Abstract
Despite the recent progress in psychiatric genetics, very few studies have focused on genetic risk factors in glial cells that, compared to neurons, can manifest different molecular pathologies underlying psychiatric disorders. In order to address this issue, we studied the effects of mutant disrupted in schizophrenia 1 (DISC1), a genetic risk factor for schizophrenia, in cultured primary neurons and astrocytes using an unbiased mass spectrometry-based proteomic approach. We found that selective expression of mutant DISC1 in neurons affects a wide variety of proteins predominantly involved in neuronal development (e.g., SOX1) and vesicular transport (Rab proteins), whereas selective expression of mutant DISC1 in astrocytes produces changes in the levels of mitochondrial (GDPM), nuclear (TMM43) and cell adhesion (ECM2) proteins. The present study demonstrates that DISC1 variants can perturb distinct molecular pathways in a cell type-specific fashion to contribute to psychiatric disorders through heterogenic effects in diverse brain cells.
Key Words: Biomarkers, Psychiatric disorders, Astrocytes, Neurons, DISC1, Proteomics
Introduction
Recent progress in psychiatric genetics has advanced our understanding of how genetic risk factors affect neurodevelopment and adult brain function [1]. However, the majority of studies have focused on neurons, and very few attempts have been made to elucidate the glial function of genes associated with psychiatric disorders despite the increasing evidence of the key roles of glial cells in mental disease [2,3]. Therefore, we began to study the roles of disrupted in schizophrenia 1 (DISC1) in both neurons and astrocytes, as both types of cells express DISC1 [4,5,6]. Previously, we reported that DISC1 binds to and stabilizes serine racemase, an enzyme that converts l-serine into d-serine. We found that expression of C terminus-truncated DISC1 (mutant DISC1), a putative product of chromosomal translocation in a Scottish pedigree [7], disrupts this binding property in a dominant-negative manner, resulting in increased degradation of serine racemase via ubiquitination and decreased production of d-serine [5]. Notably, these changes were observed in astrocytes but not in neurons that expressed mutant DISC1 [5,8], suggesting that mutant DISC1 may exert cell type-specific effects.
DISC1 is a multifunctional protein that is involved in various neuronal functions [9]. It is therefore conceivable that it may also partake in multiple molecular pathways in astrocytes and may have different interacting partners in different cell types. As a result, DISC1 variants could affect distinct pathways in neurons, as compared to glial cells, leading to cell type-specific molecular alterations. To investigate this hypothesis, we compared the effects of mutant DISC1 between neurons and astrocytes using an unbiased proteomic approach. Specifically, we performed a proteomic profiling study in which the effects of mutant DISC1 were evaluated using a label-free non-hypothesis-driven approach. Liquid chromatography (LC) combined with mass spectrometry (LC-MSE) was employed as an unbiased screening method for detecting changes in the proteome of primary neurons or astrocytes that express mutant DISC1.
Our findings demonstrate that selective expression of mutant DISC1 in primary neurons altered the expression of proteins predominantly involved in neuronal development and vesicular transport, whereas selective expression of mutant DISC1 in primary astrocytes was associated with changes in the expression of mitochondrial and nuclear proteins as well as cell adhesion proteins. Taken together, these data suggest that mutant DISC1 affects different molecular pathways in neurons and astrocytes, which is consistent with the idea that genetic variants can produce distinct molecular alterations in a cell-specific manner.
Materials and Methods
We expressed C terminus-truncated human DISC1 (mutant DISC1), which is a putative product of translocation in the original Scottish family [7]. In order to express mutant DISC1 in neurons or astrocytes, we mated either single hemizygous transgenic CaMKII-tTA (for neurons) or GFAP-tTA mice (for astrocytes, line 110) with single homozygous transgenic TRE-mutant DISC1 mice (line 1001) as described previously [5,10]. All mice were on the C57BL6/J background. This mating protocol produces litters that have about 50% of single transgenic mutant DISC1 mice that do not express mutant DISC1 (controls) and ∼50% of double transgenic mice that express mutant DISC1 (mutants) in neurons or astrocytes.
Primary Cultures
Primary neuron or astrocyte cultures were prepared from mouse embryos (embryonic days 16-18) as previously described [5,11,12]. Briefly, meninges-free cortices were isolated, trypsinized and mechanically dissociated by passing them through fire-polished Pasteur pipettes. Washed cells were plated onto poly-l-lysine (0.05 mg/ml) and laminin (0.1 mg/ml)-coated tissue culture coverslips (200,000 cells/cm2) in Neurobasal-A medium supplemented with 0.5 mm l-glutamine and B27 and penicillin-streptomycin supplements. On the 3rd day of culture, 2 μm arabinosylcytosine was added to the culture medium to inhibit the growth of nonneuronal cells. The composition of major cell types in the cultures was estimated by visual counting of cells immunostained with anti-MAP2 (neurons) or anti-GFAP (astrocytes) antibodies. Neuron-rich cultures contained >95% neurons and <4% glia.
Primary astrocytes were prepared from forebrains of neonatal (postnatal days 1-2) mice. To prepare pure astroglia, mixed glial cultures were washed every other day with fresh medium to remove loosely attached microglial cells and inhibit microglia growth. After 2-3 weeks in culture, the glial cultures were passaged (1:4 split ratio) using trypsin (0.25%) and allowed to form a confluent monolayer for 3-7 days. This monolayer consisted of ∼90-97% astrocytes.
We generated the following sets of primary cell samples: control neurons (no expression), to be compared with mutant DISC1 neurons (expression of mutant DISC1), and control astrocytes (no expression), to be compared with mutant DISC1 astrocytes (expression of DISC1). Both the control and the mutant samples were derived from the same litter to minimize potential confounding litter effects. We used 4 mutant DISC1 and 5 control samples derived from primary astrocytes as well as 12 mutant DISC1 and 8 control samples derived from primary neurons for the proteomic studies. We used 5-7 samples per group for Western blotting studies.
Sample Preparation
All biochemicals and reagents were obtained from Sigma-Aldrich (Poole, UK) unless specified otherwise. Protein extraction from cells was performed by addition of fractionation buffer (7 m urea, 2 m thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 2% ASB14 and 70 mm dithiothreitol), followed by sonication for 10 s using a Branson Sonifier 150 (Thistle Scientific, Glasgow, UK) and vortexing for 30 min at 4°C. The homogenates were centrifuged for 3 min at 17,000 g and the supernatants were collected for precipitation of the proteins using 4:1 volumes of ice-cold acetone. The resulting pellets were suspended in 100 µl of 50 mm NH4HCO3 (pH 8.0). Sulfhydryl groups on the proteins were reduced by incubation with 100 mm dithiothreitol for 30 min at 60°C and alkylated with 200 mm iodoacetamide for 30 min at 37°C. The proteins were cleaved into peptides by incubation with 1:50 (trypsin:protein) porcine trypsin (Promega, Madison, Wis., USA) for 17 h at 37°C and stopped after 16 h by addition of 0.80 µl of 8.8 m HCl. The samples were stored at −80°C. Prior to the MS analyses, 0.1% formic acid was added to a final concentration of 0.12 µg/µl protein.
Label-Free LC-MSE Profiling
LC-MSE was used as a first step for the identification of changed proteins between the DISC1 model and the wild type. Quality control (QC) samples were generated using equal aliquots of all samples for assessing MS performance. The cell samples and QCs (0.6 µg protein) were analyzed in duplicate using a splitless nano ultrahigh-performance LC system (10K psi nanoACQUITY; Waters Corporation, Milford, Mass., USA). The LC comprised a 0.18 × 20 mm C18 trap column (5 µm particle size) and a 0.075 × 200 mm analytical C18 BEH nanocolumn (17 µm particle size). The separation buffers were H2O + 0.1% formic acid (buffer A) and acetonitrile + 0.1% formic acid (buffer B). Samples were desalted for 2 min with 100% buffer A, which was followed by a 2-step gradient at a flow rate of 300 nl/min over 133 min. The analytical LC column was coupled online to a 7-cm nanoESI emitter (10-µm tip; New Objective, Woburn, Mass., USA) on a quadrupole time-of-flight (Q-TOF) Premier mass spectrometer (Waters Corporation), and data were acquired in an alternate scanning, data-independent acquisition mode (MSE). During each run, 500 fmol/µl [Glu1]-fibrinopeptide B was infused every 30 s using the LockSpray to maintain mass accuracy. The mass spectrometer was operated in the V mode, and analyses were performed using the positive nanoESI ion mode. Low collision energy (MS) generated information about intact precursor ions (5 eV), while high collision energy (MSE) provided information about peptide fragments (ramped from 17 to 40 eV). The cycling time of the low and the high energy was 0.6 s, and the mass range was 50-1,990 Da.
Data Analysis
LC-MSE data were processed using the ProteinLynx Global Server v2.5 (Waters Corporation) and the Rosetta Elucidator v3.3 (Rosetta Biosoftware, Seattle, Wash., USA) for time and mass/charge alignment of the MS data as described previously [13]. The Mus musculus complete proteome FASTA sequence Integr8 database was used for the assignment of protein identities. Quantitative peptide measurements for each replicate were normalized against the total ion volume of all deconvoluted spectra. The criteria for protein identification were set to ≥3 fragment ions per peptide as well as ≥7 fragment ions per protein and ≥2 peptides per protein. The data were also searched against a randomized decoy database, which was created using the original database, thus conserving amino acid frequencies. Only peptides that were present in all samples of each treatment group were considered for further analysis.
The final part of data processing was a principal component analysis (SIMCA P+ v2.12; Umetrics, Malmö, Sweden), which is used to identify unwanted variability due to sample nonhomogeneity or inconsistent manipulation during the preparation and analytical stages. No outliers were detected by principal component analysis when testing for outlying samples. Subsequently, the LC-MSE data were analyzed in the R statistical programming language (v2.15.3; R 2013) using the MS stats package (Purdue University, West Lafayette, Ind., USA), which provides wrapper functions to simplify the fitting of the linear mixed-effects models. The data of the identified proteins were log2 transformed to stabilize the variance, and normalized for the intensities of the peaks. For this label-free experiment, constant normalization was performed based on endogenous signals across runs among all proteins. Peptide transitions were excluded based upon a between-run interference score <0.8, where the score was based on the correlation between the mean of peptides by run and peptide transition intensity [14]. Visualization of the processed data occurred using profile plots, QC plots and condition plots. Profile plots are capable of identifying the potential source of variation of each protein. The exploratory QC plots can reveal any systematic sample run variation of transition intensities between the runs, which are shown as box plots. The condition plots show the systematic difference between conditions. Analysis was performed using linear mixed models to detect differentially abundant proteins between groups. The interference for biological replicates and technical replicates was used in expanded scope, which expands the conclusion from the model to the population of biological units [15]. To test the model assumptions, diagnostic plots were generated, such as residual plots to check the assumption of a constant variance and normal quantile-quantile plots to indicate whether the errors were well approximated by a normal distribution. These analyses resulted in p values, q-values (allowing for multiple testing) and ratio changes (mutant DISC1/control). As the study was underpowered, we used a 10% change in protein abundance and a p value of 0.05 as a threshold to identify proteins that will be considered for further analysis. The number of mice used to derive the cells from each group was not sufficient to reach a significant p value of 0.05 after multiple testing. An analysis of the results of Western blotting was made using Student's t test, with p < 0.05 being set as a significance level.
Western Blotting
Selection of the proteins detected by LC-MSE was based on functional significance, disease relevance, previous associations with DISC1 as well as the availability and quality of specific antibodies. The proteins chosen for validation were: Rab37 (Abcam, ab124413; 1:500) and SOX1 (R&D, MAB3369; 1:1,000) for neurons and TMM43 (Abcam, ab184164; 1:500), GDPM (Abcam, ab188585; 1:1,000) and ECM2 (Proteintech Group, 21376-1-AP; 1:500) for astrocytes. Primary cells were cultured for 2 weeks and were collected, frozen on dry ice and kept at −80°C until used. The optical density of the protein bands was normalized to the optical density of the loading control (β-actin, 1:20,000; Sigma-Aldrich, St. Louis, Mo., USA). Densitometry was performed with the help of ImageJ (NIH, Bethesda, Md., USA). All samples were run in triplicate, and the mean values were used for statistical analyses.
Results
Cell Type-Specific Proteomic Profile in Mutant DISC1 Neurons and Astrocytes
LC-MSE profiling was carried out to determine the effects of mutant DISC1 protein expression on proteomes of cortical neurons and astrocytes. After data filtering, this analysis resulted in the identification of a total of 600 proteins in neurons and 520 proteins in astrocytes. Following data quality assessment, 46 proteins were significantly altered in neurons (table 1) and 10 proteins in astrocytes (table 2). In the neurons, the main changes were detected in proteins involved in neuronal development (SOX1, HTRA3, TBC24, DCX, RAC1 and KCC2B) and transport (RAB37, RAB35, ACTZ, STX1B, SYT1 and RAB3B). In astrocytes, the main alterations were found in proteins involved in nuclear transport, mitochondrial function and the extracellular matrix.
Table 1.
Identified proteins in control or mutant DISC1 primary neurons
| Function | Protein | Ratio mutant/control | p value |
|---|---|---|---|
| Transport | Ras-related protein Rab-37 (RAB37) | 2.68 | 0.0104 |
| Ras-related protein Rab-3B (RAB3B) | 0.43 | 0.0192 | |
| Pleckstrin homology domain-containing family A member 8 (PKHA8) | 3.73 | 0.0207 | |
| Fatty acid-binding protein, brain (FABP7) | 0.29 | 0.0289 | |
| Ras-related protein Rab-35 (RAB35) | 1.46 | 0.0319 | |
| Syntaxin-1B (STX1B) | 0.53 | 0.0330 | |
| Transmembrane protein 106B (T106B) | 4.94 | 0.0353 | |
| Synaptotagmin-1 (SYT1) | 0.46 | 0.0378 | |
| Alpha-centractin (ACTZ) | 0.54 | 0.0436 | |
| Metabolism | Electron transfer flavoprotein subunit beta (ETFB) | 1.84 | 0.0032 |
| Pyruvate kinase PKM (KPYM) | 0.71 | 0.0086 | |
| Transketolase (TKT) | 0.43 | 0.0101 | |
| V-type proton ATPase 116 kDa subunit a isoform 1 (VPP1) | 0.68 | 0.0102 | |
| Peroxisomal NADH pyrophosphatase NUDT12 (NUD12) | 2.79 | 0.0199 | |
| V-type proton ATPase subunit d 1 (VA0D1) | 0.41 | 0.0208 | |
| Fatty acid-binding protein, epidermal (FABP5) | 0.54 | 0.0376 | |
| Mitochondrial glutamate carrier 1 (GHC1) | 0.56 | 0.0415 | |
| Perilipin-2 (PLIN2) | 2.78 | 0.0486 | |
| Transcription/translation | DNA polymerase epsilon subunit 2 (DPOE2) | 11.09 | 0.0067 |
| Transcription factor SOX-1 (SOX1) | 4.64 | 0.0191 | |
| Lethal(3)malignant brain tumor-like protein 4 (LMBL4) | 0.49 | 0.0360 | |
| Transformer-2 protein homolog beta (TRA2B) | 4.46 | 0.0456 | |
| 3-Hydroxyacyl-CoA dehydrogenase type-2 (HCD2) | 1.71 | 0.0468 | |
| Development | Centrosomal protein of 63 kDa (CEP63) | 0.39 | 0.0162 |
| Transcription factor SOX-1 (SOX1) | 4.64 | 0.0191 | |
| Neuronal migration protein doublecortin (DCX) | 0.58 | 0.0267 | |
| ADP-ribosylation factor-like protein 8B (ARL8B) | 2.46 | 0.0308 | |
| TBC1 domain family member 24 (TBC24) | 2.79 | 0.0399 | |
| Calcium/calmodulin-dependent protein kinase II subunit beta (KCC2B) | 0.47 | 0.0483 | |
| Cell-cell interaction | Ras-related C3 botulinum toxin substrate 1 (RAC1) | 0.51 | 0.0187 |
| Sodium/potassium-transporting ATPase subunit beta-2 (AT1B2) | 0.42 | 0.0282 | |
| Thy-1 membrane glycoprotein (THY1) | 0.55 | 0.0391 | |
| Cytoskeleton | Actin, cytoplasmic 2 (ACTG) | 1.40 | 0.0112 |
| Tubulin beta-4B chain (TBB4B) | 0.49 | 0.0235 | |
| Tubulin beta-4A chain (TBB4A) | 0.54 | 0.0296 | |
| Tubulin beta-1 chain (TBB1) | 1.70 | 0.0342 | |
| Apoptosis | WD repeat-containing protein 92 (WDR92) | 3.04 | 0.0313 |
| RanBP-type and C3HC4-type zinc finger-containing protein 1 (HOIL1) | 3.35 | 0.0445 | |
| Extracellular matrix | Serine protease HTRA3 (HTRA3) | 2.82 | 0.0381 |
| Signaling | Guanine nucleotide-binding protein G(o) subunit alpha (GNAO) | 0.53 | 0.0149 |
| Unknown | PNMA-like protein 1 (PNML1) | 11.26 | 0.0053 |
| 14-3-3 protein epsilon (1433E) | 0.50 | 0.0159 | |
| Lymphocyte antigen 6H (LY6H) | 0.32 | 0.0169 | |
| Transmembrane protease serine 7 (TMPS7) | 4.32 | 0.0232 | |
| Leucine-rich repeat-containing protein 71 (LRC71) | 3.53 | 0.0333 | |
| Keratin, type II cuticular Hb6 (KRT86) | 3.02 | 0.0338 | |
| Prenylcysteine oxidase (PCYOX) | 0.40 | 0.0405 | |
The table includes UniProt IDs, ratios (calculated based on the average) and p values. The adjusted p values for the neurons were 0.4975. Significant proteins with an increase/decrease greater than 10% are depicted here.
Table 2.
Identified proteins in control or mutant DISC1 primary astrocytes
| Function | Protein | Ratio mutant/control | p value |
|---|---|---|---|
| Metabolism | Glycerol-3-phosphate dehydrogenase, mitochondrial (GPDM) | 1.52 | 0.0396 |
| Transcription/translation | Heat shock cognate 71 kDa protein (HSP7C) | 1.53 | 0.0406 |
| Development | Neuronal membrane glycoprotein M6-a (GPM6A) | 3.66 | 0.0033 |
| Cell-cell interaction | Extracellular matrix protein 2 (ECM2) | 0.28 | 0.0090 |
| Cytoskeleton | Tubulin beta-2B chain (TBB2B) | 1.71 | 0.0341 |
| Tubulin beta-5 chain (TBB5) | 1.27 | 0.0360 | |
| Cytoskeleton-associated protein 4 (CKAP4) | 1.44 | 0.0373 | |
| Actin, cytoplasmic 2 (ACTG) | 0.64 | 0.0434 | |
| Unknown | Transmembrane protein 43 (TMM43) | 1.80 | 0.0152 |
| Epoxide hydrolase 1 (HYEP) | 1.38 | 0.0179 | |
The table includes UniProt IDs, ratios (calculated based on the average) and p values. The adjusted p values for the astrocytes were 0.9834. Significant proteins with an increase/decrease greater than 10% are depicted here.
Validation of Cell Type-Specific Alterations
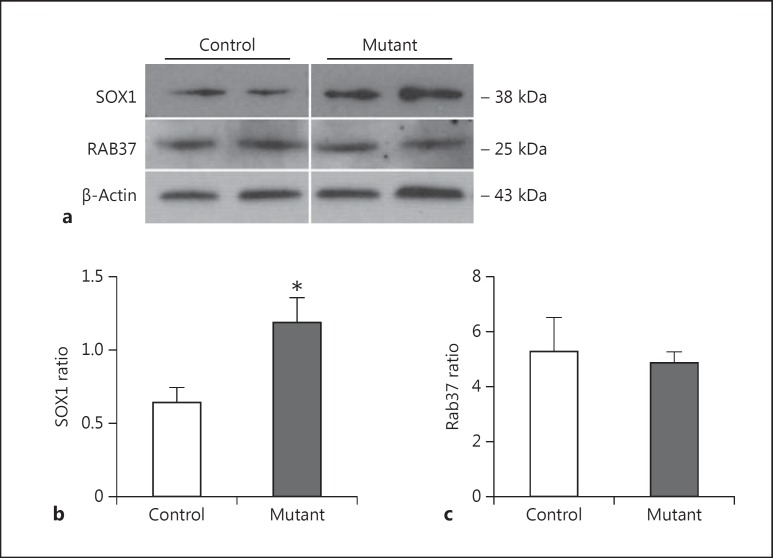
Validation of the changed proteins as indicated by the LC-MSE profiling study was performed using Western blot analysis. We found a significant upregulation of SOX1 in samples derived from neurons that expressed mutant DISC1. The levels of expression of SOX1 were significantly higher in mutant DISC1 samples than in those derived from control neurons (p < 0.05). Although the proteomic data demonstrate an upregulation of Rab37 in neurons expressing mutant DISC1, no such increase in expression of Rab37 was observed by Western blotting (fig. 1).
Fig. 1.
Expression of neuronal markers. a Representative Western blot images. b Quantitative analysis of expression of SOX1 (n = 5-9 per group). c Quantitative analysis of expression of Rab37 (n = 5 per group).
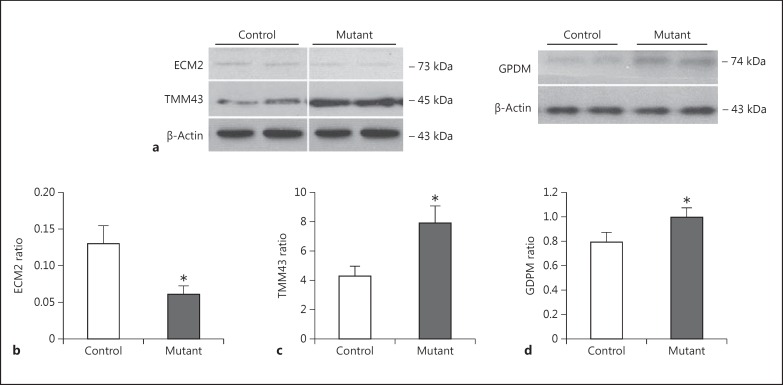
The Western blot experiments on the samples derived from astrocytes were more consistent with the proteomic analysis. We found a significant decrease in the levels of ECM2 in astrocytes derived from newborn mutant DISC1 pups as compared to those from control mouse pups (p < 0.05) (fig. 2). In line with the proteomic results, we observed a significant increase in expression of TMM43 and GPDM in mutant DISC1 astrocytes as compared to control astrocytes (fig. 2).
Fig. 2.
Expression of astrocyte markers. a Representative Western blot images. b Quantitative analysis of expression of ECM2 (n = 5 per group). c Quantitative analysis of expression of TMM43 (n = 5 per group). d Quantitative analysis of expression of GDPM (n = 5 per group).
Discussion
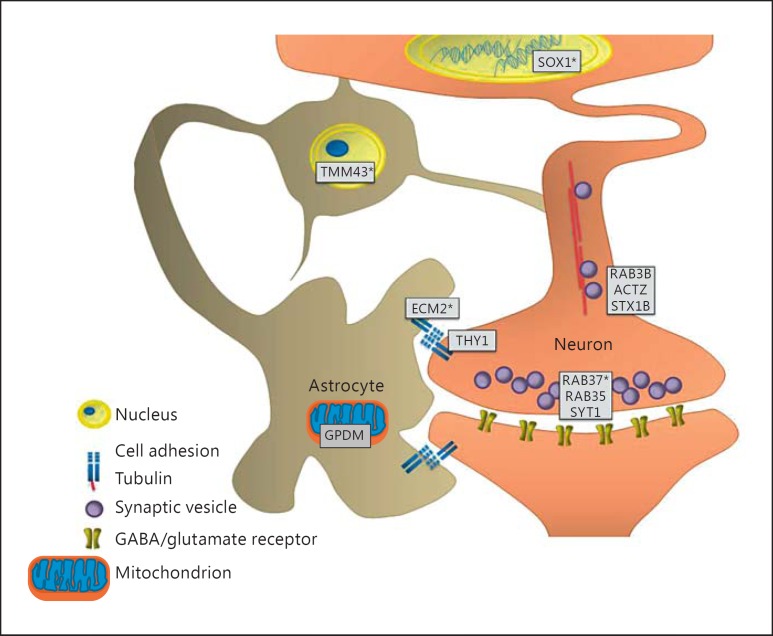
Herein, we present the first comprehensive proteomic study characterizing the effects of mutant DISC1 in a cell type-specific manner. DISC1 has been shown to have an extended protein-protein interaction network consistent with DISC1 involvement in multiple brain functions [9], suggesting that it may have different protein partners in various brain cells; in addition, DISC1 variants might affect distinct pathways in diverse cell types. Previous studies have mainly focused on identifying global molecular changes in neurons or total brain tissue of DISC1 mouse models. In this study, we compared the effects of mutant DISC1 on protein profiles between neurons and astrocytes and found cell type-specific alterations in proteins involved in neuronal development, vesicular transport and cell adhesion as well as mitochondrial and nucleus functioning (fig. 3).
Fig. 3.
Overview of the key results for mutant DISC1 neurons and astrocytes. The main changes are observed in pathways associated with cell-cell contact (ECM2 and THY1), synaptic vesicle transport and docking (RAB3B, ACTZ, STX1B, RAB37, RAB35 and SYT1), nuclear membrane protein (TMM43) and neuronal development (SOX1). Proteins indicated with an asterisk are proteins that were validated by Western blotting.
In primary neurons, mutant DISC1 affected the expression of proteins involved in synaptic vesicular transport (table 1). For example, RAC1 and SYT1 have previously been associated with DISC1, and have been implicated in synaptic vesicle transport [16,17]. In addition, using LC-MSE we detected a changed expression of proteins of the GTPase Rab protein family (e.g., RAB37, RAB35 and RAB3B, of which Rab37 was validated by Western blot) that are also involved in vesicle formation and docking [18,19]. Although there are no data to indicate an interaction of DISC1 with members of the Rab family or other significantly changed proteins involved in cellular transport such as pleckstrin homology domain-containing family A member 8 (PKHA8) and transmembrane protein 106B (T106B), DISC1 has been implicated in mitochondrial trafficking [20,21,22]. These findings point to potentially new DISC1 partners and mechanisms whereby DISC1 might be involved in neuronal transport.
Our study also identifies proteins involved in transcription. We validated the upregulation of the sex determining region Y-box 1 protein (SOX1), a transcription factor that plays a critical role in neurodevelopment [23,24,25]. Our prior study demonstrated an 11-fold upregulation of another member of the SOX family (SOX10) in mice that express mutant DISC1 in early neurodevelopment [26]. Thus, it is tempting to speculate that DISC1 functions as a repressor of expression of SOX proteins, and mutant DISC1 disinhibits their expression via accelerating the degradation of endogenous DISC1 as a result of dominant-negative effects [26]. Upregulation of SOX proteins in mutant DISC1 neurons might result in abnormal neuronal proliferation, which is consistent with a prior report that knockdown of DISC1 in proliferating neurons leads to increased neurogenesis in the adult hippocampus [27]. We also detected a remarkable upregulation (>11-fold) of the transcription factor DNA polymerase epsilon subunit 2 (DPOE2) and significant alterations in the expression of proteins involved in RNA processing such as transformer-2 protein homolog beta (TRA2B) and lethal(3)malignant brain tumor-like protein 4 (LMBL4). These results are consistent with previous findings that DISC1 is involved in transcriptional regulation [28].
In astrocytes, LC-MSE profiling indicated that the affected proteins are involved in cell-cell adhesion, mitochondria and the nucleus. Using Western blotting, we validated significantly decreased levels of extracellular matrix 2 protein (ECM2), a protein that is involved in cell-cell adhesion [29]. This result suggests that mutant DISC1 may affect the extracellular matrix, potentially impacting neuronal migration, the integrity of the blood-brain barrier and synaptic neurotransmission [30,31]. Interestingly, recent findings point to an altered expression of ECM proteins in postmortem samples from schizophrenia patients [32,33], and a new genome-wide association study showed that astrocytic ECM genes are implicated in the etiology of schizophrenia [34].
Furthermore, in astrocytes we validated a significant upregulation of the mitochondrial protein glycerol-3-phosphate dehydrogenase (GPDM), which catalyzes the conversion of glycerol-3-phosphate into dihydroxyacetone phosphate, using flavin adenine dinucleotide as a cofactor [35,36]. Mitochondrial abnormalities have been reported in individuals with schizophrenia, bipolar disorder and major depressive disorder [37,38,39]. Although previous studies have implicated DISC1 in mitochondrial function in neurons [40,41], our findings in astrocytes are new. Given that astrocytes provide most of the energy necessary for neurons [42,43], the metabolic functions of DISC1 in astrocytic mitochondria deserve further study.
In the nuclear envelope, transmembrane protein 43 (TMM43, also called LUMA) [44] levels were increased in mutant DISC1 astrocytes in Western blot analysis. A previous study indicated a predominantly nuclear localization of DISC1 in astrocytes [5]. In line with this, one can speculate that DISC1 may be involved in the structural organization of the astrocytic nuclear membrane, a hypothesis that could be further investigated.
One limitation of the present study includes differences (e.g., Rab37) between results generated by two different methods, i.e., LC-MSE and Western blotting. MS analyses are frequently used in experimental proteomics and continue to progress in sensitivity, throughput, type and depth of proteomic analysis [45]. As compared to Western blotting, LC-MSE measures several peptides from the same protein instead of targeting a specific region on the protein with an antibody, excluding this technique from cross-reactivity [46]. Moreover, LC-MSE is capable of scanning over a large dynamic range across protein sizes and charges. Therefore, LC-MSE was used as a profiling study for the identification and quantification of changed proteins between the DISC1 model and the wild type. Western blotting was used as a qualitative method to validate the proteins indicated in the LC-MSE method, since Western blotting uses antibodies that specifically target proteins and is less sensitive to quantifying changes in protein levels.
In conclusion, our findings demonstrate cell type-specific effects of mutant DISC1 in neurons compared to astrocytes. The present study highlights the possible, distinct roles of DISC1 in diverse brain cells and encourages cell type-specific molecular profiling of psychiatric disorders in order to gain new insights into the heterogeneous effects of human genetic variation and to facilitate the search for more reliable biomarkers and treatment targets.
Statement of Ethics
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Disclosure Statement
The authors declare no conflict of interest. The funding agencies did not have any role in the study design, in the collection, analysis and interpretation of the data, in the writing of the report and in the decision to submit the paper for publication.
Acknowledgements
This study was prepared with support by the following grants: MH-083728, MH-094268 Silvo O. Conte Center; the Brain and Behavior Research Foundation; the Stanley Medical Research Institute (M.P. and S.B.), and the Dutch Fund for Economic Structure Reinforcement [No. 0908; NeuroBasic PharmaPhenomics project (S.B. and J.A.C.B.)].
References
- 1.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry. 2011;69:134–139. doi: 10.1016/j.biopsych.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50:291–301. doi: 10.1016/j.neuint.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda K, Yamada S, Tanaka M, Iizuka M, Yano H, Mori D, Tsuboi D, Nishioka T, Namba T, Iizuka Y, Kubota S, Nagai T, Ibi D, Wang R, Enomoto A, Isotani-Sakakibara M, Asai N, Kimura K, Kiyonari H, Abe T, Mizoguchi A, Sokabe M, Takahashi M, Yamada K, Kaibuchi K. Behavioral alterations associated with targeted disruption of exons 2 and 3 of the Disc1 gene in the mouse. Hum Mol Genet. 2011;20:4666–4683. doi: 10.1093/hmg/ddr400. [DOI] [PubMed] [Google Scholar]
- 5.Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, Sawa A, Snyder SH, Pletnikov MV. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2013;18:557–567. doi: 10.1038/mp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshadri S, Kamiya A, Yokota Y, Prikulis I, Kano S, Hayashi-Takagi A, Stanco A, Eom TY, Rao S, Ishizuka K, Wong P, Korth C, Anton ES, Sawa A. Disrupted-in-Schizophrenia-1 expression is regulated by β-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc Natl Acad Sci USA. 2010;107:5622–5627. doi: 10.1073/pnas.0909284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 8.Abazyan S, Yang EJ, Abazyan B, Xia M, Yang C, Rojas C, Slusher B, Sattler R, Pletnikov M. Mutant Disrupted-in-Schizophrenia 1 in astrocytes: focus on glutamate metabolism. J Neurosci Res. 2014;92:1659–1668. doi: 10.1002/jnr.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abazyan B, Dziedzic J, Hua K, Abazyan S, Yang C, Mori S, Pletnikov MV, Guilarte TR. Chronic exposure of mutant DISC1 mice to lead produces sex-dependent abnormalities consistent with schizophrenia and related mental disorders: a gene-environment interaction study. Schizophr Bull. 2014;40:575–584. doi: 10.1093/schbul/sbt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- 12.Ovanesov MV, Ayhan Y, Wolbert C, Moldovan K, Sauder C, Pletnikov MV. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. J Neuroinflammation. 2008;5:50. doi: 10.1186/1742-2094-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamurthy D, Harris LW, Levin Y, Koutroukides TA, Rahmoune H, Pietsch S, Vanattou-Saifoudine N, Leweke FM, Guest PC, Bahn S. Metabolic, hormonal and stress-related molecular changes in post-mortem pituitary glands from schizophrenia subjects. World J Biol Psychiatry. 2013;14:478–489. doi: 10.3109/15622975.2011.601759. [DOI] [PubMed] [Google Scholar]
- 14.Surinova S, Hüttenhain R, Chang CY, Espona L, Vitek O, Aebersold R. Automated selected reaction monitoring data analysis workflow for large-scale targeted proteomic studies. Nat Protoc. 2013;8:1602–1619. doi: 10.1038/nprot.2013.091. [DOI] [PubMed] [Google Scholar]
- 15.Chang CY, Picotti P, Hüttenhain R, Heinzelmann-Schwarz V, Jovanovic M, Aebersold R, Vitek O. Protein significance analysis in selected reaction monitoring (SRM) measurements. Mol Cell Proteomics. 2012;11:M111.014662. doi: 10.1074/mcp.M111.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores R, 3rd, Hirota Y, Armstrong B, Sawa A, Tomoda T. DISC1 regulates synaptic vesicle transport via a lithium-sensitive pathway. Neurosci Res. 2011;71:71–77. doi: 10.1016/j.neures.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Disrupted-in-Schizophrenia-1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res. 2014;328:1–19. doi: 10.1016/j.yexcr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6:a022616. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkin TA, MacAskill AF, Brandon NJ, Kittler JT. Disrupted in Schizophrenia-1 regulates intracellular trafficking of mitochondria in neurons. Mol Psychiatry. 2011;16:122–124. doi: 10.1038/mp.2010.110. 121. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa F, Malavasi EL, Crummie DK, Eykelenboom JE, Soares DC, Mackie S, Porteous DJ, Millar JK. DISC1 complexes with TRAK1 and Miro1 to modulate anterograde axonal mitochondrial trafficking. Hum Mol Genet. 2014;23:906–919. doi: 10.1093/hmg/ddt485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahani N, Pryor W, Swarnkar S, Kholodilov N, Thinakaran G, Burke RE, Subramaniam S. Rheb GTPase regulates β-secretase levels and amyloid β generation. J Biol Chem. 2014;289:5799–5808. doi: 10.1074/jbc.M113.532713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–8846. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Katsel P, Tan W, Abazyan B, Davis KL, Ross C, Pletnikov MV, Haroutunian V. Expression of mutant human DISC1 in mice supports abnormalities in differentiation of oligodendrocytes. Schizophr Res. 2011;130:238–249. doi: 10.1016/j.schres.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-in-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K, Shimoda M, Toda H, Sawamura-Yamamoto T, Makuch LA, Hayashi A, Ishizuka K, Cascella NG, Kamiya A, Ishida N, Tomoda T, Hai T, Furukubo-Tokunaga K, Sawa A. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry. 2008;13:1138–1148. doi: 10.1038/mp.2008.101. 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manabe R, Tsutsui K, Yamada T, Kimura M, Nakano I, Shimono C, Sanzen N, Furutani Y, Fukuda T, Oguri Y, Shimamoto K, Kiyozumi D, Sato Y, Sado Y, Senoo H, Yamashina S, Fukuda S, Kawai J, Sugiura N, Kimata K, Hayashizaki Y, Sekiguchi K. Transcriptome-based systematic identification of extracellular matrix proteins. Proc Natl Acad Sci USA. 2008;105:12849–12854. doi: 10.1073/pnas.0803640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobeih MM, Corfas G. Extracellular factors that regulate neuronal migration in the central nervous system. Int J Dev Neurosci. 2002;20:349–357. doi: 10.1016/s0736-5748(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 31.Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012;62:1584–1597. doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, Smit AB, Posthuma D, Verheijen MH. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 2014;40:925–935. doi: 10.1093/schbul/sbt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharadwaj MS, Zhou Y, Molina AJ, Criswell T, Lu B. Examination of bioenergetic function in the inner mitochondrial membrane peptidase 2-like (Immp2l) mutant mice. Redox Biol. 2014;2C:1008–1015. doi: 10.1016/j.redox.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valadi A, Granath K, Gustafsson L, Adler L. Distinct intracellular localization of Gpd1p and Gpd2p, the two yeast isoforms of NAD+-dependent glycerol-3-phosphate dehydrogenase, explains their different contributions to redox-driven glycerol production. J Biol Chem. 2004;279:39677–39685. doi: 10.1074/jbc.M403310200. [DOI] [PubMed] [Google Scholar]
- 37.Daoud H, Gruchy N, Constans JM, Moussaoui E, Saumureau S, Bayou N, Amy M, Védrine S, Vu PY, Rötig A, Laumonnier F, Vourc'h P, Andres CR, Leporrier N, Briault S. Haploinsufficiency of the GPD2 gene in a patient with nonsyndromic mental retardation. Hum Genet. 2009;124:649–658. doi: 10.1007/s00439-008-0588-3. [DOI] [PubMed] [Google Scholar]
- 38.Goncalves VF, Andreazza AC, Kennedy JL. Mitochondrial dysfunction in schizophrenia: an evolutionary perspective. Hum Genet. 2015;134:13–21. doi: 10.1007/s00439-014-1491-8. [DOI] [PubMed] [Google Scholar]
- 39.Marchbanks RM, Mulcrone J, Whatley SA. Aspects of oxidative metabolism in schizophrenia. Br J Psychiatry. 1995;167:293–298. doi: 10.1192/bjp.167.3.293. [DOI] [PubMed] [Google Scholar]
- 40.James R, Adams RR, Christie S, Buchanan SR, Porteous DJ, Millar JK. Disrupted in Schizophrenia 1 (DISC1) is a multicompartmentalized protein that predominantly localizes to mitochondria. Mol Cell Neurosci. 2004;26:112–122. doi: 10.1016/j.mcn.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Park YU, Jeong J, Lee H, Mun JY, Kim JH, Lee JS, Nguyen MD, Han SS, Suh PG, Park SK. Disrupted-in-schizophrenia 1 (DISC1) plays essential roles in mitochondria in collaboration with Mitofilin. Proc Natl Acad Sci USA. 2010;107:17785–17790. doi: 10.1073/pnas.1004361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adam-Vizi V, Tretter L. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochem Int. 2013;62:757–763. doi: 10.1016/j.neuint.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Mráček T, Drahota Z, Houšĕk J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta. 2013;1827:401–410. doi: 10.1016/j.bbabio.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Meinke P, Nguyen TD, Wehnert MS. The LINC complex and human disease. Biochem Soc Trans. 2011;39:1693–1697. doi: 10.1042/BST20110658. [DOI] [PubMed] [Google Scholar]
- 45.Mesri M. Advances in proteomic technologies and its contribution to the field of cancer. Adv Med. 2014;2014:238045. doi: 10.1155/2014/238045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aebersold R, Burlingame AL, Bradshaw RA. Western blots versus selected reaction monitoring assays: time to turn the tables? Mol Cell Proteomics. 2013;12:2381–2382. doi: 10.1074/mcp.E113.031658. [DOI] [PMC free article] [PubMed] [Google Scholar]