparisons (r = −0.10, n.s.). No further significant relationships were identified for the pre- and neonatal variables. Of note, in contrast to a recent report on a United States sample (23), parental income in this Norwegian sample had no impact on either GCA (r = −0.02, n.s.) or cortical area in the GCA region (r = −0.01, n.s.). Finally, BW, gestational age, Apgar score, parental education, parental income, and single parenthood were entered in regression analyses simultaneously, along with age and sex as independent variables, and with GCA and cortical area in the GCA region as dependent variables, respectively. These analyses showed that only parental education had a unique effect on GCA (β = 0.193, P = 0.00039), and besides age and sex, only BW had a unique effect on cortical area in the GCA region (β = 0.199, P = 0.00025).
Genetic Influences on the Relationships.
The observed relations between early life factors and cortical area across the lifespan could be genetically mediated (7). We therefore estimated the heritability of cortical area of the GCA region. Participants in this heritability analysis were 515 middle-aged men from the Vietnam Era Twin Study of Aging (VETSA; sample 3) (Table 1) (23, 42, 43). The sample included 131 monozygotic and 96 dizygotic twin pairs and an additional 61 individual twins from two sites (Supporting Information). Imaging data were acquired with 1.5-Tesla Siemens scanners. T1-weighted anatomical scans were processed and analyzed with FreeSurfer (Supporting Information) (33, 34). Analyses were run controlling for scanner and age. The heritability for cortical area was high within the GCA region [additive genetic contribution = 0.94, 95% confidence interval (CI) = 0.91; 0.95]. We also ran a bivariate model with GCA in this sample based on the Wechsler Abbreviated Scale of Intelligence Full-Scale IQ (two-subtest version) (22) and cortical area within the GCA region identified within the child sample (subsample 1) to investigate the phenotypic, genetic, and environmental correlations between GCA and cortical area in the adult twin sample. We observed a significant phenotypic correlation (r = 0.16, 95% CI = 0.06; 0.25), and a significant genetic correlation (r = 0.21, 95% CI = 0.08; 0.36). There was minimal unique environmental correlation (r = 0.06, 95% CI = −0.13; 0.24), indicating that the majority of the observed phenotypic association was a result of shared genes between GCA and the brain region.
Discussion
In summary, we identified an extensive cortical region wherein surface area related positively to GCA in development. A regional pattern of cortical area–cognition relationships was present in all lobes. This finding fits with the notion that GCA is supported by distributed brain networks (44, 45). Using genetically defined cortical clusters (6, 35), there was evidence that especially prefrontal and medial and posterolateral temporal clusters related more strongly to GCA. Most previous studies have focused on cortical volume or thickness, but the present results correspond with previously reported findings on area–cognition relationships (24) in a sample not overlapping with the current developmental cohort wherein the region was currently identified. This relationship was identified in the youngest part of the sample only, to enable observation of whether the cortical area–GCA relationship defined in childhood would hold through the entire age range. There were remarkable similarities in the age-trajectories of this cortical region in the two GCA groups throughout the lifespan. Previous studies have observed very high stability in IQ scores across life (10, 11). However, because IQ is standardized to age, this should not be taken to mean that general cognitive ability does not change. Still, it changes predictably, so that the functioning level relative to same age individuals is quite stable. The present results yield a possible brain substrate for this stability: that is, stability of change.
There has been uncertainty as to what degree age-specific mechanisms affect brain and cognition (3, 4, 46). The present study indicates a high extent of stability in the age trajectories of cortical characteristics underlying GCA. We cannot by this study pinpoint the relative roles of nature and nurture, early biological embedding and plasticity through life. Nor can this study on normal variation inform on how trajectories may be affected by specific diseases and traumatic injuries affecting brain and cognition. However, the fact that the cortical change trajectories of different GCA groups were parallel can be taken as an indication of continued influence of early life factors throughout the lifespan.
Specifically, among the tested early factors, BW and parental education were identified as predictors of brain and cognitive development. These influences can be both environmentally and genetically mediated. For cortical thickness, we recently showed that the coordination of changes in maturation and aging adhered to the genetic organization of the cortex (6). These findings open new possibilities to identify genes and pathways that influence brain development and aging (47). Although cortical area is a metric distinct from thickness, genetically (19), phylogenetically, and ontogentically (20), the current findings on area may also be in part genetically governed. Using the genetic clusters identified in the VETSA twin sample (35), significantly stronger relationships of area and GCA were identified in the prefrontal and medial posterolateral temporal clusters. Support for a genetic component of the present results was also obtained in the twin sample. The identified GCA region showed a high additive genetic contribution, and the phenotypic correlation between GCA and the area of the GCA region in the twin sample was also genetically mediated. Parental genes, as indexed by parental height and weight, make contributions to infant BW (48). However, importantly, BW had an effect on cortical area also when controlling for part of the possibly genetic differences in terms of parental height, which may suggest additional environmental influence.
Interestingly, although parental education related positively to GCA, parental income in this Norwegian sample had no impact on either GCA or cortical area in the GCA region. This finding is in contrast to a recent report on a United States sample from the Pediatric Imaging, Neurocognition, and Genetics (PING) study (23). There are important differences between the two studies. The present study focused on pre- and neonatal variables, and hence parental income at the prenatal stage was used as a predictor. In the PING study, parental income was reported at the time of the child’s scanning. Moreover, Norway as a nation is characterized by less income inequality (49) and a greater degree of welfare services than the United States, which may work against effects of income on child well-being (50), and possibly either differences in brain development or GCA. It is also likely that SES may influence pre- and neonatal characteristics differently in different populations. However, aside from this likelihood, the discrepant findings should serve as a reminder that SES variables may not in and of themselves be causal, and may serve as proxies for different variables and causal relationships across space and time.
The present samples were in part recruited so as to be representative of specific populations (i.e., subsample 1, MoBa; and sample 3, VETSA), and in part they were a convenience sample (subsample 2). The samples may not be fully representative of the broader population. Some selection effects are likely, as also reflected in the somewhat higher than average mean IQ of all samples. However, there was much variation in cognitive functioning across all samples, as indicated by the SDs. Further details and considerations on representativeness are given in Supporting Information.
Conclusion
In conclusion, although exact mechanisms remain to be uncovered, our results demonstrate a high extent of stability in brain–cognition relationships throughout life. The same differences in cortical area for participants with higher versus lower general cognitive ability were seen throughout life. Importantly, this observation was based on a brain–cognition relationship identified in the child sample only, but still generalized to the whole age range. Furthermore, the analyses of the health and social variables indicate that early life factors can have a significant impact on brain structure and general cognitive function, likely for the entire life course of individuals.
Materials and Methods
Full-Lifespan Sample, Including Subsamples 1 and 2.
After movement and surface reconstruction control, leading to the exclusion of 55 participants, a total of 1,633 scans from 974 participants, 4–89 y of age, were drawn from Norwegian studies coordinated from the Research Group for Lifespan Changes in Brain and Cognition, Department of Psychology, University of Oslo, Norway (see Table 1 and below). The majority had repeated MRI-scans, with a mean follow-up interval of 2.30 y (SD 1.19). The studies were approved by the Regional Committee for Medical and Health Research Ethics. Written informed consent was obtained from all participants older than 12 y of age and from a parent/guardian of volunteers under 18 y of age. Oral informed consent was obtained from all participants under 12 y of age.
Subsample 1 was recruited through MoBa, a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (32, 51). Participants in the cohort study living in Oslo and Sør-Trøndelag counties were invited to participate in the MRI study given that certain criteria were met (Supporting Information). Valid MRI scans, examined by a neuroradiologist and deemed free of significant injuries or conditions, were obtained from 472 participants (mean age 6.7 y, range 4.1–10.7 y, at baseline, 231 girls and 241 boys). Of these participants, 449 had valid scans at baseline, and these were used in the initial analyses with GCA. For 301 participants, valid scans at both baseline and follow-up were acquired. Data from the Medical Birth Registry (gestational age, BW, and Apgar score) were available for 444 participants. When extracting area data for the cortical regions wherein area significantly related to GCA in the sample of 449 children, ROI-extraction yielded extremely deviant area data for one child, with standardized value > 4 SD. As the ROI extraction was deemed to yield flawed results for this one case, it was omitted from further analysis on individual ROI area data.
Parental education was indicated as the highest level completed at the time of completing the form during pregnancy; mean values of maternal and paternal education and income were calculated and used as described in Supporting Information and Table S1. For the variable single parenthood, a scale on partner information was recoded to reflect the absence (1) or presence (0) of the other parent in the household. When taking parental height into account in the relation between BW and cortical areas, parental report on maternal and paternal height were used as regressors on BW, and the standardized residuals for BW were used in analyses (Supporting Information).
Participants for subsample 2 (n = 502, 334 with one follow-up, 24 with two follow-ups, age at scanning 8.2–88.5 y, 277 females) were recruited through newspaper advertisements and local schools and workplaces. Participants were screened using a standardized health interview before inclusion. Participants with a history of self- or parent-reported neurological or psychiatric conditions, including clinically significant stroke, serious head injury, untreated hypertension, diabetes, and use of psychoactive drugs within the last 2 y were excluded. Furthermore, participants reporting worries concerning their cognitive status, including memory function, were excluded.
GCA was assessed by the Wechsler Abbreviated Scale of Intelligence (22) for participants aged 6.5–89 y of age, and scores for corresponding subtests (vocabulary, similarities, block design, and matrices) from the Wechsler Preschool and Primary Scale of intelligence–III (WPPSI-III) (52) were used for the youngest participants (<6.5 y). All participants scored within normal IQ range (82–145), or normal range of scaled scores (mean of subtests, s = 6.7–16.5). The age-standardized GCA score was calculated by z-transforming each subtest according to age group, and then calculating the mean of the z-scores (Supporting Information). If one subscore was missing, (e.g., some subtests were rated by tester as nonvalid for the youngest children), GCA would be computed based on the existing scores. The estimated IQ for the younger part of subsample 1 (<6.5 y) using different subtests would be close to identical to that of the older part of subsample 1 (Table 1 and Supporting Information). Hence, we do not believe that the use of different subtests caused substantial differences in results. However, the use of partly different measures of cognitive function at different ages constitutes a limitation of the lifespan approach. For all samples for lifespan analysis, MRI scans were examined by a neuroradiologist, and all included scans were deemed free of injuries and pathological conditions.
Sample for Heritability Analysis.
The VETSA MRI sample used in this study is a subsample of participants from the main VETSA study, which includes a total of 1,237 male twins, of whom 534 had MRI data and for whom 19 scans did not pass quality control and were discarded, resulting in the current sample of 515 (23, 42, 43), aged 51–59 y. All participants gave written informed consent to be in the study. The study protocol was approved by the Institutional Review Boards at the participating institutions: University of California, San Diego; Boston University; and Massachusetts General Hospital. Imaging was conducted at the University of California, San Diego and Massachusetts General Hospital.
MRI Data Acquisition and Processing for the Lifespan Analyses.
Imaging data for the 1,633 scans in the full-lifespan sample analyses were acquired using a 12-channel head coil on a 1.5-Tesla Siemens Avanto scanner (Siemens Medical Solutions) at Oslo University Hospital-Rikshospitalet and, for a part (n = 129) of subsample 1 (MoBa MRI study), St. Olav’s University Hospital in Trondheim. Controlling for site in addition to age and sex in the correlation analyses performed on subsample 1, inclusive of analyses on pre- and neonatal variables, yielded correlations of similar magnitude, and did not affect the significance of any result. Two 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) were used (Supporting Information).
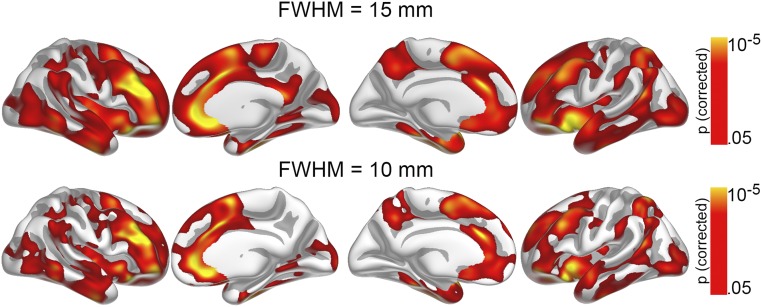
MRI data were processed and analyzed with FreeSurfer 5.3 (surfer.nmr.mgh.harvard.edu/), described in detail elsewhere (33, 34). This process yields a measure of cortical area or arealization (areal expansion factor) for each person at each point on the reconstructed surface. To extract reliable arealization estimates for each time point for the longitudinal observations, images were automatically processed with the longitudinal stream in FreeSurfer (Supporting Information). Surface maps were smoothed using a circularly symmetric Gaussian kernel with a full-width at half maximum (FWHM) of 15 mm. The smoothing level was chosen both to improve signal-to-noise ratio for the vertex-wise comparisons and based on an expectation of relatively broad effects, in line with previous observations of a polyregional substrate for GCA (36). However, the initial analysis on the relationship of GCA and cortical area in subsample 1 was rerun also with less smoothing (i.e., a kernel with FWHM of 10 mm) and this yielded similar, yet as expected somewhat smaller effect areas (Fig. S6). Because FreeSurfer is an almost fully automated processing tool, manual editing was not performed to avoid introducing errors. For the children especially, movement could potentially induce bias in the analyses. All scans were manually rated for movement on a 1–4 scale, and only scans rated 1 (no movement) and 2 (adequate quality, minor movement) were included in the analyses, reducing the risk of movement affecting the results. In addition, all reconstructed surfaces were inspected and discarded if they did not pass internal quality control. This process lead to the exclusion of 46 participants from subsample 1 and 9 from subsample 2, reducing the number of scans to the now reported 1,633 observations. Example images included as well as excluded are shown in Fig. S7, along with descriptions of ratings. The correlation analyses on the relationship of early life factors to cortical area and GCA in subsample 1 were repeated with movement (rating 1: 70.8%; rating 2: 29.2%) partialled out. The correlations remained very similar and this did not affect the significance of any result.
Fig. S6.
The relationship of GCA and regional cortical area as observed when using a smaller smoothing kernel. The initial analysis on the relationship of GCA and cortical area in subsample 1, with a kernel with FWHM of 15 mm, was rerun also with less smoothing (i.e., a kernel with FWHM of 10 mm), and this yielded similar, yet as expected somewhat smaller effect areas. With FWHM = 15, the total effect covered 90.883 mm2, whereas FWHM = 10 yielded an effect of 73.593 mm2, of the cortical surface of 130.437 mm2.
Fig. S7.
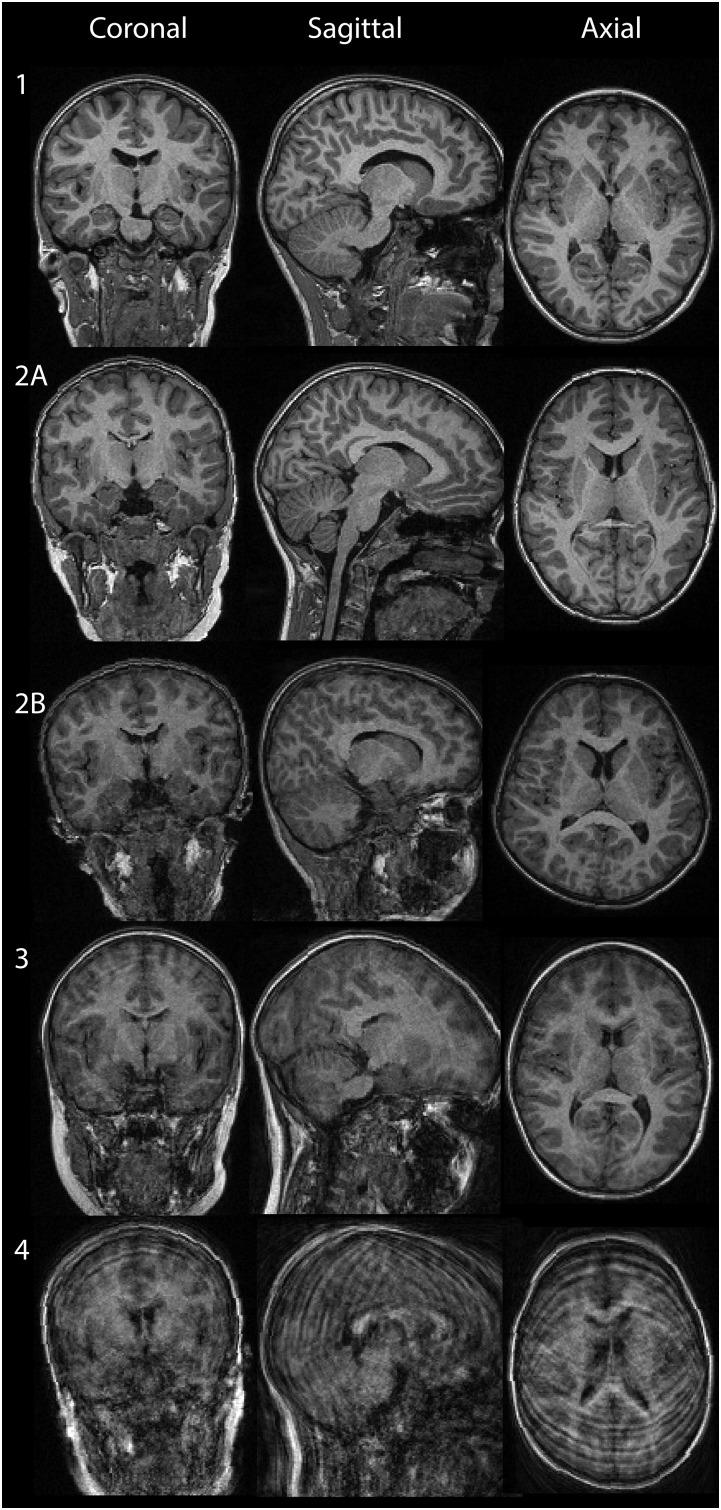
Examples of images rated for movement distortions. Images were rated for presence of movement. Ratings were performed in the Lifespan Changes in Brain and Cognition laboratory by three trained graduate-level operators with experience in MR processing. From left to right: coronal, sagittal, and axial views. Rows represent degrees of movement: 1: No sign of movement. 2: Adequate quality, minor movement; here we include two example images to show the possible quality included: 2A, example of best quality rated 2; 2B, example of worst quality rated 2. 3: Substantial movement artifacts, excluded. 4: Completely distorted image because of movement, excluded. Only scans deemed to be of adequate quality, and without substantial movement (i.e., rated 1 and 2) were included in analyses.
MRI Data Acquisition and Processing for VETSA, “Sample 3.”
Images were acquired on Siemens 1.5T scanners. The 3D cortical surface was reconstructed to measure area at each surface location or vertex using a semiautomated approach in the FreeSurfer software package. The resulting cortical surface model was manually reviewed and edited for technical accuracy. Minimal manual editing was performed (Supporting Information).
Further Details on Statistical Analyses.
GAMM was chosen because these models allow nonparametric fits with relaxed assumptions about the actual relationship between cortical arealization and age. The framework used for fitting cortical arealization to age basically includes regression analyses with automatic smoothness constrains (see Mixed GAM Computation Vehicle With GCV/AIC/REML Smoothness Estimation, cran.r-project.org/web/packages/mgcv/index.html).
Additional Information on the Calculation of GCA
The age-standardized GCA score was calculated by z-transforming each subtest according to age group, and then calculating the mean of the z-scores. Because we had a very high sampling density in the younger age ranges where we expected rapid cognitive development, z-transformations were performed separately for small age-ranges for the youngest participants, and then for gradually increasing age ranges for older children and adults. Each age group covered 0.5 (below 9 y of age), 1 (9–15 y), 2 (15–21), 5 (21–30 y), or 10 (30 y and above) y.
Additional Analyses on the Specificity of the GCA–Cortical Area Relationship
When directly contrasted, the GCA–cortical area relationship was significantly stronger for the GCA region than the rest of the cortex (r = 0.15) or the total cortical surface area (r = 0.24) in subsample 1 (both zs > 6, P < 0.00001), also as evidenced by significant GCA group × region interaction in a repeated-measures general linear models [effect ROI vs. noneffect ROI t = 47.76 (P < 0.001)/effect ROI vs. total surface area t = 16.86 (P < 0.001)]. The same was true if the analyses were run for the full-lifespan sample [effect ROI vs. noneffect ROI t = 30.27 (P < 0.001)/effect ROI vs. total surface area t = 16.71 (P < 0.001)]. Importantly, however, although the relationship to GCA was stronger for the GCA region; in addition, the rest of the cortical surface area was significantly related to GCA. Using GAMM to predict surface area for the noneffect ROI for the full sample confirmed this, yielding a significant main effect of GCA on cortical area (t = 2.84, P = 0.005).
Additional Information on Pre- and Neonatal Variables Used in Subsample 1 Analyses
We chose to use mean parental education, where the value of either parent’s education would be used if a value for either parent was missing (n = 425 had data for maternal education, n = 414 for paternal education). Parental education was indicated as the highest level completed at the time of completing the form during pregnancy, where 1 = 9 y of primary education, 2 = 1–2 y of high school, 3 = occupational high school, 4 = 3 y of high school, 5 = up to 4 y of higher education at university/college level, 6 = greater than 4 y of higher education at university/college level. Income level was indicated for mother and father separately, and we chose to use mean income, where the value of either parent´s income would be used if a value for either parent was missing (n = 433 had data for maternal income, n = 426 for paternal income). However, note that using the total parental income (n = 420, M = 9.69, SD = 2.52, range 2–14 on the above scale) would not affect the significance of any result. Income level was indicated on a scale which in American dollars (USD) would correspond to approximately: 1 = no income, 2 < $19,300, 3 = $19,300–25,700, 4 = $19,300–38,600, 5 = $38,600–51,400, 6 = $51,400–64,300, 7 = greater than $64,300. For the variable single parenthood, a scale on partner information was recoded to reflect the absence (1) or presence (0) of the other parent in the household, as cohabiting without being married is very common in Norway (43.8% of parents in this sample, versus 49.6% being married). Only 3.1% in the current MoBa-MRI sample indicated single parenthood. For parental height, there were instances of missing data, and one case was excluded because of what was likely flawed entry (below 100 cm), resulting in this information being available for n = 434.
Additional Information on the Samples and on Representativeness of Samples
Subsample 1.
Participants for the MoBa, a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (32, 51), were recruited from all over Norway from 1999 to 2008. Briefly, pregnant women were invited to participate in the cohort study by mail before a routine ultrasound examination at their local hospital, usually at around 18-wk gestation (51). Women who agreed to participate received self-administered questionnaires by mail during the pregnancy, and during the infant’s early childhood. The women consented to participation in 40.6% of the pregnancies. The cohort now includes 114.500 children, 95.200 mothers, and 75.200 fathers. Participants in the MoBa cohort study living in Oslo and Sør-Trøndelag counties were invited to participate in the MRI study (see also refs. 6 and 21), given that the following criteria were met: (i) the families had not withdrawn from the study (i.e., were listed as current participants); (ii) the 3-y questionnaire had been returned (indicative of continued interest for the study); (iii) singleton pregnancy. In case of multiple siblings being part of the study, invitations were sent for one child, the oldest sibling in the study population, with the exception of invitations sent for 36 siblings. A total of 2,530 invitations were sent. Of the 449 children with valid MRI data at baseline, recruited by invitation from the MoBa study, two older siblings not being part of the MoBa study specifically were tested, so that MoBa data were not available for these children. The family of one participating child asked not to have the information linked to the MoBa database, reducing the number of children with MoBa data for the study to 446.
The mean BW of this sample is slightly higher (26 g) than in the broader MoBa cohort, where BW was 3,570 g (SD = 611 g); in the total population of births in Norway 2000–2006 this was significantly lower than in the MoBa cohort (mean BW = 3,528 g, SD = 624). The percentage with low BW (<2,500 g) in the present MoBa MRI-cohort (11 of 444) was 2.5%, whereas in the broader MoBa, this was 4.5% and in the full population 5.1% (30). Of the 11 children with low BW in the present analyses, 9 were born prematurely (before 37 wk of gestation). The average GCA of the subsample (n = 11) of low BW children did not appear to deviate from that of the broader sample (mean z-score = 0.02, SD = 0.71). Average Apgar score at 5 min in the current study appears similar to that of the broader MoBa cohort (9.4, SD = 1.0), which was significantly higher than that of the general population (9.3, SD = 1.1) (51). Only 3.1% in the current MoBa-MRI sample indicated single parenthood, whereas in the total MoBa database, 3.5% reported single parenthood, a lower percentage than that of the total population of women giving birth in Norway (6.4%) (51). Collectively, these parameters suggest a slight selection bias in the present subsample 1 relative to the broader MoBa cohort, and again, relative to the general population.
Subsample 2.
For detailed exclusion criteria at baseline, see ref. 14. All subjects above 20 y of age scored <16 on Beck Depression Inventory (53) and subjects above 40 y of age >26 on Mini Mental State Examination (54).
Relative Representativeness of Subsamples 1 and 2.
Values of education for subsample 1 (original scale described above) were recoded to the four-point scale shown in Table 1 so as to be on the same scale as those for subsample 2. As shown in Table 1, participant and, for children, parental, education, in subsamples 1 and 2 was identical and on average at (lower) university/college level, which is higher than in the general population in Norway (Statistics Norway, ssb.no). However, most of these participants resided in the Oslo area, where the majority of the population in their 30s, and almost half the overall adult population have university/college level education (Statistics Norway, ssb.no). Because subsample 2 also contains a number of older participants for whom average population education levels are lower in Norway, it seems if anything that subsample 2 may have a somewhat greater selection bias with regard to greater education level. There is, however, likely some education selection bias relative to the broader populations for both subsamples 1 and 2. For subsample 1, these values were obtained at pre/neonatal stage (values are recoded from a scale used in Table S1 for comparability), whereas for subsample 2, this information was obtained, later (i.e., at the age of testing and scanning). Mean parental age at birth in subsample 1, MoBa (n = 432) was 32.5 y (SD = 4.5), an age when most people have finished their education, so this is likely relatively stable and can be compared across the two Norwegian subsamples.
Income was originally coded on different scales for subsamples 1 and 2. The original scale for subsample 1 is described above under Additional Information on Pre- and Neonatal Variables Used in Subsample 1 Analyses, whereas for subsample 2, the original 7-point scale was 1 ≤ 200,000, 2 = 200–299,000, 3 = 300–399,000, 4 = 400–499,000, 5 = 500–599,000, 6 = 600–699,000, 7 = 700k,000+.
For Table 1, the two scales used have been recoded for both samples into a 5-point scale for tentative comparison. However, for income, one needs to take into account the increase in monthly wages in Norway because the subsample 1 income values were obtained, across the about 7 y passed. For example, there was ∼35.8% wage increase in Norway 2005–2012 (Statistics Norway, ssb.no), although the numbers suggest about 26% higher income in subsample 2 than in subsample 1. Hence, the true income level in subsample 1 may likely be somewhat higher than that in subsample 2. However, this may not necessarily suggest different representativeness across subsamples 1 and 2, as subsample 2 also includes other age groups, both younger and older, that have lower population level income (Statistics Norway, ssb.no).
Sample 3 for Heritability Analyses
VETSA participants are similar to American men in their age range in terms of health and sociodemographic characteristics (National Health and Nutrition Examination Survey) (23, 42, 55). All VETSA participants served in the military ∼35 y before the study; nearly 80% reported no combat experience. Overall, the participants who underwent brain imaging did not differ from the VETSA participants who did not undergo the MRI protocol in terms of sociodemographic characteristics (19). Income for the VETSA sample as presented in Table 1 was originally coded on a 13-point scale. Median participant income for the previous year was $50,000 to $59,000, and median family income for the previous year was $60,000 to $69,000, which is near the median US household income for persons in their age range. For mean income, a new variable was created to reflect midpoint of range for indicated category, upon which mean income was calculated to be $53,904, SD = $29,556, range = $5,000–125,000, and combined family income mean was $70,575, SD = $32,036, range = $5,000–125,000. The mean individual income would correspond to about 450.00 NOK (i.e., about 4.5 on the Norwegian scale, which is higher than the Norwegian samples). Note however, that range of individual differences in salaries is more restricted in Norway and different welfare systems apply in Norway and the United States, so income values cannot be directly compared across Norwegian and United States samples. For the 515 men included in the present analysis, mean level of education was 13.81 y (SD = 2.10; range: 8–20 y).
Further Details on MRI Acquisition and Processing for the Lifespan Analyses
The pulse sequences used for morphometric analysis were two repeated 3D T1-weighted MPRAGE, with the following parameters: repetition time (TR) 2,400 ms, echo time (TE) 3.61 ms, inversion time (TI) 1,000 ms, flip angle 8°, matrix 192 × 192, field-of-view 240 mm. Each volume consisted of 160 sagittal slices with voxel sizes 1.25 × 1.25 × 1.2 mm. Scanning time for each of these sequences was 7 min, 42 s. For the children between 4 and 9 y old in the MoBa sample, we used a parallel imaging technique (iPAT), acquiring multiple T1 scans within a short scan time (4 min, 18 s), enabling us to discard scans with residual movement and average the scans with sufficient quality. Previous studies have shown that accelerated imaging does not introduce significant measurement bias in surface-based measures when using FreeSurfer for image analysis, compared with a standard MPRAGE protocol with otherwise identical voxel dimensions and sequence parameters (56), which is in accordance with our own analyses. The protocol also included a 25-slice coronal T2-weighted fluid-attenuated inversion recovery sequence (TR/TE = 7,000–9,000/109 ms) to aid the neuroradiological examination.
The longitudinal processing stream (57) in FreeSurfer includes creation of an unbiased within-subject template space and image using robust, inverse consistent registration. Several processing steps, such as skull stripping, Talairach transforms, atlas registration, as well as spherical surface maps and parcellations, are then initialized with common information from the within-subject template, substantially increasing reliability and statistical power (57).
Further Details on MRI Acquisition and Processing for the VETSA, “Sample 3” Analyses
Two sagittal T1-weighted MPRAGE sequences were used with a TI = 1,000 ms, TE = 3.31 ms, TR = 2,730 ms, flip angle = 7°, slice thickness = 1.33 mm, and voxel size 1.3 × 1.0 × 1.3 mm. To increase the signal-to-noise ratio, the two MPRAGE acquisitions were rigid-body registered to each other (motion corrected) and then averaged. Cortical surface reconstruction (33, 34) was based on the publicly available software package. The cortical surface was covered with a polygonal tessellation and smoothed to reduce metric distortions. The MRI processing for the VETSA sample was undertaken at a different site and machine than those for the full-lifespan samples (subsamples 1 and 2), and included minor edits not undertaken in the MRI processing for the full-lifespan sample. However, for reasons of data security, the original raw data of this sample could not readily be merged with those of the other samples, and the minor differences were seen as unlikely to have any substantial impact on the results, as this sample was used for a separate analysis. MRI image acquisition and processing for the VETSA sample is also explained in more detail elsewhere (58).
Acknowledgments
We thank all participants at all ages. This work was supported by the Department of Psychology, University of Oslo (K.B.W. and A.M.F.); the Norwegian Research Council (K.B.W. and A.M.F.); and the European Research Council’s Starting Grant scheme, ERC Grants 313440 (to K.B.W.) and 283634 (to A.M.F.). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/National Institute of Environmental Health Sciences (Contract N01-ES-75558), NIH/National Institute of Neurological Disorders and Stroke (Grants UO1 NS 047537-01 and UO1 NS 047537-06A1). VETSA is supported by US National Institute on Aging Grants R01 AG022381, AG018386, AG050595, and K08 AG047903, and with resources from the VA San Diego Center of Excellence for Stress and Mental Health, VA Cooperative Studies Program, and Vietnam Era Twin Registry.
Footnotes
Conflict of interest statement: A.M.D. is a founder and holds equity in CorTechs Laboratories and also serves on its Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California at San Diego, in accordance with its conflict of interest policies.
This article is a PNAS Direct Submission.
See Commentary on page 9148.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524259113/-/DCSupplemental.
References
- 1.Lindenberger U. Human cognitive aging: Corriger la fortune? Science. 2014;346(6209):572–578. doi: 10.1126/science.1254403. [DOI] [PubMed] [Google Scholar]
- 2.Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs GG, et al. Linking pathways in the developing and aging brain with neurodegeneration. Neuroscience. 2014;269:152–172. doi: 10.1016/j.neuroscience.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Fjell AM, et al. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci USA. 2015;112(50):15462–15467. doi: 10.1073/pnas.1508831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16(6):332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller M, et al. Birth size and brain function 75 years later. Pediatrics. 2014;134(4):761–770. doi: 10.1542/peds.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batty GD, Deary IJ. Early life intelligence and adult health. BMJ. 2004;329(7466):585–586. doi: 10.1136/bmj.329.7466.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deary IJ, et al. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 2012;482(7384):212–215. doi: 10.1038/nature10781. [DOI] [PubMed] [Google Scholar]
- 11.Lyons MJ, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20(9):1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karama S, et al. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Mol Psychiatry. 2014;19(5):555–559. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown TT, et al. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22(18):1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storsve AB, et al. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: Regions of accelerating and decelerating change. J Neurosci. 2014;34(25):8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384(2):312–320. [PubMed] [Google Scholar]
- 16.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 17.Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 18.Tamnes CK, et al. The brain dynamics of intellectual development: Waxing and waning white and gray matter. Neuropsychologia. 2011;49(13):3605–3611. doi: 10.1016/j.neuropsychologia.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 21.Amlien IK, et al. Organizing principles of human cortical development-thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cereb Cortex. 2014;26(1):257–267. doi: 10.1093/cercor/bhu214. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 23.Vuoksimaa E, et al. The genetic association between neocortical volume and general cognitive ability is driven by global surface area rather than thickness. Cereb Cortex. 2015;25(8):2127–2137. doi: 10.1093/cercor/bhu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fjell AM, et al. High-expanding cortical regions in human development and evolution are related to higher intellectual abilities. Cereb Cortex. 2015;25(1):26–34. doi: 10.1093/cercor/bht201. [DOI] [PubMed] [Google Scholar]
- 25.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5(1):87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 26.Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci USA. 2012;109(28):11366–11371. doi: 10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walhovd KB, et al. Pediatric Imaging, Neurocognition, and Genetics Study Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci USA. 2012;109(49):20089–20094. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phua DY, Rifkin-Graboi A, Saw SM, Meaney MJ, Qiu A. Executive functions of six-year-old boys with normal birth weight and gestational age. PLoS One. 2012;7(4):e36502. doi: 10.1371/journal.pone.0036502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart A, Otterblad Olausson P, Källen K. Apgar scores at 5 minutes after birth in relation to school performance at 16 years of age. Obstet Gynecol. 2011;118(2 Pt 1):201–208. doi: 10.1097/AOG.0b013e31822200eb. [DOI] [PubMed] [Google Scholar]
- 30.Noble KG, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarsour K, et al. Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. J Int Neuropsychol Soc. 2011;17(1):120–132. doi: 10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- 32.Magnus P, et al. MoBa Study Group Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 33.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, et al. Genetic topography of brain morphology. Proc Natl Acad Sci USA. 2013;110(42):17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuoksimaa E, et al. Is bigger always better? The importance of cortical configuration with respect to cognitive ability. Neuroimage. 2016;129:356–366. doi: 10.1016/j.neuroimage.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood SN. Generalized Additive Models: An Introduction with R. Chapman and Hall/CRC; Boca Raton, FL: 2006. [Google Scholar]
- 38.Team TRC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
- 39.Bartsch H, Thompson WK, Jernigan TL, Dale AM. A web-portal for interactive data exploration, visualization, and hypothesis testing. Front Neuroinform. 2014;8:25. doi: 10.3389/fninf.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed Lawrence Earlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 42.Kremen WS, et al. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA) Twin Res Hum Genet. 2006;9(6):1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- 43.Kremen WS, Franz CE, Lyons MJ. VETSA: The Vietnam Era Twin Study of Aging. Twin Res Hum Genet. 2013;16(1):399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vakhtin AA, Ryman SG, Flores RA, Jung RE. Functional brain networks contributing to the parieto-frontal integration theory of intelligence. Neuroimage. 2014;103:349–354. doi: 10.1016/j.neuroimage.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 45.Gläscher J, et al. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci USA. 2010;107(10):4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutter M. Achievements and challenges in the biology of environmental effects. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17149–17153. doi: 10.1073/pnas.1121258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson PM. Cracking the brain’s genetic code. Proc Natl Acad Sci USA. 2015;112(50):15269–15270. doi: 10.1073/pnas.1520702112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice F, Thapar A. Estimating the relative contributions of maternal genetic, paternal genetic and intrauterine factors to offspring birth weight and head circumference. Early Hum Dev. 2010;86(7):425–432. doi: 10.1016/j.earlhumdev.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Insititute CSR. 2014. Credit Suisse Global Wealth Databook (Credit Suisse Group, Credit Suisse Research Institute, Zurich) p 156.
- 50.Pickett KE, Wilkinson RG. The ethical and policy implications of research on income inequality and child well-being. Pediatrics. 2015;135(Suppl 2):S39–S47. doi: 10.1542/peds.2014-3549E. [DOI] [PubMed] [Google Scholar]
- 51.Nilsen RM, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence - III, Norwegian Manual. Pearson Assessment; Stockholm: 2008. [Google Scholar]
- 53.Beck AT, Steer R. Beck Depression Inventory Scoring Manual. The Psychological Corporation; New York: 1987. [Google Scholar]
- 54.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 55.Schoenborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004-2007. Natl Health Stat Rep. 2009;(16):1–31. [PubMed] [Google Scholar]
- 56.Wonderlick JS, et al. Reliability of MRI-derived cortical and subcortical morphometric measures: Effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44(4):1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kremen WS, et al. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. Neuroimage. 2010;49(2):1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]