Significance
4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA), a potent antiviral with an unusually long half-life, is currently in phase I clinical trials. It inhibits reverse transcriptase (RT) through a mechanism of action different from all approved anti-HIV drugs: After incorporation in the nascent DNA chain, it suppresses the rate of viral replication by diminishing RT translocation on the nucleic acid substrate. Here, we present four crystal structures of various inhibition intermediates. The structures provide insight into the structural basis of the unusual mechanism of inhibition and the exceptional potency of this promising antiviral.
Keywords: EFdA, HIV-1 reverse transcriptase, inhibitors, NRTIs, X-ray crystallography
Abstract
4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) is the most potent nucleoside analog inhibitor of HIV reverse transcriptase (RT). It retains a 3′-OH yet acts as a chain-terminating agent by diminishing translocation from the pretranslocation nucleotide-binding site (N site) to the posttranslocation primer-binding site (P site). Also, facile misincorporation of EFdA-monophosphate (MP) results in difficult-to-extend mismatched primers. To understand the high potency and unusual inhibition mechanism of EFdA, we solved RT crystal structures (resolutions from 2.4 to 2.9 Å) that include inhibition intermediates (i) before inhibitor incorporation (catalytic complex, RT/DNA/EFdA-triphosphate), (ii) after incorporation of EFdA-MP followed by dT-MP (RT/DNAEFdA-MPP•dT-MPN), or (iii) after incorporation of two EFdA-MPs (RT/DNAEFdA-MPP•EFdA-MPN); (iv) the latter was also solved with EFdA-MP mismatched at the N site (RT/DNAEFdA-MPP•EFdA-MP*N). We report that the inhibition mechanism and potency of EFdA stem from interactions of its 4′-ethynyl at a previously unexploited conserved hydrophobic pocket in the polymerase active site. The high resolution of the catalytic complex structure revealed a network of ordered water molecules at the polymerase active site that stabilize enzyme interactions with nucleotide and DNA substrates. Finally, decreased translocation results from favorable interactions of primer-terminating EFdA-MP at the pretranslocation site and unfavorable posttranslocation interactions that lead to observed localized primer distortions.
HIV reverse transcriptase (RT) converts viral genomic RNA into viral DNA, which can then be integrated into the host genome. Most clinically used drugs that block HIV replication targeting RT are nucleoside RT inhibitors (NRTIs) that inhibit further DNA polymerization. Once incorporated into the viral DNA, NRTIs act as chain terminators due to the lack of a 3′-OH. Although current NRTIs are highly effective, treatment adherence often leads to the development of drug-resistant HIV variants. New and even more potent antiretrovirals are needed, especially drugs effective against current clinically significant drug-resistant HIV strains.
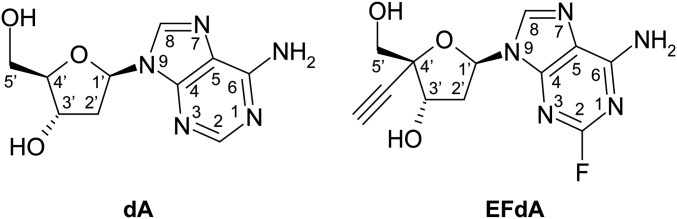
The most potent nucleoside analog antivirals possess 3′-OH groups. Entecavir is the most potent anti-hepatitis B NRTI drug, and sofosbuvir is the most successful anti-hepatitis C virus nucleotide drug (1–5). 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) extends this concept as an anti-HIV agent. EFdA is a deoxyadenosine (dA) analog with 3′-OH, 4′-ethynyl (4′-E), and 2-fluoro (2-F) groups (Fig. 1). EFdA blocks HIV replication in peripheral blood mononuclear cells (PBMCs) at picomolar concentrations, making it orders of magnitude more potent than current clinical anti-HIV NRTIs (6–8). Recent phase I clinical data with EFdA (also known as MK-8591) demonstrate that it is well tolerated in healthy adult subjects (9). Moreover, it has exceptionally favorable pharmacokinetic properties and an unusually long intracellular half-life compared with other NRTIs, mainly due to its resistance to degradation by adenosine deaminase (6, 9–12). Hence, it is targeted for use as a single 10-mg dose for >7 d (9). Remarkably, ongoing studies with slow release parenteral formulations suggest the potential for a single injection to provide coverage for durations up to 1 y (9).
Fig. 1.
Chemical structures of 2′-deoxyadenosine (dA) and EFdA.
EFdA is effective against wild-type and clinical drug-resistant HIV strains (6), and acts synergistically with the second-generation nonnucleoside RT inhibitor rilpivirine (13). EFdA blocks simian immunodeficiency virus replication in vitro in macaque PBMCs better than tenofovir (TFV), zidovudine (AZT), and emtricitabine (FTC) (14). In vivo, EFdA outperforms Truvada (a two-NRTI combination) in several humanized mouse models for HIV infection (12). EFdA was highly effective at treating simian immunodeficiency virus-infected macaques with advanced AIDS. Furthermore, no toxicities have been noted in animals treated with EFdA (12, 14).
Biochemical data indicate that, depending on the context of template sequence, EFdA can act either as a de facto immediate chain terminator (ICT) or as a delayed chain terminator (DCT) that allows incorporation of a single nucleotide following EFdA-monophosphate (MP) and slows nucleotide extension after incorporation into the nascent primer (15). Moreover, RT misincorporates EFdA-MP more efficiently than dA-triphosphate (TP), resulting in mismatched primers that are difficult to extend. Finally, when EFdA acts as a DCT, EFdA-MP–terminated primers are protected from the phosphorolytic excision that can lead to NRTI resistance (15).
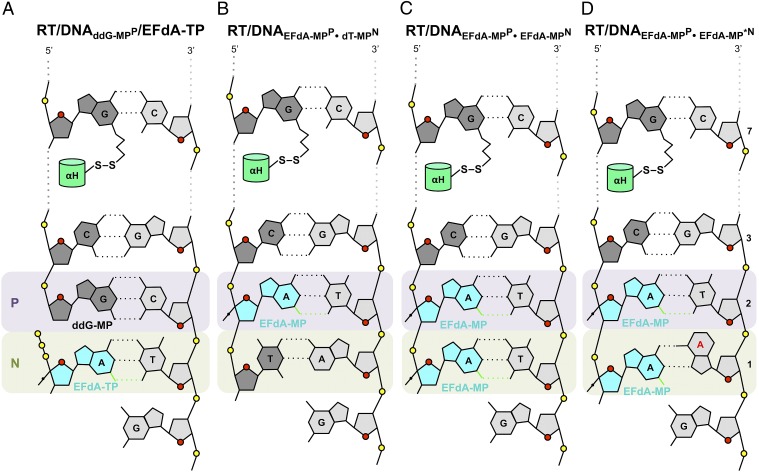
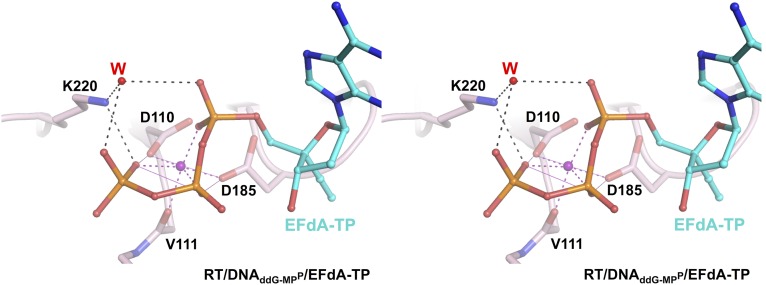
To determine the structural mechanisms of HIV inhibition by EFdA, we solved crystal structures that include intermediates of the RT inhibition reaction (Fig. 2). The first structure is a precatalytic complex of RT bound to a dideoxyguanosine (ddG)-terminated double-stranded DNA and to incoming EFdA-TP (RT/DNAddG-MPP/EFdA-TP, 2.4-Å resolution; Figs. 2A, 3, and 4).
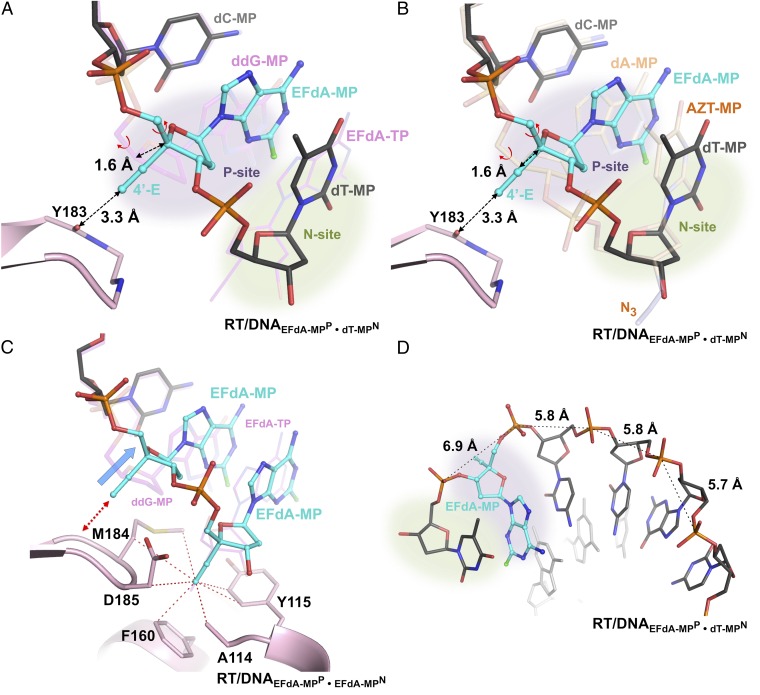
Fig. 2.
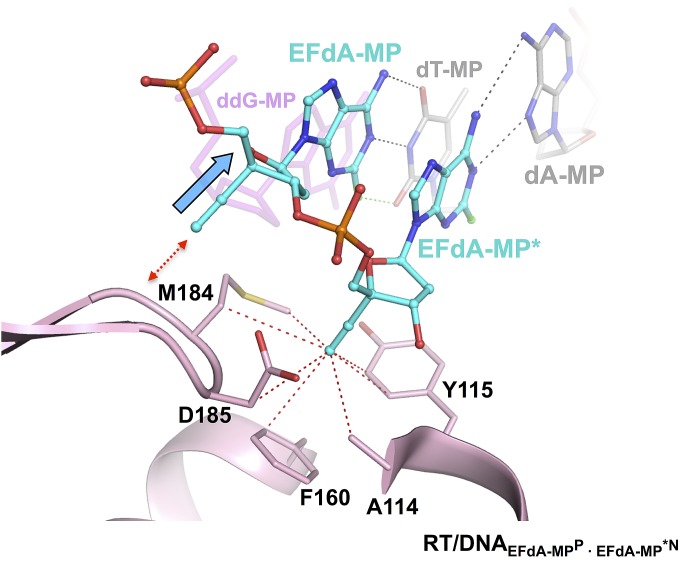
Schematic representation of crystallized reaction intermediates. (A) Ternary complex of RT with ddG-MP–terminated DNA and EFdA-TP (RT/DNAddG-MPP/EFdA-TP). The thioalkyl-tethered guanosine at the seventh position from the primer 3′-end is covalently cross-linked to RTQ258C on the αH helix through formation of a disulfide bond (hydrogen bonds, black dotted lines; halogen bond, green dotted lines). (B) Binary complex of RT/DNA with EFdA-MP incorporated at the 3′ primer end in the posttranslocation site (priming site or P site) and further extended by a dT-MP at the pretranslocation site (nucleotide-binding site or N site) (RT/DNAEFdA-MPP•dT-MPN). (C) Binary complex of RT/DNA with EFdA-MP incorporated at the P site and further extended by a second EFdA-MP correctly base paired against dT-MP at the N site (RT/DNAEFdA-MPP•EFdA-MPN). (D) RT/DNA with EFdA-MP incorporated at the P site and further extended by a second EFdA-MP mismatched against dA-MP at the N site (RT/DNAEFdA-MPP•EFdA-MP*N).
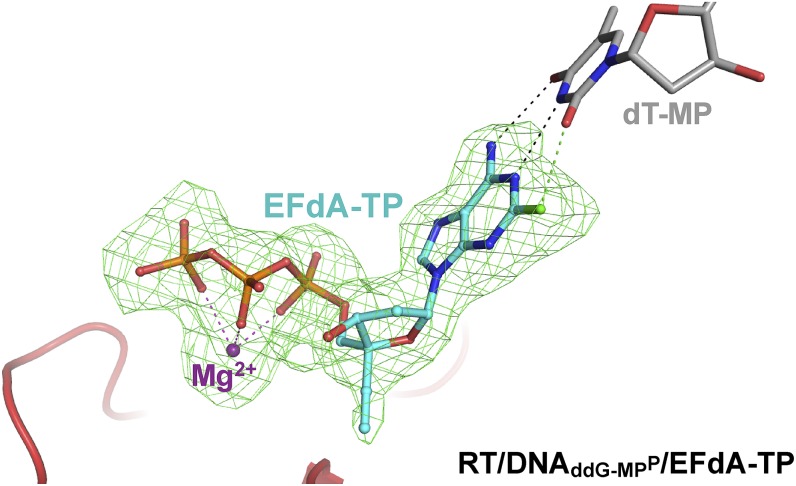
Fig. 3.
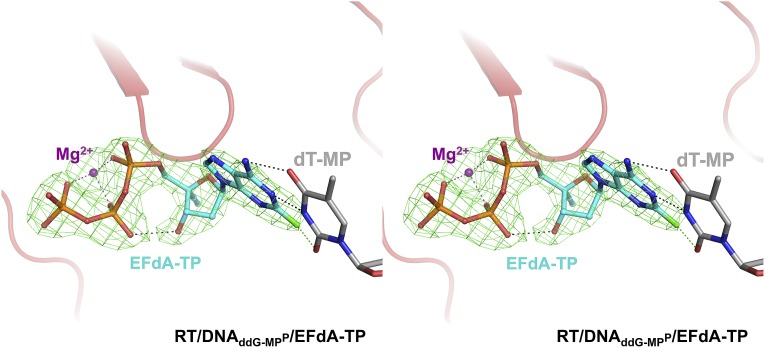
Simulated annealing omit map (green; 2.4 Å) for EFdA-TP in the RT/DNAddG-MPP/EFdA-TP complex (carbons, cyan; phosphorus, orange; fluorine, green) at σ = 2.2.
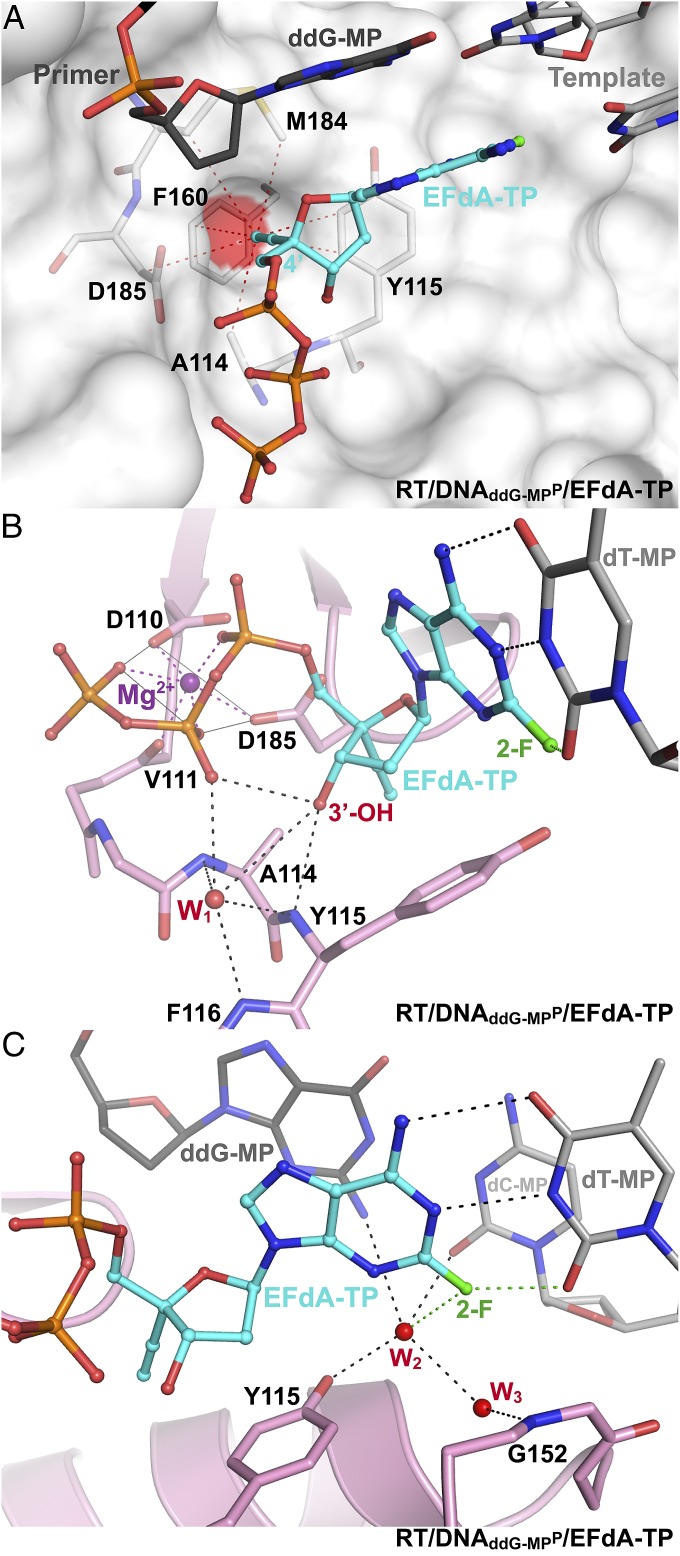
Fig. 4.
EFdA-TP interactions at the polymerase active site of RT/DNAddG-MPP/EFdA-TP. (A) Hydrophobic interactions (atoms within 4 Å; red dotted lines) of 4′-E at a hydrophobic pocket (red surface) near the polymerase active site. (B) Polar interactions between the EFdA-TP 3′-OH, the β-phosphate, ordered water W1, and peptide backbone atoms are shown as dotted lines (H bonds, black; metal binding, purple; halogen bond, green). (C) Interactions of water W2 with Y115, 2-F, the P-site base pair, and water W3, which is also bound to main-chain N-H of G152.
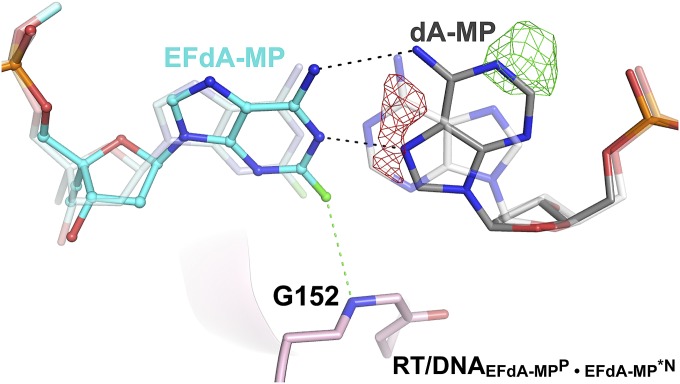
The other structures are postincorporation complexes with EFdA-MP bound at the primer-binding or P site (EFdA-MPP). They have primers further extended by nucleotides that are positioned at the nucleotide-binding, N site: either a deoxythymidine-MP (dT-MPN) (RT/DNAEFdA-MPP•dT-MPN, 2.9 Å; Fig. 2B) or an additional EFdA-MPN (RT/DNAEFdA-MPP•EFdA-MPN, 2.5 Å; Fig. 2C). These structures elucidate how EFdA affects RT translocation by describing interactions of EFdA-MP bound at the posttranslocation P site (EFdA-MPP) or at the N site (EFdA-MPN) (Fig. 5). In a fourth structure, EFdA is mismatched against a dA template base at the N site (EFdA-MP*N) (RT/DNAEFdA-MPP•EFdA-MP*N, 2.8 Å; Figs. 2D and 6). This structure provides insight into why RT favors EFdA-MP misincorporation and why EFdA-misincorporated primers are difficult to extend.
Fig. 5.
Structural changes in EFdA-containing complexes. (A) Superposition of N-complex RT/DNAEFdA-MPP•dT-MPN and ternary complex RT/DNAddG-MPP/EFdA-TP (magenta DNA). (B) Superposition of two N complexes of RT, in the presence or absence of EFdA-MP at the P site [RT/DNAEFdA-MPP•dT-MPN and RT/DNAdA-MPP•AZT-MPN (PDB ID 1N6Q in light brown) (17)]. In both A and B, without the localized 1.6-Å primer shift (marked dotted arrow) of EFdA-MPP, the currently observed 3.3-Å distance between the 4′-E and main-chain carbonyl of Y183 would have been highly unfavorable at ∼1.7 Å. This shift is accomplished through a change in the γ-torsion angle about the C4′-C5′ (Fig. S6A vs. Fig. S6B; Table S1). (C) Primer distortion due to interactions of P-site EFdA-MP of RT/DNAEFdA-MPP•EFdA-MPN with Y183 (red arrow) compared with RT/DNAddG-MPP/EFdA-TP (magenta DNA); hydrophobic interactions of the 4′-E at the active-site hydrophobic pocket (red dotted lines connect atoms within 4 Å). (D) The change in γ results in increased distance between neighboring phosphates near the polymerase active site.
Fig. 6.
Interactions of EFdA-MP•dA-MP mismatch. Hoogsteen base pairing between EFdA-MP and dA-MP in the RT/DNAEFdA-MPP•EFdA-MP*N structure, superposed on a hypothetical non-Hoogsteen base pairing. Green/red Fo-Fc maps (σ = ±2.4) indicate that the mismatched adenine (light gray) should be flipped and modeled as Hoogsteen base paired as it better fits the crystallographic data.
Results and Discussion
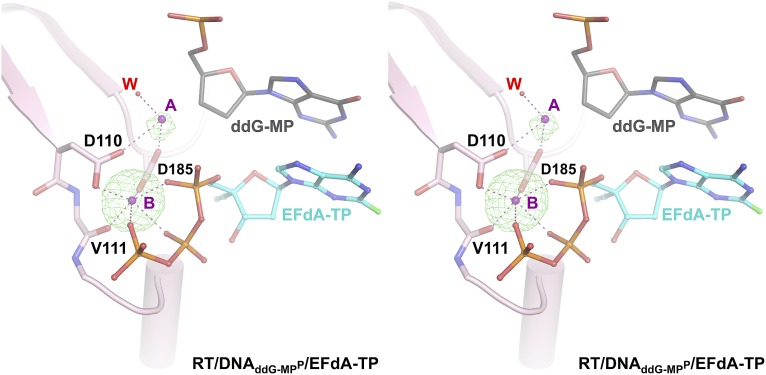
RT/DNAddG-MPP/EFdA-TP is the highest resolution structure (2.4 Å) of HIV-1 RT in complex with DNA and a nucleotide TP to date. This resolution enables reliable modeling of ordered water molecules at the polymerase active site. We observed two divalent metals bound at the polymerase active site (Fig. S1). Metal ion B, also known as a structural metal, is coordinated in an octahedral geometry, bound by the side chains of D110 and D185, the main chain carbonyl of V111, and three oxygen atoms from the α, β, and γ phosphates of the incoming nucleotide triphosphate (Fig. S1). Due to lack of 3′-OH group in the dideoxyguanosine monophosphate (ddG-MP) at P site, the coordination of magnesium ion A (known as a catalytic metal) is incomplete and likely low occupancy (Fig. S1). The structure shows strong electron density for the 4′-E, 3′-OH, and 2-F of EFdA-TP, and reveals interactions of the incoming inhibitor at the N site (Fig. 3 and Fig. S2). The 4′-E of EFdA-TP is bound at a preformed hydrophobic pocket defined by conserved residues A114, Y115, F160, and M184, and the aliphatic part of D185 (Fig. 4A). Such extensive interactions of EFdA-TP at the N site are consistent with the previously reported high kon and low koff rates that result in RT binding EFdA-TP even better than dNTP substrates (15). These interactions contribute to the inhibition mechanism of EFdA-TP as an ICT or translocation-defective RT inhibitor as they would favor binding of the EFdA-MP–terminated primer at the pretranslocation N site.
Fig. S1.
Cross-eyed stereoview of the divalent metal electron density at the polymerase active site. Simulated annealing omit electron density map (2.4-Å resolution) contoured at σ = 4.5 (green) is shown for two polymerase active site magnesium ions (A, or catalytic metal; B, or structural metal; metal binding, purple dotted lines) in the RT/DNAddG-MPP/EFdA-TP complex. The octahedral coordination geometry of metal ion B (also known as a structural metal ion) is complete. One water molecule (W) contributes to the coordination of magnesium ion A; due to lack of the primer 3′-OH, the coordination of catalytic metal ion A is incomplete.
Fig. S2.
Cross-eyed stereoview showing electron density for EFdA-TP at the RT polymerase active site in the RT/DNAddG-MPP/EFdA-TP complex. Simulated annealing omit electron density map at 2.4-Å resolution, contoured at σ = 5.5 (green) is shown for EFdA-TP (carbons, cyan; phosphorus, orange; fluorine, green; hydrogen bond, black dotted lines; halogen bonds, green dotted lines).
A unique feature of EFdA-TP compared with clinical anti-HIV NRTIs is its 3′-OH, which interacts through a hydrogen bond with the β-phosphate of the inhibitor (Fig. 4B and Fig. S2). This interaction is further stabilized by ordered water W1, which in turn is held in position by conserved protein backbone atoms of A114, Y115, and F116 (Fig. 4B). We propose that NRTIs that lack a 3′-OH (such as dideoxynucleosides, AZT, d4T, 3TC, tenofovir, and abacavir) are less efficient in (i) conformationally stabilizing and optimally positioning the α-phosphate of the nucleotide substrate for nucleophilic attack by the primer 3′-OH, and (ii) stabilizing the pyrophosphate leaving group. Thus, the presence of a 3′-OH in EFdA-TP allows more efficient incorporation of the inhibitor into the viral DNA. In addition to the previously reported interactions of RT with the α, β, and γ phosphates of the incoming nucleotide [K65, R72, main-chain V111, D113, and Q151 (16)], improved resolution and good-quality electron density allowed assignment of additional interactions of K220 with the γ-phosphate (Fig. S3). We also report another previously undescribed network of water molecules, which includes interactions between ordered waters W2 and W3, the 2-F of EFdA-TP at the N site, the Y115 hydroxyl, the G152 main-chain amide and minor groove atoms of the nucleotide dG-dC base pair at the P site. dG-dC–specific interactions at the P site (with the 2-amino of dG and 2-carbonyl of dC; Fig. 4C) may further stabilize pretranslocation complexes and explain the reported preference for the ICT rather than DCT mechanism of inhibition for such nucleic acid substrates (15).
Fig. S3.
Cross-eyed stereoview of the interactions between K220 and the triphosphate of EFdA-TP in the RT/DNAddG-MPP/EFdA-TP complex. The side chain of K220 directly interacts with the γ-phosphate of EFdA-TP, as well as with the α- and γ-phosphates through a water molecule (W), thus contributing to optimal binding of the incoming nucleotide.
The RT/DNAEFdA-MPP•dT-MPN and RT/DNAEFdA-MPP•EFdA-MPN structures have EFdA-MP at only the P or at both the P and N sites (Figs. 2 B and C, and 5). As in RT/DNAddG-MPP/EFdA-TP, the 4′-E at the N site of RT/DNAEFdA-MPP•EFdA-MPN engages RT through strong hydrophobic interactions (Fig. 5C).
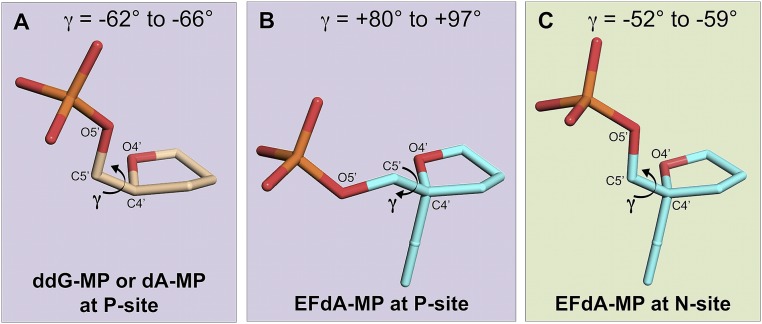
To assess the role of 4′-E at the P site, we compared structures with EFdA-MPP, such as RT/DNAEFdA-MPP•dT-MPN, to structures without EFdA-MPP, such as the previously reported related intermediate RT/DNAdA-MPP•AZT-MPN (Fig. 5B) or RT/DNAddG-MPP/EFdA-TP (Fig. 5A) (17). AZT is a thymidine analog with a 3′-azido group replacing the 3′-OH. The 3′-azido interaction is not through the hydrophobic pocket but through residues A114, Y115, and F116. These comparisons suggest that a 1.6-Å shift of EFdA-MPP is required to relieve the potential steric clash between the 4′-E of EFdA-MPP and the main-chain carbonyl of Y183 (Fig. 5 A and B). Without such shift, the 4′-E − Y183 distance would be a highly unfavorable 1.7 Å (3.3 − 1.6 = 1.7 Å). Interestingly, the primer distortion is restricted to EFdA-MP at the P site and is not present upstream or downstream of EFdA-MPP (Fig. 5). It is concurrent with a change in the EFdA-MPP γ-torsion dihedral angle (O4′-C4′-C5′-O5′; Fig. S4), which differs significantly from the γ-angle of other nucleotides at the P site (by up to |−66| + |+97| = 163°; Fig. S4A vs. Fig. S4B; Table S1). The difference in γ-angle is not an inherent property of EFdA-MP, as the γ-angle of EFdA-MPN is different from that of EFdA-MPP and similar to that of nucleotides other than EFdA-MP bound at the P site (Fig. S4C vs. Fig. S4B, Table S1). Notably, the changed γ-torsion affects an interphosphate distance at the polymerase active site, from ∼5.8 Å (typical A DNA distance) to 6.9 Å (closer to B DNA; Fig. 5D), which may also affect the translocation and DNA-binding properties of RT.
Fig. S4.
The γ-torsion dihedral angles of various nucleotides at the RT P or N sites. The γ-angle is defined by atoms O4′-C4′-C5′-O5′ of a nucleotide and changes through rotation about C4′-C5′. Changes in the value of γ result in a different positioning of the phosphate group. (A) The γ-angles for nucleotides other than EFdA-MP (ddG-MP or dAMP) at the P site. These range from −62° for ddG-MP (RT/DNAddG-MPP/EFdA-TP) to −66° for dA-MP (RT/DNAdA-MPP•AZT-MPN) (Table S1) (17). (B) The γ-angles for EFdA-MP at the P site (EFdA-MPP). These range from +80° in RT/DNAEFdA-MPP•dT-MPN, to +97° in RT/DNAEFdA-MPP•EFdA-MP*N (Table S1). (C) The γ-angles for EFdA-MP at the N site (EFdA-MPN). These range from −52° in RT/DNAEFdA-MPP•EFdA-MP*N to −59° in RT/DNAEFdA-MPP•EFdA-MPN (Table S1).
Table S1.
γ-Torsion dihedral angle of nucleotides at various primer sites
| Complex | P-1 site, ° | P site, ° | N site, ° |
| RT/DNAddG-MPP/EFdA-TP | −67 | −62 | −57† |
| RT/DNAEFdA-MPP•dT-MPN | −60 | +80 | −62 |
| RT/DNAEFdA-MPP•EFdA-MPN | −64 | +81 | −59 |
| RT/DNAEFdA-MPP•EFdA-MP*N | −60 | +97 | −52 |
| RT/DNAdA-MPP•AZT-MPN | −72 | −66 | −74 |
N site, pretranslocation site (nucleotide-binding or N site); P site, posttranslocation site (primer-binding or P site); P-1 site, site immediately upstream of the P site.
Value for triphosphate.
These findings provide structural insights regarding the ICT and DCT inhibition mechanisms: in the case of ICT, we propose that strong interactions of RT with EFdA-MPN prevent translocation of the enzyme, thus inhibiting DNA synthesis. This hypothesis is supported by hydroxyl radical footprinting experiments showing that, at template sites favoring ICT, EFdA-MP–extended primers are overwhelmingly bound with EFdA-MP positioned at the N site (EFdA-MPN) (15). At template sites where EFdA acts as a DCT, EFdA-MPN is extended to EFdA-MPP•dN-MPN; in this case, we propose that inhibition occurs because of steric crowding between the 4′-E of EFdA-MP and the main chain of Y183, leading to localized primer distortions (Fig. 5 A and B) that may affect the translocation efficiency and DNA-binding properties of RT.
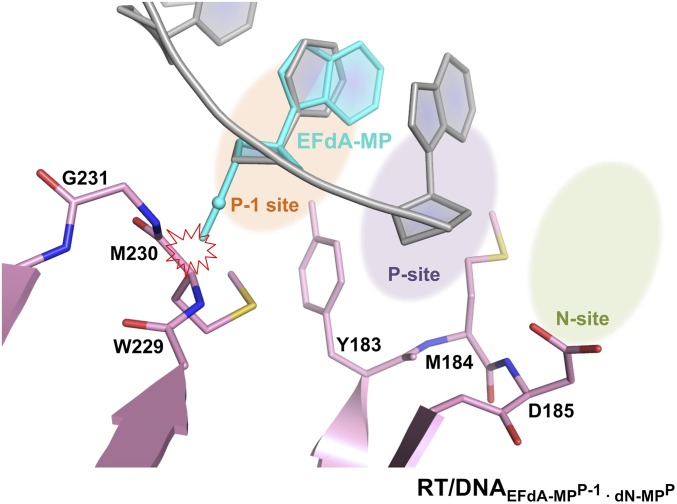
Moreover, further translocation of a DNAEFdA-MPP•dN-MPN placing the EFdA-MP at the “P-1” upstream primer site, would likely result in similar steric clashes between the 4′-E of EFdA-MPP-1 with the main chain of M230, as shown in the molecular model of RT/DNAEFdA-MPP-1•dN-MPP (Fig. S5). Thus, steric interactions of the 4′-E of EFdA-MPP or EFdA-MPP-1 with RT residues prevent further dN-MP additions, leading to DCT inhibition of DNA synthesis.
Fig. S5.
Expected steric interactions between the 4′-E of EFdA-MPP-1 and the P-1 site of RT (site immediately upstream of the P site). EFdA-MP (cyan) was modeled into the “P-1” site by superposition of its ribose ring with the ribose ring of the dC-MP (gray) residue that is present in this position of the RT/DNAddG-MPP/EFdA-TP structure. 4′-E of EFdA-MP clashes with the side chain of M230 (∼2 Å) in this RT/DNAEFdA-MPP-1•dN-MPP model.
We previously demonstrated that the type of mechanism by which EFdA inhibits RT (ICT vs. DCT) is not stochastically determined but appears to depend on the template sequence in a complex and poorly understood manner. Our present structural data suggest that sequence-dependent differences in the observed interactions of 2-F, W2, and the base pair at the P site (Fig. 4C) may affect the inhibition mechanism (ICT vs. DCT), along with other possible changes imposed by global curvature and flexibility of the nucleic acid.
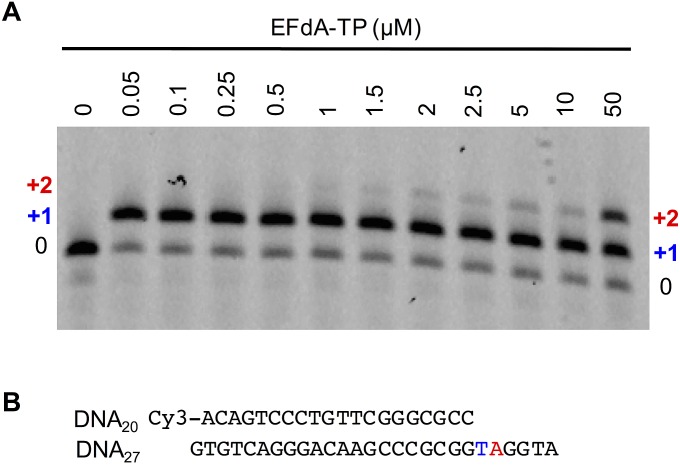
Our previous biochemical studies suggested that EFdA can also inhibit HIV RT via facile misincorporation into nascent primers that are subsequently difficult to extend (15). We confirmed this finding in primer extension assays shown in Fig. S6 using template/primers with the same sequence used in the crystallization studies. To elucidate the structural details of EFdA mismatch incorporation, we solved the RT/DNAEFdA-MPP•EFdA-MP*N structure that has EFdA-MPP extended by a mismatched second EFdA-MP against dA-MP at the N site (Fig. 2D). The structure shows that EFdA-MP*N interacts with the “flipped” purine of dA-MP through Hoogsteen base pairing (Fig. 6). In addition, the interaction of EFdA-MP with G152 through 2-F (Fig. 6) would be absent in a dA-MP•dA-MP mismatch. This may address why the EFdA-MP•dA-MP mismatch is more efficiently formed than the dA-MP•dA-MP mismatch (15). Moreover, the strong interactions of EFdA-MP at the pretranslocation site through its 4′-E, 2-F, and 3′-OH groups, even when paired with a mismatched template base, may explain the inefficient extension of EFdA-MP*N (Fig. S7).
Fig. S6.
Incorporation of EFdA-MP against a matched template base (dT-MP), followed by a misincorporation against a mismatched template base (dA-MP). (A) Primer extension reactions at different EFdA-TP concentrations. (B) Sequence of the template/primer (5′-Cy3-DNAd20/DNAd27) used in the primer extension reactions and in the crystal structure of RT/DNAEFdA-MPP•EFdA-MP*N. EFdA-MP was incorporated as a match in the first site (+1 in blue) and as a mismatch in the following site (+2, in red).
Fig. S7.
Polymerase active-site superposition between RT/DNAddG-MPP/EFdA-TP and the mismatched RT/DNAEFdA-MPP•EFdA-MP*N complex. A 1.6-Å primer shift is indicated by a blue arrow. The EFdA-TP at the N site is not shown for clarity. Hydrophobic interactions between EFdA-MP*N 4′-E and protein atoms within 4 Å (red dotted lines) are shown at the HIV RT polymerase active site.
In conclusion, our crystal structures provide the structural basis for understanding the EFdA inhibition mechanism and illuminate the roles of its three structural motifs, 4′-E, 3′-OH, and 2-F. Regarding 4′-E, it is observed in all inhibition intermediate structures to consistently and extensively interact with conserved residues of an active-site hydrophobic pocket. To our knowledge, EFdA is the first antiviral exploiting this site, leading to a previously unidentified inhibition mechanism (Fig. 4A). In addition, the 4′-E distorts the primer structure (Fig. 5 A–C), thus altering the translocation and DNA-binding properties of RT. Regarding 3′-OH, it is a unique feature among NRTIs that significantly enhances antiviral potency (EFdA has 10,000 times higher antiviral activity than EFddA, the otherwise identical nucleoside lacking a 3′-OH) (7). We propose this is due to improved binding and catalytic incorporation into the viral DNA (Fig. 4B); also, the 3′-OH of EFdA contributes to its rapid and facile activation by the deoxycytidine kinase (6, 18). Finally, regarding 2-F, it contributes to improved inhibitor binding at the polymerase active site (Fig. 4C) and it provides exceptional resistance to adenosine deaminase-catalyzed degradation in cells (6, 11). Remarkably, a single 10-mg dose of EFdA resulted in consistent viremia control for up to 10 d (9), offering exceptional promise for further development as an anti-HIV drug.
Materials and Methods
Protein Expression and Purification.
BL21(DE3)-RIL cells containing two vectors carrying the p66 and p51 subunits of HIV-1 RT were expressed in LB and induced with 1 mM isopropyl β-d-1-thiogalactopyranoside at OD600 of ∼0.6–0.8, followed by expression at 37 °C for 3 h. Cell pellets were treated with lysozyme, homogenization, and sonication. RT was purified by nickel-affinity chromatography (GE Healthcare), followed by Mono Q anion exchange (GE Healthcare), and size-exclusion chromatography (Shodex) as previously described (15). Fractions with pure RT were concentrated and stored at −80 °C.
Crystallization of the RT/DNA Complex.
We used a primer strand that contained a thioalkyl-tethered guanosine at the seventh position from the 3′-end, which was covalently cross-linked to RTQ258C through formation of a disulfide bond (Fig. 2) (19). Double-stranded DNA was prepared with template sequence 5′-ATG GTC GGC GCC CGA ACA GGG ACT GTG-3′; 5′-ATG GAT GGC GCC CGA ACA GGG ACT GTG-3′; 5′-ATG GTT GGC GCC CGA ACA GGG ACT GTG-3′; 5′-ATG GAT GGC GCC CGA ACA GGG ACT GTG-3′ for RT/DNAddG-MPP/EFdA-TP ternary complex, RT/DNAEFdA-MPP•dT-MPN and RT/DNAEFdA-MPP•EFdA-MPN binary complexes, and RT/DNAEFdA-MPP•EFdA-MP*N mismatched binary complex, respectively. The primer sequence used in all four structures was 5′-ACA GTC CCT GTT CGG G*CG CC-3′ (G* has a thioalkyl tether covalently attached to the N2 group of the guanosine; Fig. 2) (Trilink Biotechnologies). Template and primer were first annealed at 95 °C for 5 min, cooled at room temperature for 20 min, and stored at −20 °C. The 15 μM annealed template/primer (T/P) substrates were incubated with 7 μM cross-linkable HIV RT in 25 mM Tris, pH 8.0, 100 mM NaCl, 10 mM MgCl2, and 0.1 mM 2′,3′-dideoxyguanosine-5′-triphosphate (ddG-TP) (GE Health Life Science) to obtain the RT/DNAddG-MPP complex; 70 μM EFdA-TP and 0.1 mM deoxythymidine monophosphate (dT-MP) for the RT/DNAEFdA-MPP•dT-MPN binary complex; or 70 μM EFdA-TP for the RT/DNAEFdA-MPP•EFdA-MPN and RT/DNAEFdA-MPP•EFdA-MP*N complexes at 31 °C for 18 h. Cross-linked RT-dsDNA complex was purified using a Ni-Heparin tandem column strategy (20). Cross-linking efficiency was judged by an increase in the molecular weight of the p66 subunit in nonreducing SDS/PAGE. Fractions containing DNA–cross-linked RT were pooled and concentrated to 15.5 mg/mL. For the RT/DNAddG-MPP/EFdA-TP ternary complex, initial RT/DNAddG-MPP complex sample was incubated on ice for 15 min with 1.5 molar excess of EFdA-TP and 5 mM MgCl2. Hanging drops were prepared by mixing 1:1, 2:1, or 2:1 volume ratio of well solution and ternary complex. Crystals were grown at 18 °C in 8% (wt/vol) PEG 4000, 25 mM Mes, pH 6.0, and 5 mM magnesium sulfate. Crystals were dehydrated by elevating PEG 4000 up to 35% (wt/vol) over 1 h and were flash-cooled in liquid nitrogen.
Data Collection and Structure Determination.
Data were collected on a CMOS detector at Advanced Light Source (ALS) beamline 4.2.2, Lawrence Berkeley National Laboratory (Table S2). Datasets were processed using XDS (21) and indexed in C2221 (a = 167.5 Å, b = 169.8 Å, and c = 101.9 Å) with one complex per asymmetric unit. Crystal structures were solved by molecular replacement using PHASER (22) within the CCP4 package (23). The Protein Data Bank (PDB) ID 3KK1 structure was used as a starting model. Several rounds of manual rebuilding using Coot (24) and refinement with the PHENIX package (25) were performed. Coordinates and molecular topologies for EFdA-TP and EFdA-MP were generated in SYBYL (https://www.certara.com/software/molecular-modeling-and-simulation/sybyl-x-suite/) and PHENIX eLBOW (26). The positions of EFdA-TP, EFdA-MP, or dT-MP in the structure were built into difference Fourier maps calculated before inclusion of the respective structural elements in refinement. Final atomic coordinates and structure factors have been deposited in the PDB (PDB ID: RT/DNAddG-MPP/EFdA-TP, 5J2M; RT/DNAEFdA-MPP•dT-MPN, 5J2N; RT/DNAEFdA-MPP•EFdA-MPN, 5J2P; RT/DNAEFdA-MPP•EFdA-MP*N, 5J2Q). The figures showing structural information were generated in PyMOL (www.pymol.org/).
Table S2.
Crystallographic statistics
| Complex | RT/DNAddG-MPP/EFdA-TP | RT/DNAEFdA-MPP•dT-MPN | RT/DNAEFdA-MPP•EFdA-MPN | RT/DNAEFdA-MPP•EFdA-MP*N |
| PDB ID | 5J2M | 5J2N | 5J2P | 5J2Q |
| Data collection | ||||
| Beamline | ALS 4.2.2 | ALS 4.2.2 | ALS 4.2.2 | ALS 4.2.2 |
| Space group | C2221 | C2221 | C2221 | C2221 |
| a, Å | 167.5 | 166.8 | 166.0 | 165.6 |
| b, Å | 169.8 | 169.9 | 170.2 | 170.5 |
| c, Å | 102.0 | 102.7 | 99.7 | 102.2 |
| α = β = γ, ° | 90 | 90 | 90 | 90 |
| Resolution range, ņ | 65.23–2.43 (2.50–2.43) | 53.62–2.88 (3.04–2.88) | 63.78–2.53 (2.62–2.53) | 53.76–2.79 (2.91–2.79) |
| Average redundancy | 7.4 | 7.2 | 7.2 | 7.4 |
| Completeness, %† | 99.5 (94.2) | 99.0 (93.0) | 98.9 (88.6) | 99.7 (97.8) |
| Rmeas† | 0.086 (1.023) | 0.149 (0.962) | 0.111 (0.917) | 0.085 (1.067) |
| CC(1/2) | 0.999 (0.790) | 0.997 (0.669) | 0.998 (0.751) | 0.999 (0.812) |
| Average I/σI† | 19.8 (2.0) | 13.4 (2.0) | 16.7 (2.0) | 21.5 (1.9) |
| Refinement | ||||
| R/Rfree | 0.195/0.232 | 0.212/0.242 | 0.202/0.245 | 0.213/0.253 |
| Wilson B factor | 44.8 | 54.7 | 42.3 | 62.2 |
| R.m.s. deviations | ||||
| Bond lengths | 0.009 | 0.002 | 0.003 | 0.002 |
| Bond angles | 0.478 | 0.443 | 0.518 | 0.452 |
| Average B | 50.9 | 57.5 | 49.3 | 70.2 |
| No. of waters | 387 | 4 | 423 | 3 |
| Ramachandran plot | ||||
| Outliers | 0.0% | 0.1% | 0.0% | 0.0% |
| Allowed | 2.9% | 5.3% | 3.6% | 4.1% |
| Favored | 97.1% | 94.6% | 96.4% | 95.9% |
Values for highest resolution shell are shown in parentheses.
RT Primer Extension Assay.
The gel-based inhibition assay of HIV-1 RT-catalyzed DNA synthesis by EFdA-TP was carried out using 5′-Cy3–labeled DNA primer and an unlabeled template. To monitor primer extension, the 20 nM DNA/DNA was incubated at 37 °C with 20 nM RT in buffer (50 mM NaCl, 50 mM Tris⋅HCl, pH 7.8). Varying amounts of EFdA-TP were added, and the reactions were initiated by the addition of 6 mM MgCl2 in a final volume of 20 μL. The reactions were terminated after 3 h by adding an equal volume of 100% formamide containing traces of bromophenol blue. The products were resolved on a 15% polyacrylamide and 7 M urea gel and analyzed as previously described (15).
Acknowledgments
We thank Jay Nix of Advanced Light Source beamline 4.2.2 for support. The ALS is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy (DOE) under Contract DE-AC02-05CH11231. We also acknowledge the Advanced Photon Source. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US DOE Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract DE-AC02-06CH11357. This work was supported in part by grants from the National Institutes of Health (AI076119, AI099284, AI100890, AI120860, GM103368, and GM118012), Mizzou Advantage, and Trail to a Cure.
Footnotes
Conflict of interest statement: The authors declare that E.N.K. and H.M. are coinventors of EFdA.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 5J2M, 5J2N, 5J2P, and 5J2Q).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605223113/-/DCSupplemental.
References
- 1.Woo G, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: A systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139(4):1218–1229. doi: 10.1053/j.gastro.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 2.Michailidis E, et al. Antiviral therapies: Focus on hepatitis B reverse transcriptase. Int J Biochem Cell Biol. 2012;44(7):1060–1071. doi: 10.1016/j.biocel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawitz E, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 4.Gentile I, Borgia F, Buonomo AR, Castaldo G, Borgia G. A novel promising therapeutic option against hepatitis C virus: An oral nucleotide NS5B polymerase inhibitor sofosbuvir. Curr Med Chem. 2013;20(30):3733–3742. doi: 10.2174/09298673113209990178. [DOI] [PubMed] [Google Scholar]
- 5.Takamatsu Y, et al. 4′-Modified nucleoside analogs: Potent inhibitors active against entecavir-resistant hepatitis B virus. Hepatology. 2015;62(4):1024–1036. doi: 10.1002/hep.27962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamoto A, et al. 2′-Deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol. 2008;40(11):2410–2420. doi: 10.1016/j.biocel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Michailidis E, et al. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J Biol Chem. 2009;284(51):35681–35691. doi: 10.1074/jbc.M109.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohrui H. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine, a nucleoside reverse transcriptase inhibitor, is highly potent against all human immunodeficiency viruses type 1 and has low toxicity. Chem Rec. 2006;6(3):133–143. doi: 10.1002/tcr.20078. [DOI] [PubMed] [Google Scholar]
- 9.Grobler J, et al. Long-acting oral and parenteral dosing of MK-8591 for HIV treatment or prophylaxis. In Special Issue: Abstracts from the 2016 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2016;24(e-1):391. [Google Scholar]
- 10.Kageyama M, Nagasawa T, Yoshida M, Ohrui H, Kuwahara S. Enantioselective total synthesis of the potent anti-HIV nucleoside EFdA. Org Lett. 2011;13(19):5264–5266. doi: 10.1021/ol202116k. [DOI] [PubMed] [Google Scholar]
- 11.Kirby KA, et al. Effects of substitutions at the 4′ and 2 positions on the bioactivity of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2013;57(12):6254–6264. doi: 10.1128/AAC.01703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoddart CA, et al. Oral administration of the nucleoside EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque. Antimicrob Agents Chemother. 2015;59(7):4190–4198. doi: 10.1128/AAC.05036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hachiya A, et al. Evaluation of combinations of 4′-ethynyl-2-fluoro-2′-deoxyadenosine with clinically used antiretroviral drugs. Antimicrob Agents Chemother. 2013;57(9):4554–4558. doi: 10.1128/AAC.00283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphey-Corb M, et al. Response of simian immunodeficiency virus to the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine in vitro and in vivo. Antimicrob Agents Chemother. 2012;56(9):4707–4712. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michailidis E, et al. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014;289(35):24533–24548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh K, Marchand B, Kirby KA, Michailidis E, Sarafianos SG. Structural aspects of drug resistance and inhibition of HIV-1 reverse transcriptase. Viruses. 2010;2(2):606–638. doi: 10.3390/v2020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarafianos SG, et al. Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA. EMBO J. 2002;21(23):6614–6624. doi: 10.1093/emboj/cdf637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakata H, et al. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2007;51(8):2701–2708. doi: 10.1128/AAC.00277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Chopra R, Verdine GL, Harrison SC. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: Implications for drug resistance. Science. 1998;282(5394):1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 20.Sarafianos SG, et al. Trapping HIV-1 reverse transcriptase before and after translocation on DNA. J Biol Chem. 2003;278(18):16280–16288. doi: 10.1074/jbc.M212911200. [DOI] [PubMed] [Google Scholar]
- 21.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 25.Adams PD, et al. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 11):1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 26.Moriarty NW, Grosse-Kunstleve RW, Adams PD. Electronic ligand builder and optimization workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 10):1074–1080. doi: 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]