Abstract
22q11.2 deletion syndrome (22q11DS) is a neurogenetic disorder associated with elevated rates of developmental neuropsychiatric disorders and impaired executive function (EF). Disrupted brain structure-function relationships may underlie EF deficits in 22q11DS. We administered the Behavior Rating Inventory of Executive Function (BRIEF) to assess real-world EF in patients with 22q11DS and matched controls (n = 86; age 6-17 years), along with cognitive measures that tap behavioral regulation and metacognition aspects of EF. Using FreeSurfer's whole-brain vertex cortical thickness pipeline, we investigated brain structure-EF relationships in patients with 22q11DS and controls. Behaviorally, patients with 22q11DS were impaired on multiple EF measures. Right orbitofrontal cortical thickness showed a differential relationship between real-world EF in patients with 22q11DS and controls. We also observed a group difference in the relationship between behavioral regulation and metacognition measures with thickness of ventral and dorsolateral prefrontal regions, respectively. Our findings suggest that executive dysfunction characteristic of 22q11DS is underscored by altered prefrontal cortical structure.
Key Words: Neurogenetics, Cognitive function, Prefrontal cortex, Neurodevelopment, Copy number variation, Dopamine, Magnetic resonance imaging
Introduction
22q11.2 deletion syndrome (velocardiofacial/DiGeorge syndrome; 22q11DS) is one of the most prevalent chromosomal deletion syndromes known, affecting nearly 1 in 1,600 live births [1,2,3]. It results from a hemizygous 1.5- to 3-Mb deletion on the long arm of chromosome 22, and encompasses up to 60 known genes. Phenotypic expression is highly variable, ranging from congenital heart disease and palatal abnormalities to developmental delays and cognitive dysfunction, particularly in the areas of executive function (EF), attention, and arithmetic ability [4,5,6]. Affected children also have greatly elevated rates of developmental neuropsychiatric disorders relative to the general population, particularly attention deficit hyperactivity disorder (ADHD; 37%), anxiety disorder (36%), and autism spectrum disorders (ASD; 14-50%), as well as psychotic spectrum disorders (25-30%), typically diagnosed in adolescence or early adulthood [5,7,8,9,10,11]. Additionally, even those who do not meet full criteria for a clinical disorder exhibit dimensionally measured symptoms of impulsivity and inhibitory dyscontrol [12,13,14]. Although the precise mechanisms underlying the characteristic neurobehavioral manifestations of 22q11DS are not well understood, it is likely to result from reduced dosage of several genes involved in neurodevelopment within the 22q11.2 locus [15].
EF is a theoretical construct encompassing a range of higher-order cognitive processes such as motivation, planning, working memory, attention, and response inhibition. EF deficits are characteristic of psychiatric disorders prevalent in 22q11DS, such as schizophrenia and ADHD [16,17]. Notably, variability in EF has also been linked to allelic variation in genes known to play a role in dopaminergic metabolism and regulation [18,19]. Given that patients with 22q11DS are hemizygous for catechol-O-methyltransferase (COMT), a gene involved in prefrontal dopamine metabolism [20], and also have elevated rates of psychiatric conditions in which dopaminergic dysfunction is implicated [21,22], investigating dopamine-dependent frontally mediated neurocognitive functions and underlying brain structural alterations in this syndrome may provide valuable insights into gene-brain-behavior relationships. By utilizing a Research Domain Criteria Project (RDoC) approach to investigate endophenotypic differences in 22q11DS, we have the ability to cut across traditionally defined disorders and instead focus on dimensionally measured behavioral and neuroanatomic alterations that may underlie downstream psychiatric illnesses [23].
Patients with 22q11DS show alterations in brain morphology across cortical and subcortical regions, with prefrontal regions relatively preserved [24,25]. Specifically, relative to typically developing controls, 22q11DS patients show reduced cortical volume in occipitoparietal, temporal, and anterior cingulate cortices, and increases in cortical thickness in medial prefrontal regions as well as the insula [24,26,27,28]. While total brain volume is typically smaller or not different from controls [25,26,28], Jalbrzikowski et al. [24] found increased thickness of the bilateral medial orbitofrontal, middle, and inferior frontal cortices in youth with 22q11DS compared to controls. Mouse models of 22q11DS have shown altered neuronal frequency in layers II/III of the medial prefrontal cortex (PFC), a characteristic that relates directly to performance on tasks of EF [29]. The PFC, particularly the orbitofrontal region, is late to mature in healthy individuals, which is thought to be attributable to later synaptic pruning and/or myelination [30,31]. Refinement of prefrontal neuronal connections may relate to increasing EF capacities in early adulthood [32]. Specifically, dorsolateral regions of the PFC are believed to be primarily implicated in attentional and working memory functions [33], whereas ventromedial regions of the PFC are implicated in emotion regulation and impulse control [34]. Based on findings suggesting an abnormal trajectory of prefrontal cortical development in 22q11DS [35,36,37], it is hypothesized that delayed prefrontal maturation in 22q11DS may be relevant to impairments in various aspects of EF in this population.
The Behavior Rating Inventory of Executive Function (BRIEF) is a widely used and reliable parent report measure of real-world EF for children and adolescents [38]. Elevated BRIEF scores have been observed in patients with 22q11DS, reflecting greater executive dysfunction [39]. This dysfunction was even more pronounced in patients with 22q11DS who met clinical criteria for a psychiatric diagnosis (ADHD, major depressive disorder, and/or phobia) [40,41].
Notably, neuroanatomic lesion studies have demonstrated a link between executive dysfunction, as assessed by the BRIEF, and prefrontal cortical pathology. In particular, Løvstad et al. [42] found that lesions of the orbitofrontal cortex (OFC) were associated with elevated BRIEF scores (indicating greater executive dysfunction), and Anderson et al. [43] found that participants with right PFC lesions in particular showed greater day-to-day executive dysfunction, as measured by the BRIEF, as compared to those with lesions in the left PFC and controls.
It should be noted that real-world EF refers to everyday behaviors that rely on aspects of EF (e.g. goal-directed behavior, attention, impulsivity, working memory) [38]. Laboratory tests that tap into real-world EF remain limited in emulating natural, real-world experiences. For example, laboratory-based measures typically require simple responses to a single event, whereas daily life requires much more complex multitasking, which involves setting a series of goals and subgoals, and making decisions about prioritization [44]; thus, it is important to measure both aspects of EF.
The development of the frontal cortex in 22q11DS appears to be disrupted, based both on human studies and animal literature [29,36]. It is therefore plausible that abnormal prefrontal maturation may be related to characteristic impairments in EF seen in this population [36]. In healthy youth, prefrontal regions are relatively late to mature in that they continue to thin into late adolescence/early adulthood [30]; this pattern of increased thinning over this developmental period is associated with healthy cognitive development, particularly in relation to EF [45]. Relatedly, youth with ADHD have delayed rates of cortical maturation, particularly in prefrontal regions [46]. Given evidence for similarly delayed maturation of prefrontal regions in 22q11DS, we anticipated that brain-behavior relationships observed in typically developing youth would be disrupted in those with the 22q11.2 deletion.
To our knowledge, no studies to date have examined the neuroanatomic substrates of laboratory and real-world executive dysfunction in 22q11DS, nor how these brain structure-function relationships differ from those observed in typically developing youth. Here, we compared the relationship between real-world EF, as measured by the BRIEF, with structural neuroanatomic variation in patients with 22q11DS and demographically comparable healthy controls. We hypothesized that patients with 22q11DS would show an altered relationship between EF and cortical thickness, particularly in prefrontal regions implicated in EF. In order to dissect the relationship between multiply determined real-world EF and prefrontal neuroanatomy, we then investigated neurocognitive measures related to two major subcomponents of EF (behavioral regulation and metacognition), and how they may differentially relate to neuroanatomy in patients with 22q11DS and controls. We anticipated that impulsivity functions related to the behavioral regulation aspects of EF would be preferentially related to ventral and medial prefrontal regions [47], whereas attentional functions related to metacognition aspects of EF would be preferentially related to dorsal and lateral regions of the PFC [48]. Moreover, we anticipated that these relationships would be altered in patients with 22q11DS relative to typically developing controls, given their known deficits in EF and hypothesized delayed neuromaturational trajectory in the PFC.
Methods and Materials
Participants
Eighty-sixparticipants, aged 6-17 years, participated in the study: 43 with a molecularly confirmed diagnosis of 22q11.2 deletion syndrome, and 43 typically developing controls (table 1). We recruited patients with molecularly confirmed 22q11.2 deletions from two sources: (1) the population of patients followed by the UCLA and Children's Hospital, Los Angeles (CHLA) Pediatric Genetics, Allergy/Immunology and Craniofacial Clinics, and (2) local support groups (e.g. Velocardiofacial Education Foundation, 22q and You Support Network). Demographically comparable typically developing comparison subjects were recruited from the same communities as patients with 22q11DS, and were tested concurrently. This was accomplished by web-based advertisements about the research study, and by posting flyers and brochures at local schools, pediatric clinics, and other community sites. Exclusion criteria for all participants included: substance or alcohol abuse and/or dependence in the last 6 months and/or insufficient fluency in English. In addition, controls could not meet criteria for any major mental disorder, with the exception of ADHD or a single past episode of depression. Controls were also excluded if they had a neurological disorder, substance abuse/dependence, intellectual disability, and/or history of head injury with loss of consciousness. This information was collected through administration of the Structured Clinical Interview for DSM-IV Axis I Disorders, with an additional developmental disorders module [49]. All interviews were conducted by psychology PhD candidates who had undergone extensive training and reliability procedures under the supervision of the senior author (C.E.B.), as described in detail elsewhere [24,50,51].
Table 1.
Demographic characteristics of study participants
| 22q11DS (n = 43) | Controls (n = 43) | p value | |
|---|---|---|---|
| Age, years | 11.42 (3.53) | 10.74 (3.61) | 0.384 |
| Males/females, n | 23/20 | 24/19 | 0.829 |
| Education, years | 5.16 (3.59) | 4.77 (3.67) | 0.615 |
| Ethnicity, % Latino | 33 | 40 | 0.500 |
| Highest parental education, yearsa | 15.81 (2.45) | 15.56 (3.48) | 0.713 |
| Full scale IQ | 82.63 (13.87) | 107.51 (21.25) | <0.001*** |
| ADHD, nb | 18 (42%) | 2 (5%) | <0.001*** |
| Psychotic disorder, n | 1 (2%) | n.a. | |
| Subthreshold psychotic symptoms, nc | 9 (21%) | n.a. | |
| Autism spectrum disorder, n | 19 (23%) | n.a. | |
| Anxiety disorder, n | 21 (49%) | n.a. | |
| No psychiatric diagnosis, n | 9 (21%) | ||
| Psychotropic medication, n | 38/2/3d |
n.a. = Not applicable. Values for age, education, and full scale IQ are expressed as mean (SD).
p < 0.001.
Data unavailable for 6 participants.
Comorbidities: ADHD only (5), ASD only (4), anxiety disorder only (5), ADHD + ASD (3), ADHD + anxiety disorder (3), psychotic disorder + anxiety disorder (1), ASD + anxiety disorder (6), ADHD + ASD + anxiety disorder (6).
Assessed via the Structured Interview for Prodromal Syndromes (SIPS); interview conducted on youth aged 10 years and up.
None/antipsychotic/antidepressant.
All participants underwent verbal and written consent after study procedures were fully explained, and their parents or guardians also completed written consent. The UCLA Institutional Review Board approved all study procedures and informed consent documents.
BRIEF Assessment
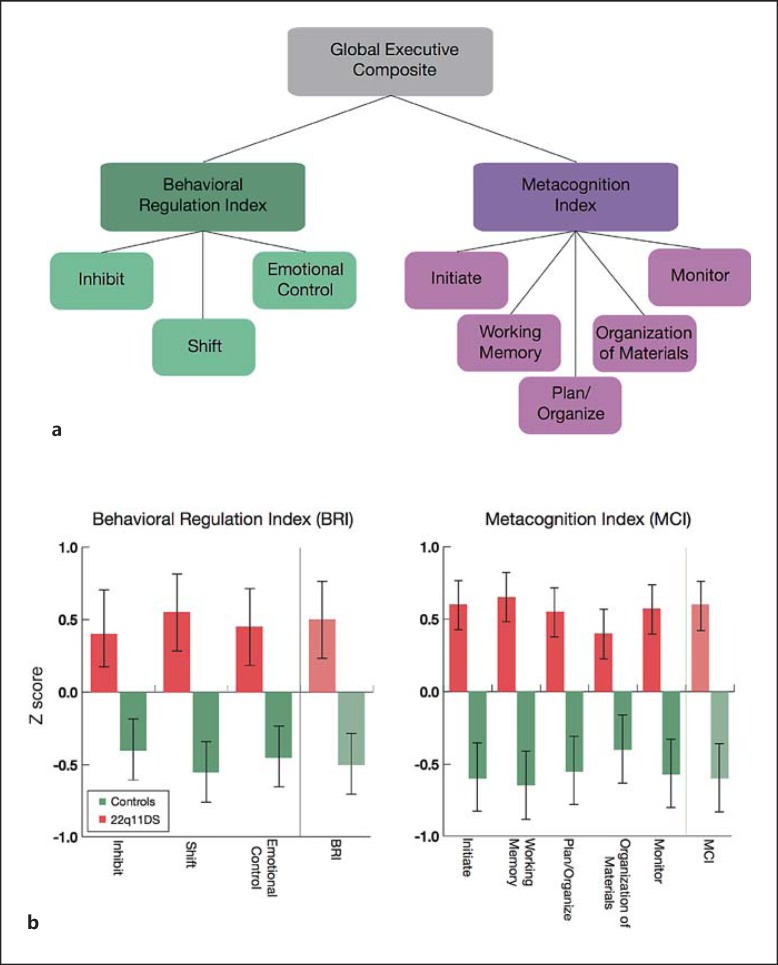
The BRIEF is a well-validated 86-item parent report questionnaire for children aged 5-17 years, which assesses real-world EF in the home and school environment [38]. Information is ascertained regarding how well a child is able to regulate his/her behavior, inhibit impulses, initiate projects, etc.; higher scores represent greater dysfunction. The Inhibit, Shift, and Emotional Control subscales sum to form the Behavioral Regulation Index, whereas the Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor subscales sum to form the Metacognition Index. These indices comprise the Global Executive Composite (GEC; fig. 1a).
Fig. 1.
a BRIEF subscales. b Scores across BRIEF subscales in youth with 22q11DS and age-matched typically developing controls. Z scores were created based on normative data [38]; higher scores indicate greater pathology. Patients with 22q11DS show greater dysfunction on all subscales (p < 0.001, Bonferroni corrected).
Laboratory-Based Measures of EF
The Continuous Performance Test-Identical Pairs (CPT-IP) [52] and Time Reproduction Task (TRT) by Barkley were employed as laboratory-based measures of subcomponents of EF. Both tasks were run on laptop computers in a quiet testing room.
The CPT-IP is a well-validated measure of sustained attention and working memory, in which sequential numeric stimuli are rapidly presented, and participants respond when two identical stimuli are presented in sequence. There are 450 total trials with varying numbers of digits. In target trials (20% total trials), two identical successive stimuli are shown; in catch trials (20% total trials), two similar successive stimuli are shown, and the other 60% of trials are random. We analyzed d-prime as our primary dependent variable, which is computed via the proportion of key presses on target trials to catch trials. Poor performance on this task has been shown to predict later development of psychotic spectrum disorder in at-risk individuals [53], and is a marker of genetic susceptibility to psychosis [54]. In patients with schizophrenia, impaired performance on this task is associated with structural and functional alterations in the medial PFC, inferior frontal gyrus (IFG), anterior cingulate cortex, postcentral gyrus, and thalamus [55,56,57]. CPT impairment is also a hallmark symptom of ADHD [58], and children with ADHD have shown abnormal prefrontal neural activity during performance of this task [59]. Because the CPT-IP was added to our battery at a later date, and is not validated in children under 10, the sample size is reduced for this measure.
The TRT is a well-established measure of one's ability to accurately reproduce a time interval in which two light bulbs appear simultaneously on the screen. The light bulb on the left is illuminated on for 4, 8, 12, 16, or 20 s, and then turned off. The subject is asked to reproduce that time with the light bulb on the right, by holding down the space bar for the same amount of time. Our primary dependent variable for the task is average absolute accuracy across all trial types. Interval timing, which occurs on the order of seconds to minutes, has been shown to depend on dopaminergic function [60,61]; thus, deficits in this domain may be an important intermediate phenotype for development of psychiatric disorders in which dopaminergic dysfunction is implicated [62]. Functional neuroimaging studies, as well as studies in animal models, have shown that interval time reproduction relies on frontostriatal-cerebellar neural circuitry [61,63,64,65].
MRI Data Acquisition
All scanning was carried out on an identical Siemens 3-tesla Tim Trio MRI at the UCLA Brain Mapping Center or at the Center for Cognitive Neuroscience (online suppl. table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000441979). Measures of brain structure were obtained with high-resolution structural MRI. Each scan began with a 10-min acquisition of standard images used for determining regional anatomy, including a sagittal localizer image (TR/TE = 500/10 ms, 192 × 256 matrix), a high-resolution T2-weighted axial image (TR/TE = 5,000/33 ms, 128 × 128 matrix, FOV = 200 × 200 mm), and a sagittal 1-mm3 T1-weighted image (MPRAGE, TR/TE = 2300/2.91, flip angle = 9°; slice thickness = 1.20 mm, 240 × 256 acquisition matrix).
MRI Analysis
The FreeSurfer image analysis suite (version 5.0, http://surfer.nmr.mgh.harvard.edu) surface-based processing pipeline was used to derive measures of cortical thickness. FreeSurfer is a well-validated processing protocol that has been previously described in detail [66,67]. In short, the following steps were taken in the processing stream: motion correction, transformation of images to standard Talairach space, intensity normalization, removal of non-brain tissue, segmentation of white matter and subcortical structures, and final segmentation of cortical surfaces. Final segmentation is based on both a subject-independent probabilistic atlas and subject-specific measured values. Raters (R.K.J., M.J., A.P.) blind to diagnosis visually inspected the scans at several points along the processing pipeline, and any errors were manually edited (see online suppl. information). We focused on cortical thickness as our primary neuroanatomic measure of interest due to the close link between changes in cortical thickness and cognitive development [68].
Statistical Analyses
Analyses of demographic and behavioral data were performed in SPSS software v21 (Chicago, Ill., USA). We conducted independent samples t tests for continuous variables and χ2 tests for categorical variables.
For the analyses of behavioral measures, we conducted univariate ANCOVAs in SPSS with each behavioral measure as the dependent variable, diagnosis as the between-group factor, and age and gender as covariates. In secondary analyses, we additionally covaried for IQ in order to determine whether global cognitive abilities accounted for differences in EF task performance.
For the whole-brain vertex-wise neuroanatomic analysis, all statistics were performed in FreeSurfer. A vertex refers to the spatial point of measurement resolution on the cortical surfaces derived in FreeSurfer. For each vertex, thickness measurements for each subject were mapped onto a common spherical coordinate system, and smoothed using a Gaussian kernel of 10 mm. In order to determine whether the relationship between regional cortical thickness and behavioral and neurocognitive measures differed between patients with 22q11DS and controls, we conducted whole-brain general linear model (GLM) analyses to test for measure by group interactions at each vertex across the whole brain. In all analyses, we covaried for age and scanner location. For each analysis, we first investigated the main effect of gender, and if no main effect was found, it was not included in the final models. To control for multiple comparisons, cluster correction was completed using Monte Carlo simulation with 10,000 iterations (vertex-wise threshold of p < 0.05), in order to determine the distribution of maximum cluster size under the null hypothesis, as described in Hagler et al. [69]. Right and left hemispheres were tested separately. We then conducted secondary analyses, which included adding IQ as a covariate into our models, and also investigated the main effect of psychiatric diagnoses (ADHD, anxiety disorder, and ASD) in three separate models so that we could investigate the main effects of each diagnosis separately.
Results
As shown in table 1, patients with 22q11DS and controls were well matched in terms of age, gender, ethnicity, and education. As expected, participants with 22q11DS had decreased IQ relative to controls. Further, patients with 22q11DS had elevated rates of neuropsychiatric disorders, consistent with rates observed in the literature [7]. We compared global measures of structural neuroanatomy in patients with 22q11DS and controls, and found no differences in intracranial volume or overall cortical thickness (online suppl. table 3). Finally, for all of the analyses described below, there was not a significant main effect of gender, and thus gender was not included as a covariate (online suppl. fig. 1).
BRIEF Results
Behavioral
Results of group comparisons on the BRIEF are provided in figure 1b. Patients with 22q11DS showed significantly elevated scores on each subscale, all of which survived Bonferroni correction for multiple comparisons (p < 0.001). In order to minimize the number of comparisons in our neuroanatomic analyses, we used the GEC score as the primary dependent measure.
Relationship with Cortical Thickness
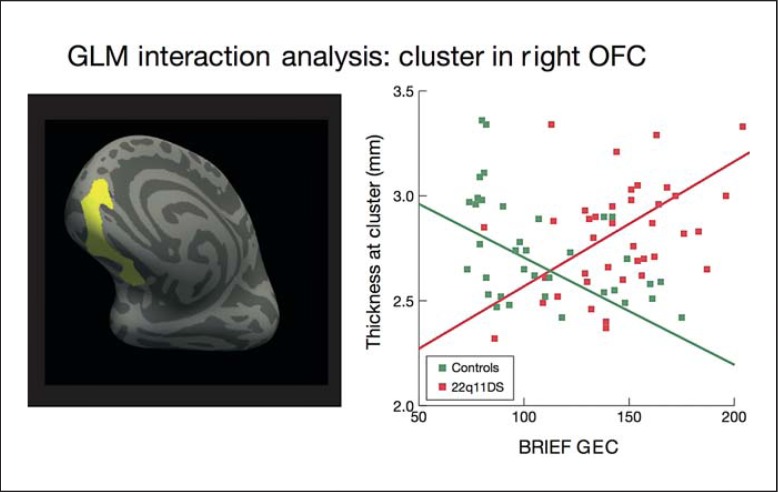
Whole-brain vertex-wise analysis revealed a significant BRIEF by group interaction cluster in the right OFC (p < 0.05, Monte Carlo correction; fig. 2), which survived correction for multiple comparisons. In this region, increased cortical thickness was associated with more severe executive dysfunction in patients with 22q11DS, whereas greater cortical thickness was associated with better EF in controls. We report the individual group correlations between thickness and BRIEF scores in online supplementary table 4.
Fig. 2.
Relationship between BRIEF GEC score and cortical thickness in patients with 22q11DS vs. controls. Analysis was conducted using an unbiased, whole-brain approach. There was a significant interaction between group and BRIEF GEC score in the right OFC (cluster size 65,020.8 mm2; p < 0.05, corrected), indicating that increased cortical thickness in the right OFC was associated with more severe real-world executive dysfunction (higher BRIEF score) in patients with 22q11DS, whereas thicker OFC was associated with better EF (lower BRIEF score) in controls. The left panel shows the location of the cluster, and the right panel displays the relationship between mean thickness of the cluster and BRIEF scores in patients with 22q11DS and controls.
CPT Results
Behavioral
On the CPT-IP, patients with 22q11DS had significantly lower d-prime scores (p < 0.001) than controls, indicating a higher proportion of hits on catch trials to hits on target trials, as well as a higher false alarm rate (p = 0.022; online suppl. table 1).
Relationship with Cortical Thickness
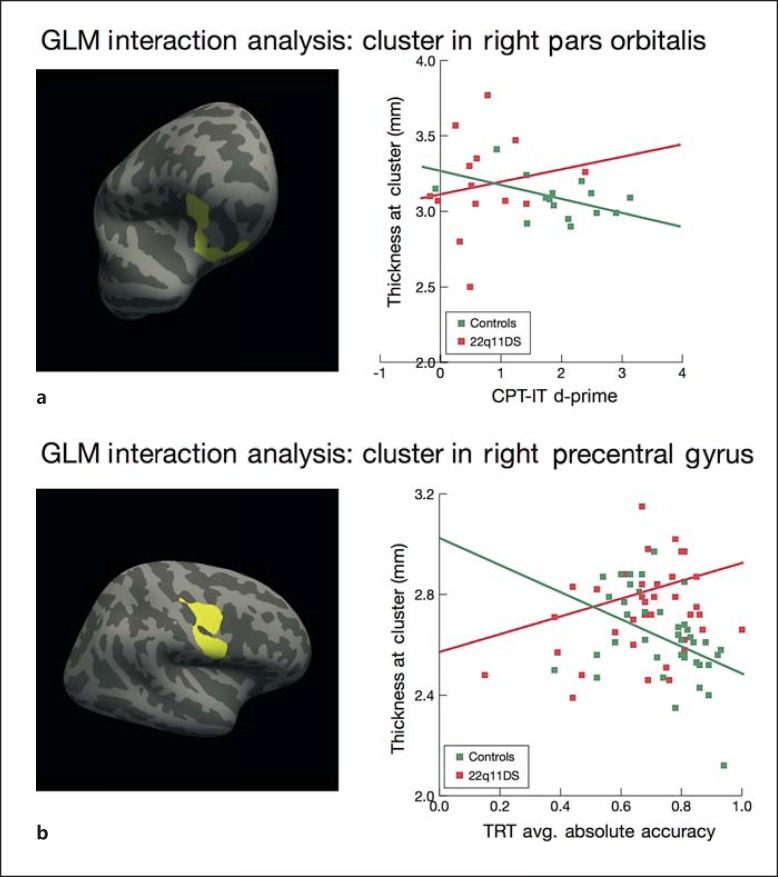
We found two significant CPT-IP by group clusters that survived correction for multiple comparisons, in the right pars orbitalis of the IFG and the superior temporal gyrus (p < 0.05, Monte Carlo correction; fig. 3a). In these regions, increased thickness was associated with better CPT-IP performance in patients with 22q11DS, whereas increased thickness was associated with worse performance in controls (p < 0.05). Given our focus on prefrontal cognitive functions, follow-up analyses focus on the larger cluster in the pars orbitalis.
Fig. 3.
a Relationship between performance on the CPT-IP and cortical thickness in patients with 22q11DS vs. controls. Whole-brain vertex-wise analysis revealed a significant group by task interaction in the right pars orbitalis region of the IFG (cluster size 919.46 mm2, p < 0.05, corrected), indicating that the relationship between CPT-IP performance, as assessed by d-prime, and thickness in this region differs between patients with 22q11DS and controls. In patients with 22q11DS, increased cortical thickness in the IFG was associated with higher d-prime scores, whereas in controls, increased cortical thickness in this region was associated with lower d-prime scores. b Relationship between performance on the TRT and cortical thickness in patients with 22q11DS vs. controls. Whole-brain vertex-wise analyses revealed a significant group by task interaction in the right precentral gyrus (cluster size 1,434.62 mm2, p < 0.05, corrected), indicating that the relationship between TRT and thickness in this region significantly differs between patients with 22q11DS and controls. In patients with 22q11DS, increased cortical thickness in the precentral gyrus was associated with higher accuracy scores, whereas in controls, increased cortical thickness in this region was associated with lower accuracy scores.
TRT Results
Behavioral
Patients with 22q11DS showed significantly reduced consistency of responses on the TRT relative to controls (p = 0.025), as well as a trend toward reduced accuracy (p = 0.065; online suppl. table 2).
Relationship with Cortical Thickness
We performed the same whole-brain GLM interaction analysis as described above in order to determine whether the relationship between performance on the TRT and cortical thickness differs between patients with 22q11DS and controls. We found a significant cluster that survived correction for multiple comparisons in the right precentral gyrus (p < 0.05, Monte Carlo correction; fig. 3b). In this region, increased thickness was associated with better time reproduction performance in patients with 22q11DS, whereas increased thickness was associated with worse performance in controls.
Secondary Analyses: IQ and Psychiatric Disorder
Including IQ as a covariate, in addition to age, group differences on the BRIEF GEC and CPT-IP remained significant (p < 0.001, p = 0.014, respectively), whereas the trend-level group difference for TRT accuracy was no longer present.
Due to the high prevalence of psychiatric illness in 22q11DS, we investigated effects of ADHD, anxiety disorder, and ASD, and did not find a significant effect of any of these diagnoses in our analyses.
Discussion
Here, we assessed brain-behavior relationships in patients with 22q11.2 deletion syndrome, a recurrent copy number variant associated with dysfunction in frontally mediated cognitive functions [4], dopaminergic dysregulation [70], and high rates of developmental neuropsychiatric disorders associated with executive dysfunction [7]. We found that patients with 22q11DS have significant executive dysfunction, as measured both by real-world behaviors and neurocognitive probes of distinct subcomponents of EF, and that altered prefrontal neuroanatomic structure appears to underlie these deficits. Notably, in a mouse model of 22q11DS, altered projection neuron frequency in layers II/III of the medial frontal cortex was observed, the severity of which predicted performance on a task of EF involving reversal learning [29].
Real-World EF
We found that patients with 22q11DS show deficits on multiple indices of real-world EF, as measured by the BRIEF, and these appear to be underscored by abnormal prefrontal neuroanatomy. The GEC score, which is comprised of both behavioral regulation and metacognition subscales, was significantly elevated in patients with 22q11DS relative to typically developing controls. Using an unbiased, whole-brain approach, we found that the relationship between GEC scores and cortical thickness uniquely differed between patients with 22q11DS and controls in the right OFC: in controls, increased right OFC thickness was associated with better EF, while in patients the opposite relationship was observed, adjusting for the effects of age. Notably, this finding is consistent with prior literature that found a relationship between right hemisphere OFC lesions and real-world EF, as measured by the BRIEF [42,43]. The OFC is critical for the formation of reward representation and decision-making [71,72], and is also involved in responding appropriately to social cues [73]. A recent structural MRI study found that OFC volume was correlated with one's tendency to conform to others' values [72]. The OFC is relatively late to mature in typically developing adolescents [30]. This maturational process may be altered in patients with 22q11DS [24,36], a possibility which warrants further investigation in prospective longitudinal studies.
Cognitive Tasks of EF
In order to deconstruct these broad deficits in behavioral manifestations of EF, we took a targeted approach to examine how performance on specific cognitive tasks tapping different subcomponents of EF relates to structural neuroanatomy in patients with 22q11DS relative to controls. Specifically, we were interested in how tasks indexing behavioral regulation and metacognition aspects of EF may differentially relate to subregions of the PFC between patients with 22q11DS and controls.
We investigated the CPT-IP as a measure of behavioral regulation and found that patients with 22q11DS had higher levels of impulsive responding, as indicated by increased false alarm rates and lower d-prime scores. Task performance was differentially related to cortical thickness in the right pars orbitalis of the IFG, such that increased thickness in controls was associated with worse performance, whereas increased thickness in patients with 22q11DS was associated with better CPT-IP performance. The IFG, particularly in the right hemisphere, has been implicated in impulsivity, in both lesion and functional neuroimaging studies [74]. Our finding is consistent with a study of patients with idiopathic schizophrenia that found a positive relationship between CPT-IP performance and IFG gray matter density in patients, but not in controls [55]. This result is also in line with a study of healthy children and adolescents that found an association between performance on a task of EF (Keep Track task) and thinner cortex in frontal regions, including the IFG [75].
Next, as a putative measure of metacognition (which is comprised of attention and working memory [38,76]), we used the TRT to investigate accuracy in the reproduction of time intervals. We found that controls tended to be more accurate in time reproduction, and that this measure was differentially related to thickness of the right precentral gyrus; specifically, in controls increased thickness was associated with reduced accuracy, whereas in patients with 22q11DS, increased thickness in this region was associated with better accuracy on the TRT. Interestingly, neural activity in the precentral gyrus has been linked to performance on attentional measures [77], and thinning of the precentral gyrus has been associated with poorer outcome in children with idiopathic ADHD [78], indicating that the attentional aspect of time reproduction may be driving the observed relationship.
Taken together, the cluster differentially associated with CPT-IP in patients with 22q11DS was located in a more ventral region of the PFC, whereas the cluster associated with TRT was located in a more dorsal and lateral region, suggesting that altered brain structure-function relationships in distinct regions of PFC may underlie deficits in task performance in metacognitive versus behavioral regulation aspects of EF in 22q11DS.
22q11DS presents an ideal context for a translational RDoC-based approach, given its well-characterized, homogeneous genetic etiology and phenotypic expression of neuropsychiatric symptoms that cut across multiple DSM categories [12,79]. Multilevel investigation of dimensionally measured traits relevant to EF deficits may better elucidate the pathophysiological mechanisms that underlie psychiatric phenotypes.
Altered Brain Structure - EF Relationships in 22q11DS
Prior neuroanatomic studies in 22q11DS have focused primarily on volume of cortical structures, but recent investigations have decomposed these differences into measures of cortical thickness and surface area, which are thought to be reliant on different genetic mechanisms and reflect distinct cortical characteristics [24,80,81]. Here, we focused on cortical thickness given prior studies indicating that it has a stronger relationship with global cognitive performance, relative to surface area measures [68].
Interestingly, all of the clusters in which we found an altered relationship between brain structure and EF performance in 22q11DS were located in the right hemisphere, suggesting there may be a differential hemispheric specialization of EF in 22q11DS. Of note, previous studies have found that lesions of the PFC in otherwise healthy individuals are related to EF, even more so in the right hemisphere than the left [43]. Children with right-sided prefrontal lesions have been shown to have worse performance than those with left prefrontal insults of similar severity on measures of EF and attention [82,83], suggesting that the right PFC may play an essential role in the development of basic attentional skills. Anderson et al. [43] have hypothesized that the right PFC may be particularly involved in mediating EFs during childhood development, whereas the left hemisphere is differentially involved in language tasks.
Additionally, a previous study on a partially overlapping sample found that prefrontal cortical thickness in the medial PFC, IFG, and middle frontal gyrus is increased in patients with 22q11DS relative to controls, and that this effect was stronger in the right hemisphere than the left [24]. This same study found that thickness of the right medial OFC was associated with psychotic symptoms in patients with 22q11DS, further supporting the functional role of this region in the neurobehavioral presentation of this syndrome.
It has been hypothesized that reduced dosage of 22q11.2 genes may compromise projection neuron integrity (layers II/III), specifically in frontal association cortices [29]. The precise mechanism is not fully understood, but it is plausible that abnormalities in cortical thickness may result from reduced dosage of genes involved in cortical development. These differences in cortical thickness between 22q11DS patients and controls are likely to play a role in the executive dysfunction characteristic of the disorder [84]. The relationship between cortical thickness and cognition in healthy individuals is complex, and a relatively thinner cortex may have different consequences, depending on the developmental context [45,72,78,85]. Findings in healthy individuals are sometimes contradictory, depending on age range and function investigated, but there is some evidence that thicker PFC is associated with better EF. In the case of real-word EF, a thicker cortex is advantageous for healthy individuals, and the opposite is seen in patients with 22q11DS. Thus, while the precise mechanisms underlying the altered relationships between cortical thickness and various components of executive cognition in 22q11DS are not yet known, we can speculate that they are related to abnormal development of the PFC.
It was particularly surprising that relationships with cortical thickness were in opposite directions for parent-reported versus performance-based assessments of EF. In particular, while we found that thinner OFC was associated with better real-world EF in patients with 22q11DS, the opposite pattern was found for the relationship with performance-based cognitive measures. This implies that various subregions of the PFC may play different roles in EF in patients with 22q11DS and controls, and that real-world and cognitive aspects of EF may be related to distinct neuroanatomic intermediate phenotypes. Given the variability in findings of associations between cortical thickness and EF across ages and specific domains, this inconsistency warrants further investigation in a larger, prospectively followed sample.
Study Limitations
Several limitations of this study should be noted. First, not all participants received all three measures of EF. Second, given the cross-sectional nature of the data, questions about distinct developmental trajectories of EF processes in 22q11DS could not be addressed. Prospective longitudinal studies are now underway in order to examine trajectories of both behavioral and neuroanatomic changes.
We investigated the effects of three major psychiatric diagnoses in 22q11DS: ADHD, anxiety disorder, and ASD. Given the relatively young sample included in the current study (age 6-18 years), only 1 of our patients had a psychotic disorder diagnosis at the time of assessment. While it is not yet known whether additional subjects in this cohort will develop a psychotic disorder, we are continuing to follow this cohort longitudinally; as such, baseline differences that are associated with subsequent development of psychotic illness can be investigated in future studies.
Future Directions
The genetic basis of these findings is unknown. In particular, given the role of dopamine in prefrontal ‘tuning’ [86], future studies should further explore the role of dopaminergic dysregulation in 22q11DS and how it may relate to these behavioral and neuroanatomic findings. It is widely theorized that the relationship between dopamine levels in the brain and PFC functioning follows an ‘inverted-U’ pattern such that levels that are too low or too high are maladaptive [87,88]. Investigating how genetic variation and resulting changes in gene expression may contribute to individual differences in brain structure, function, and downstream behavior in both patients with 22q11.2 deletions and animal models will help elucidate the neurobiological mechanisms relevant to behavioral pathology in 22q11DS and the broader population.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Supplementary Material
Supplementary data
References
- 1.Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, et al. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- 2.Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Bin Xu, et al. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2011;29:259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shashi V, Veerapandiyan A, Schoch K, Kwapil T, Keshavan M, Ip E, et al. Social skills and associated psychopathology in children with chromosome 22q11.2 deletion syndrome: implications for interventions. J Intellect Disabil Res. 2011;56:865–878. doi: 10.1111/j.1365-2788.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 4.Chow EWC, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr Res. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 6.Gothelf D, Schaer M, Eliez S. Genes, brain development and psychiatric phenotypes in velo-cardio-facial syndrome. Dev Disabil Res Rev. 2008;14:59–68. doi: 10.1002/ddrr.9. [DOI] [PubMed] [Google Scholar]
- 7.Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree M, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171:627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorstman JA, Morcus ME, Duijff SN, Klaassen PWJ, Heineman-de Boer JA, Beemer FA, et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 9.Green T, Gothelf D, Glaser B, Debbané M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 10.Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Fung W, McEvilly R, Fong J. Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome. Am J Psychiatry. 2010;167:998. doi: 10.1176/appi.ajp.2010.09101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry. 2014;75:351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antshel KM, Hendricks K, Shprintzen R, Fremont W, Higgins AM, Faraone SV, et al. The longitudinal course of attention deficit/hyperactivity disorder in velo-cardio-facial syndrome. J Pediatr. 2013;163:187–193. doi: 10.1016/j.jpeds.2012.12.026. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gothelf D, Hoeft F, Hinard C, Hallmayer JF, Van Dover Stoecker J, Antonarakis SE, et al. Abnormal cortical activation during response inhibition in 22q11.2 deletion syndrome. Hum Brain Mapp. 2007;28:533–542. doi: 10.1002/hbm.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meechan DW, Maynard TM, Tucker ES, LaMantia AS. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: Patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci. 2011;29:283–294. doi: 10.1016/j.ijdevneu.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetta NDJ, Hurks PPM, Krabbendam L, Jolles J. Interference control, working memory, concept shifting, and verbal fluency in adults with attention-deficit/hyperactivity disorder (ADHD) Neuropsychology. 2008;22:74–84. doi: 10.1037/0894-4105.22.1.74. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2009;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes JJM, Dean AJ, Nandam LS, O'Connell RG, Bellgrove MA. The molecular genetics of executive function: role of monoamine system genes. Biol Psychiatry. 2011;69:e127–e143. doi: 10.1016/j.biopsych.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–e157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Casey BJ, Craddock N, Cuthbert BN, Hyman SE, Lee FS, Ressler KJ. DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci. 2013;14:810–814. doi: 10.1038/nrn3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, et al. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: relationship with psychotic symptoms. Neuroimage Clin. 2013;3:405–415. doi: 10.1016/j.nicl.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome) Schizophr Res. 2009;115:173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Kates WR, Bansal R, Fremont W, Antshel KM, Hao X, Higgins AM, et al. Mapping cortical morphology in youth with velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2011;50:272–282. doi: 10.1016/j.jaac.2010.12.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bearden CE, van Erp TGM, Dutton RA, Tran H, Zimmermann L, Sun D, et al. Mapping cortical thickness in children with 22q11.2 deletions. Cerebral Cortex. 2007;17:1889–1898. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry. 2000;157:409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- 29.Meechan DW, Rutz HLH, Fralish MS, Maynard TM, Rothblat LA, LaMantia AS. Cognitive ability is associated with altered medial frontal cortical circuits in the LgDel mouse model of 22q11.2DS. Cereb Cortex. 2015;25:1143–1151. doi: 10.1093/cercor/bht308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Mills KL, Goddings A-L, Clasen LS, Giedd JN, Blakemore S-J. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 33.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 34.Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. Ventromedial prefrontal cortex encodes emotional value. J Neurosci. 2013;33:11032–11039. doi: 10.1523/JNEUROSCI.4317-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shashi V, Veerapandiyan A, Keshavan MS, Zapadka M, Schoch K, Kwapil TR, et al. Altered development of the dorsolateral prefrontal cortex in chromosome 22q11.2 deletion syndrome: an in vivo proton spectroscopy study. Biol Psychiatry. 2012;72:684–691. doi: 10.1016/j.biopsych.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaer M, Debbané M, Cuadra MB, Ottet M-C, Glaser B, Thiran J-P, et al. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr Res. 2009;115:182–190. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Kates WR, Krauss BR, Abdulsabur N, Colgan D, Antshel KM, Higgins AM, et al. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11.2 deletion syndrome) Neuropsychologia. 2007;45:2863–2873. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 39.Lajiness-O'Neill R, Beaulieu I, Asamoah A, Titus JB, Bawle E, Ahmad S, et al. The neuropsychological phenotype of velocardiofacial syndrome (VCFS): relationship to psychopathology. Arch Clin Neuropsychol. 2006;21:175–184. doi: 10.1016/j.acn.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Antshel KM, Conchelos J, Lanzetta G, Fremont W, Kates WR. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome) Psychiatry Res. 2005;138:235–245. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Antshel KM, Fremont W, Roizen NJ, Shprintzen, Higgins AM, Dhamoon A, et al. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45:596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- 42.Løvstad M, Funderud I, Endestad T, Due-Tønnessen P, Meling TR, Lindgren M, et al. Executive functions after orbital or lateral prefrontal lesions: neuropsychological profiles and self-reported executive functions in everyday living. Brain Inj. 2012;26:1586–1598. doi: 10.3109/02699052.2012.698787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson V, Jacobs R, Harvey AS. Prefrontal lesions and attentional skills in childhood. J Int Neuropsychol Soc. 2005;11:817–831. doi: 10.1017/s1355617705051052. [DOI] [PubMed] [Google Scholar]
- 44.Chan RCK, Shum D, Toulopoulou T, Chen EYH. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev Cogn Neurosci. 2013;6:61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, et al. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2012;26:479–487. doi: 10.1007/s10548-012-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming SM, Dolan RJ. The neural basis of metacognitive ability. Philos Trans R Soc Lond B Biol Sci. 2012;367:1338–1349. doi: 10.1098/rstb.2011.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.First MB, Spitzer RL, Gibbon M, Williams J, Benjamin LS. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) New York: Biometrics Research; 1994. [Google Scholar]
- 50.Jalbrzikowski M, Carter C, Senturk D, Chow C, Hopkins JM, Green MF, et al. Social cognition in 22q11. 2 microdeletion syndrome: relevance to psychosis? Schizophr Res. 2012;142:99–107. doi: 10.1016/j.schres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho JS, Radoeva PD, Jalbrzikowski M, Chow C, Hopkins J, Tran W-C, et al. Deficits in mental state attributions in individuals with 22q11.2 deletion syndrome (velo-cardio-facial syndrome) Autism Res. 2012;5:407–418. doi: 10.1002/aur.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keilp JG, Herrera J, Stritzke P, Cornblatt BA. The continuous performance test, identical pairs version (CPT-IP): III. Brain functioning during performance of numbers and shapes subtasks. Psychiatry Res. 1997;74:35–45. doi: 10.1016/s0925-4927(96)02881-8. [DOI] [PubMed] [Google Scholar]
- 53.Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am J Med Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- 54.Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry. 1998;155:1214–1220. doi: 10.1176/ajp.155.9.1214. [DOI] [PubMed] [Google Scholar]
- 55.Salgado-Pineda P, Baeza I, Pérez-Gómez M, Vendrell P, Junqué C, Bargalló N, et al. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19:365–375. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 56.Salgado-Pineda P, Junqué C, Vendrell P, Baeza I, Bargalló N, Falcón C, et al. Decreased cerebral activation during CPT performance. Neuroimage. 2004;21:840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 57.Volz H, Gaser C, Häger F, Rzanny R, Pönisch J, Mentzel H, et al. Decreased frontal activation in schizophrenics during stimulation with the continuous performance test - a functional magnetic resonance imaging study. Eur Psychiatry. 1999;14:17–24. doi: 10.1016/s0924-9338(99)80711-1. [DOI] [PubMed] [Google Scholar]
- 58.Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- 59.Wang S, Yang Y, Xing W, Chen J, Liu C, Luo X. Altered neural circuits related to sustained attention and executive control in children with ADHD: an event-related fMRI study. Clin Neurophysiol. 2013;124:2181–2190. doi: 10.1016/j.clinph.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Gómez J, Marín-Méndez JJ, Molero P, Atakan Z, Ortuño F. Time perception networks and cognition in schizophrenia. A review and a proposal. Psychiatry Res. 2014;220:737–744. doi: 10.1016/j.psychres.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 61.Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62:1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 63.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 64.Radua J, del Pozo NO, Gómez J, Guillen-Grima F, Ortuño F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia. 2014;58:14–22. doi: 10.1016/j.neuropsychologia.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 66.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 67.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 68.Burgaleta M, Johnson W, Waber DP, Colom R, Karama S. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. Neuroimage. 2014;84:810–819. doi: 10.1016/j.neuroimage.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagler DJ, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boot E, Booij J, Zinkstok J, Abeling N, de Haan L, Baas F, et al. Disrupted dopaminergic neurotransmission in 22q11 deletion syndrome. Neuropsychopharmacology. 2007;33:1252–1258. doi: 10.1038/sj.npp.1301508. [DOI] [PubMed] [Google Scholar]
- 71.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 72.Campbell-Meiklejohn DK, Kanai R, Bahrami B, Bach DR, Dolan RJ, Roepstorff A, et al. Structure of orbitofrontal cortex predicts social influence. Curr Biol. 2012;22:R123–R124. doi: 10.1016/j.cub.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hornak J. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 74.Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- 75.Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48:2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002;8:249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- 77.Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 79.Cuthbert BN. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology. 2014;51:1205–1206. doi: 10.1111/psyp.12342. [DOI] [PubMed] [Google Scholar]
- 80.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- 82.Anderson V, Levin H, Jacobs R. Developmental and acquired lesions of the frontal lobes in children: neuropsychological implications. In: Stuss D, Knight R, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 504–527. [Google Scholar]
- 83.Vargha-Khadem F, Polkey CE. A review of cognitive outcome after hemidecortication in humans. In: Rose FD, Johnson DA, editors. Recovery from Brain Damage. Boston: Springer; 1992. pp. 137–151. [DOI] [PubMed] [Google Scholar]
- 84.Meechan DW, Maynard TM, Fernandez A, Karpinski BA, Rothblat LA, LaMantia AS. Modeling a model: mouse genetics, 22q11.2 deletion syndrome, and disorders of cortical circuit development. Prog Neurobiol. 2015;130:1–29. doi: 10.1016/j.pneurobio.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Bäckman L, Li S-C, et al. Cortical thickness is linked to executive functioning in adulthood and aging. Hum Brain Mapp. 2012;33:1607–1620. doi: 10.1002/hbm.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 87.Cai JX, Arnsten AF. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283:183–189. [PubMed] [Google Scholar]
- 88.Goldman-Rakic PS, Muly EC, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data