Abstract
The transmembrane protein Vangl2, a key regulator of the Wnt/planar cell polarity (PCP) pathway, is involved in dendrite arbor elaboration, dendritic spine formation and glutamatergic synapse formation in mammalian central nervous system neurons. Cultured forebrain neurons from Vangl2 knockout mice have simpler dendrite arbors, fewer total spines, less mature spines and fewer glutamatergic synapse inputs on their dendrites than control neurons. Neurons from mice heterozygous for a semidominant Vangl2 mutation have similar but not identical phenotypes, and these phenotypes are also observed in Golgi-stained brain tissue from adult mutant mice. Given increasing evidence linking psychiatric pathophysiology to these subneuronal sites and structures, our findings underscore the relevance of core PCP proteins including Vangl2 to the underlying biology of major mental illnesses and their treatment.
Key Words: Dendrite, Forebrain, Planar cell polarity, Pyramidal neuron, Spine, Synapse, Vangl2, Wnt
Introduction
Dendrite, dendritic spine and excitatory (glutamatergic) synapse formation and plasticity are molecularly interrelated developmental prerequisites for proper brain function and behavior. Defects in spine and synapse formation and turnover are increasingly understood to be key contributors to neuropsychiatric disorders including autism, schizophrenia and major affective disorders [1,2,3,4,5].
Cell communication pathways with well-established roles in other aspects of development - including both the Wnt/β-catenin pathway [6,7,8] and the Wnt/planar cell polarity (PCP) pathway [9,10,11] - participate in these processes. The four-pass transmembrane protein Van Gogh-like 2 (Vangl2) is a key player in the PCP pathway and interacts with several proteins that influence synapse formation, including other PCP proteins such as Dishevelled (Dvl) [12] and Dapper-antagonist of catenin-1 (Dact1) [13] as well as the postsynaptic protein PSD95 [14,15,16]. Vangl2 also participates in signaling upstream of small GTPases [17,18] regulating cytoskeletal dynamics crucial to dendrite and dendritic spine formation and plasticity [19,20,21,22]. Looptail (Lp) is a missense mutation in Vangl2[23] that causes semidominant phenotypes reflective of abnormal PCP including, in heterozygous animals, the curled or kinked tail from which the mutation gets its name, and in homozygous animals, craniorachischisis, a completely open neural tube and exposed brain [23,24,25]. Genetically engineered null mutations in Vangl2 cause similar phenotypes, but recessively and with lower penetrance in homozygous mutant animals [26,27]. Using both allele types, we show here that Vangl2 functions during neural differentiation in dendrite arborization, spine formation, spine maturation and glutamatergic synapse formation.
Methods
Genetics
The Vangl2Δ allele [26] is here referred to as Vangl2KO or Vangl2-. The LtapLp allele (Jackson Laboratory stock No. 000220) [23] is here referred to as Vangl2Lp or Lp. All assays compared littermates of the designated experimental and control genotypes derived from Vangl2-/+ or Vangl2Lp/+ intercrosses.
Recombinant DNA
The mouse Vangl2 cDNA clone and expression plasmid has been described previously [28].
Primary Culture and Immunostaining
Dissociated neurons were obtained from embryos, fixed, transfected with pEGFP-C1 (Clontech) and immunostained for synaptic markers as previously described [29,30]. Hippocampal cultures were used where possible because of the ease of producing populations of predominantly pyramidal neurons and prior validation as a model system relevant to dendrite, spine and synapse formation in the forebrain [31]. The borders of the hippocampus were not consistently identifiable in mutant homozygotes (both Vangl2Δ/Δ and Vangl2Lp/Lp) due to craniorachischisis and neural precursor migration defects [27,32]. Therefore, for these genotypes the entire forebrain (cortex and hippocampus) was prepared and compared to entire forebrain cultures of wild type (WT) and heterozygous littermates.
Visualization and Quantification
Cells were visualized on a Nikon CS1i upright spectral confocal at ×40 magnification or a custom-built spinning disc confocal microscope (Zeiss Axiovert 200M with Perkin-Elmer spinning disc and Melles Griot 43 series ion laser, Cascade 512B digital camera; Roper Scientific) at ×40 magnification. Golgi images were obtained on an Olympus IX51 compound inverted fluorescence microscope, also at ×40. Images were analyzed with ImageJ software (NIH). Sholl analysis, dendritic spine binning and spine and synapse quantification were performed as previously described [30].
Golgi Staining
Golgi-Cox silver staining was performed on 4-month-old littermates using the FD GolgiStain kit (FD Neurotechnologies) according to the manufacturer's instructions.
Statistics
All p values were calculated by unpaired parametric t tests (2-way comparison) or one-way ANOVA (≥3 comparisons) with Tukey's post hoc analysis using Graphpad Prism software. Each comparison entailed ≥8 neurons and ≥11 dendrites per condition derived from multiple independent experiments; all reported differences reflect a minimal p ≤ 0.05 for experimental vs. control mice.
Results
Genetic Elimination of Vangl2 Reduces Dendrite Arbor and Spine Formation
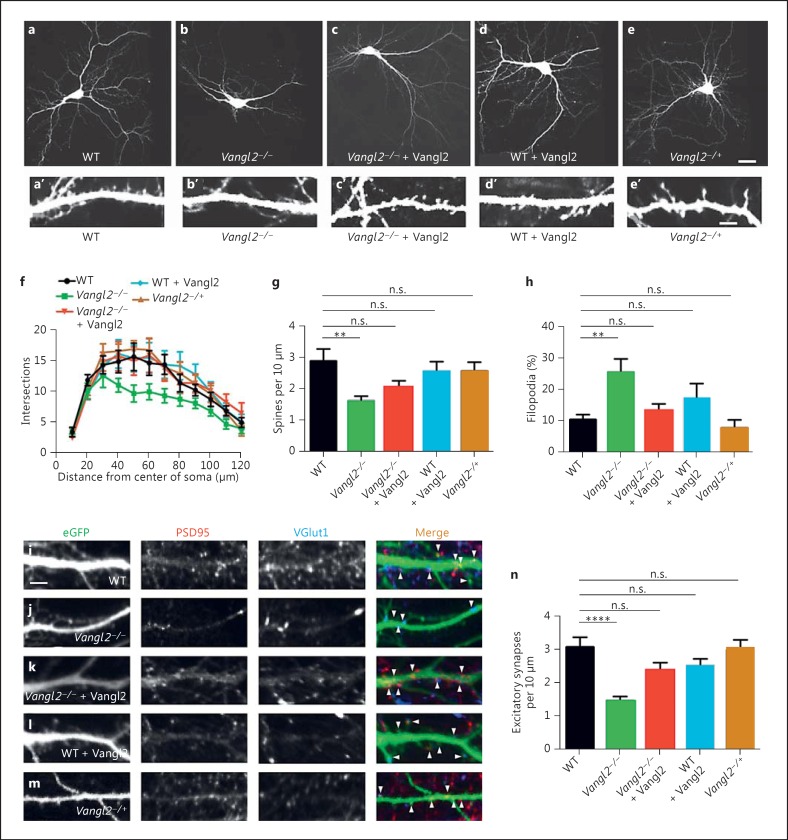
On inspection, cultured Vangl2-/- forebrain pyramidal neurons had simpler dendrite arbors than controls (fig. 1a vs. b). Sholl analysis confirmed that mutant neurons had reduced numbers of dendrite branch crossings (fig. 1f). The maturation and density of dendritic spines were also affected (fig. 1a′ vs. b′): the density of spines along dendrites was lower in Vangl2-/- neurons compared to WT (fig. 1g). Moreover, Vangl2-/- neurons had an increased percentage of immature (i.e. filopodial) relative to mature (i.e. thin, mushroom or stub-shaped) spines (fig. 1h).
Fig. 1.
Differentiation phenotypes in cultured Vangl2KO forebrain neurons. a-e EGFP-transfected cultured forebrain neurons from WT (a), Vangl2-/- (b), Vangl2-/- + Vangl2 (c), WT + Vangl2 (d) and Vangl2-/+ (e). a′-e′ Corresponding dendritic segments at a higher magnification. f-h Vangl2-/- neurons have simpler dendritic arbors as quantified by Sholl analysis (f), fewer dendritic spines (g) and less mature spines (h) than controls. i-m Immunostaining for glutamatergic synapse markers along dendrite segments from WT (i), Vangl2-/- (j), Vangl2-/- + Vangl2 (k), WT + Vangl2 (l) and Vangl2-/+ (m) neurons. n Quantification. Scale bars = 30 μm (a-e); 5 μm (a′-e′, i-m). n.s. = p > 0.05, ** p ≤ 0.01, **** p ≤ 0.0001.
Recombinant expression of Vangl2 rescued the dendrite arbor phenotype in Vangl2-/- neurons (fig. 1c, f). It also rescued spine density and spine maturity (fig. 1a′, c′, g, h).
Vangl2 Overexpression and Heterozygosity Do Not Alter Dendrites or Spines
To investigate effects of other genetic manipulations expected to alter (but not eliminate) Vangl2 levels, we examined phenotypes in WT neurons recombinantly overexpressing Vangl2, and also in heterozygous (Vangl2-/+) neurons. Neither of these genetic manipulations had any effect on dendrite complexity (fig. 1a vs. d, e; f), spine density (fig. 1a′ vs. d′, e′; g) or spine maturity (fig. 1a′ vs. d′, e′; h).
Vangl2-/- Dendrites Have Fewer Glutamatergic Synaptic Contacts
We quantified density of glutamatergic synapses along dendrites by visualization with antibodies specific for VGlut1 (presynaptic marker) and PSD95 (postsynaptic marker). Vangl2-/- neurons had reduced glutamatergic synapse density (fig. 1i vs. j; n). Recombinant expression of Vangl2 in Vangl2-/- neurons rescued glutamatergic synapse density (fig. 1j vs. k; n). Neither WT neurons recombinantly overexpressing Vangl2 (fig. 1l) nor Vangl2-/+ neurons (fig. 1m) were significantly different from WT in this assay (fig. 1n). In contrast to this glutamatergic synapse phenotype, Vangl2-/- neurons had no significant reduction in inhibitory (GABAergic) synapse density on their dendrites measured similarly (data not shown).
Lp Causes Mixed Effects on Dendrite Arbors, Spines and Glutamatergic Synapses
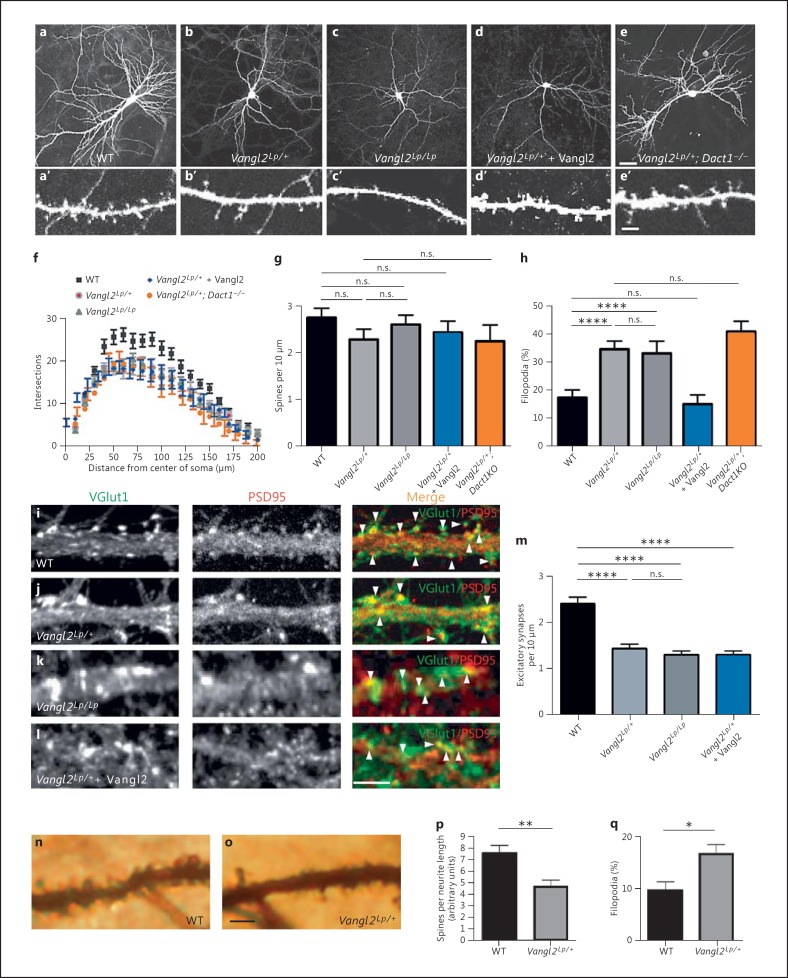
As with Vangl2-/- neurons, upon visual inspection, Vangl2Lp/+ neurons had simpler dendrite arbors than WT (fig. 2a, b). Interestingly, although homozygous (Vangl2Lp/Lp) mice have more severe cell polarity phenotypes than heterozygous (Vangl2Lp/+) mice in embryonic axis elongation, inner ear epithelia, and neural precursor proliferation and migration [26,32], neurons from Vangl2Lp/Lp mice did not have a greater decrease in dendrite complexity than those from Vangl2Lp/+ mice (fig. 2b, c). Sholl analysis revealed that Lp neurons, whether heterozygous or homozygous, had similar reductions in number of dendrite branch crossings (fig. 2f). Dendritic spines were also affected by the Lp mutation, not in terms of density (fig. 2a′, b′, c; g) but in terms of maturity (fig. 2h).
Fig. 2.
Differentiation phenotypes in Vangl2Lp forebrain neurons. a-e EGFP-transfected cultured neurons from WT (a), Vangl2Lp/+ (b), Vangl2Lp/Lp (c), Vangl2Lp/+ + Vangl2 (d) and Vangl2Lp/+; Dact1-/- (e). a'-e' Corresponding dendritic segments at a higher magnification. f-h Quantification. Vangl2Lp neurons have simpler dendritic arbors (f), no reduction in total density of dendritic projections (spines + filopodia) (g), but a larger proportion of immature (filopodial) dendritic projections (h) than controls; only the last phenotype is rescued by recombinant expression of Vangl2 (blue bar; color refers to the online version only). i-l Immunostaining for glutamatergic synapse markers along dendrite segments from WT (i), Vangl2Lp/+ (j), Vangl2Lp/Lp (k) and Vangl2Lp/++ Vangl2 (l) cultured neurons. m Quantification. n, o Segments of apical dendrite from a Golgi-stained pyramidal neuron in hippocampal CA1 of WT (n) and Vangl2Lp/+ (o) littermates. Quantification of total spines (p) and immature (filopodial) spines (q). Scale bars = 30 μm (a-e); 5 μm (a'-e', i-l, n, o). n.s. = p > 0.05, * p ≤ 0.05; ** p ≤ 0.01, **** p ≤ 0.0001.
Similar to Vangl2-/- neurons, glutamatergic synapse density along dendrites of Vangl2Lp/+ and Vangl2Lp/Lp neurons was reduced compared to WT (fig. 2i-k; m). As in Vangl2-/- neurons, GABAergic synapse density was unaffected (data not shown).
Vangl2 Overexpression Rescues Only Some Lp Neurodevelopmental Phenotypes
As stated above, neurons carrying the Vangl2Lp allele, whether heterozygous or homozygous, displayed similar decreases in dendrite complexity, spine maturation and glutamatergic synapse density. Interestingly, only the spine maturation phenotype was rescued by recombinant expression of Vangl2: recombinant overexpression of Vangl2 did not rescue dendrite complexity (fig. 2e, f), nor did it rescue glutamatergic synapse density along dendrites in Lp mutant neurons (fig. 2l, m). In contrast, overexpression of WT Vangl2 did rescue spine maturity in these neurons (fig. 2b′, d′; h).
Elimination of Dact1 Does Not Rescue Lp Neurodevelopmental Phenotypes
We previously showed that genetic loss of the Wnt signal pathway scaffold protein Dact1 can rescue embryonic phenotypes in Vangl2Lp/+mice [13]. Dact1 is expressed in differentiating forebrain neurons, and its loss causes reductions in dendrite arbor complexity, dendritic spine maturity and glutamatergic synapse formation [30] similar to the Vangl2 mutant phenotypes reported here. Nonetheless, neurons from Vangl2Lp/+; Dact1-/- mice had no rescue of dendrite complexity (fig. 2b vs. e; f) and no rescue of spine maturity (fig. 2b′ vs. e′; h) relative to Vangl2Lp/+ neurons.
Golgi Staining Confirms Spine Reductions in the Vangl2 Mutant Forebrain
All the preceding assays were conducted using cultured forebrain neurons. To confirm that similar phenotypes occur in intact mammalian forebrain tissue, we analyzed the morphology of pyramidal neurons in the CA1 region of the hippocampus via Golgi-Cox staining on brains taken from adult (4- to 6-month-old) Vangl2Lp/+ and littermate control mice. (The prenatal death of Vangl2-/- and Vangl2Lp/Lp mice precluded such analysis.) CA1 pyramidal neurons in Vangl2Lp/+ mice had decreased spine density on apical dendrites compared to controls (fig. 2n-p). They also had an increased percentage of immature (filopodial) projections (fig. 2q).
Discussion
Loss of Vangl2 function, whether via the semidominant Lp missense mutation or a targeted knockout, leads to decreased dendrite arbor complexity, spine maturity and glutamatergic synapse density without similar losses in GABAergic synapse density on forebrain pyramidal neuron dendrites. As dendritic spines are the specific subcellular site of glutamatergic synapses in pyramidal neurons, these data are consistent with previous findings that Vangl2 localizes to the postsynaptic compartment of glutamatergic synapses, where it interacts with PSD95, transsynaptic adhesion molecules and the PCP pathway protein Prickle2 [14,15,16]. Our genetic data corroborate previous reports of similar phenotypes following shRNA-mediated knockdown of Vangl2 in cultured neurons [15,33] and have allowed us to compare and contrast neurodevelopmental phenotypes induced by two molecularly distinct (engineered null vs. spontaneous missense) alleles at this locus.
Unlike the KO (null) allele, the Lp allele of Vangl2 is not a simple loss of function: it causes dominant phenotypes to varying degrees in different biological contexts and exerts complex cell biological and biochemical effects on the encoded protein [12,26,27,34]. Unlike homozygous Vangl2KO neurons, Lp neurons do not have reductions in spine density and their dendrite complexity and glutamatergic synapse phenotypes are not rescued by recombinant overexpression of Vangl2; this suggests that Vangl2 has a molecularly distinct role in spine formation and maturation compared to dendrite arborization and glutamatergic synapse formation. The differences in rescue of these Lp neurodevelopmental phenotypes cannot be explained by different temporal requirements for Vangl2 in these subneuronal compartments and processes, because recombinant expression of Vangl2 in the same manner rescues all four phenotypes (dendrite complexity, spine number, spine maturity and glutamatergic synapse density) in Vangl2-/- neurons.
Conclusions
PCP Pathway Proteins Play Important Divergent Roles in Neurons
Genetically altering Vangl2 function, whether by semidominant missense (Vangl2Lp) or engineered knock-out (Vangl2KO), results in forebrain pyramidal neurons with simpler dendrite arbors, a larger proportion of immature spines and fewer glutamatergic synapses. Genetic disruption of other PCP genes in mammals, including Dvl1 [35] and Dact1 [30], causes similar phenotypes. Given the widespread neural expression of Vangl2 and several other PCP genes during prenatal development, postnatal development and in the mature brain, it is plausible that the neural functions of these molecules are similarly widespread and continuous over the lifespan. In prior work, we have demonstrated requirements for the Vangl2 partner Dact1 during dendrite, spine and synapse development in both pyramidal neurons [30] and interneurons of the cerebral cortex [36,37]. However, mutations in Vangl2 and Dact1 that exhibit strong mutual rescue during gastrulation [13] do not exhibit similar reciprocal functional relationships during neurodevelopment, suggesting that the molecular mechanisms underlying these phenotypes differ. Consistent with other studies [15,38,39] our genetic work therefore suggests that although components of the PCP pathway play important roles in the nervous system, the molecular pathways by which they function in developing neurons differ substantially from the PCP pathway established in studies of basic embryonic development.
The Goldilocks Principle in Molecular Neuropsychiatry
Evidence increasingly supports that neurodevelopmental and neuroplastic processes regulating spine and glutamatergic synapses contribute to the pathogenesis of psychiatric conditions including autism, schizophrenia and major affective disorders [2,3,4,40,41,42,43]. In line with this, several PCP proteins contributing to these processes have been implicated in psychiatric pathophysiology. For example, in mice, the elimination of Dvl1 reduces social behavior [44] and Prickle2 sequence variants associated with autism lead to dendrite and glutamatergic synapse phenotypes [45]. Our work with Vangl2 accords with these findings and underscores that at some genetic loci and in some biochemical pathways different molecular defects can lead to similar neural phenotypes. We refer to the general idea that similar neural and behavioral phenotypes can result from functionally different and even opposite molecular defects as the ‘Goldilocks Principle’ - i.e. either too much or too little can be deleterious in molecular neuropsychiatry. This is, in fact, a well-established theme for Wnt/PCP signaling in other developmental contexts [13,46,47]. A similar phenomenon has been observed for loci contributing to neuropsychiatry through entirely different mechanisms, including ion channel proteins such as KCNA2 for which both gain- and loss-of-function mutations cause epilepsy [48] and CACNA1C for which both a gain-of-function mutation and reduced expression variants are associated with bipolar disorder [49]. This is now also firmly established for several copy number variants, such as 7q11.23 and 22q11.2, that contribute to psychiatric susceptibility either when deleted or duplicated [3,50].
Statement of Ethics
All procedures involving live animals were carried out in accordance with an IACUC-approved animal use protocol at the University of California, San Francisco.
Disclosure Statement
The authors have no conflicts of interest to report.
Acknowledgments
N.D.O. was supported in part by Autism Speaks Predoctoral Fellowship 4654. R.E.S. was supported in part by supplement R01HD55300-S1 to B.N.R.C. This research was also supported by a Simons Foundation Autism Research Initiative Grant (B.N.R.C.). We thank all members of the Cheyette laboratory for advice and support, and Yingzi Yang (NHGRI) for providing the Vangl2Δ allele.
References
- 1.Williams AJ, Umemori H. The best-laid plans go oft awry: synaptogenic growth factor signaling in neuropsychiatric disease. Front Synaptic Neurosci. 2014;6:4. doi: 10.3389/fnsyn.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, Thiruvahindrapuram B, Xu X, Ziman R, Wang Z, Vorstman JA, Thompson A, Regan R, Pilorge M, Pellecchia G, Pagnamenta AT, Oliveira B, Marshall CR, Magalhaes TR, Lowe JK, Howe JL, Griswold AJ, Gilbert J, Duketis E, Dombroski BA, De Jonge MV, Cuccaro M, Crawford EL, Correia CT, Conroy J, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders SJ. First glimpses of the neurobiology of autism spectrum disorder. Curr Opin Genet Dev. 2015;33:80–92. doi: 10.1016/j.gde.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Ben Fredj N, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- 8.Farias GG, Valles AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, Barrantes FJ, Inestrosa NC. Wnt-7a induces presynaptic colocalization of α7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27:5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okerlund ND, Cheyette BN. Synaptic Wnt signaling - a contributor to major psychiatric disorders? J Neurodev Disord. 2011;3:162–174. doi: 10.1007/s11689-011-9083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farias GG, Alfaro I, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5a (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- 13.Suriben R, Kivimae S, Fisher DA, Moon RT, Cheyette BNR. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009;41:977–985. doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka T, Hagiwara A, Hida Y, Ohtsuka T. Vangl2, the planar cell polarity protein, is complexed with postsynaptic density protein PSD-95 [corrected] FEBS Lett. 2013;587:1453–1459. doi: 10.1016/j.febslet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Nagaoka T, Ohashi R, Inutsuka A, Sakai S, Fujisawa N, Yokoyama M, Huang YH, Igarashi M, Kishi M. The Wnt/planar cell polarity pathway component Vangl2 induces synapse formation through direct control of N-cadherin. Cell Rep. 2014;6:916–927. doi: 10.1016/j.celrep.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 16.Nagaoka T, Tabuchi K, Kishi M. PDZ interaction of Vangl2 links PSD-95 and Prickle2 but plays only a limited role in the synaptic localisation of Vangl2. Sci Rep. 2015;5:12916. doi: 10.1038/srep12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movement during development of the proximal outflow tract. Circ Res. 2009;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 18.Lindqvist M, Horn Z, Bryja V, Schulte G, Papachristou P, Ajime R, Dyberg C, Arenas E, Yamaguchi TP, Lagercrantz H, Ringstedt T. Vang-like protein 2 and Rac1 interact to regulate adherens junctions. J Cell Sci. 2009;123:472–483. doi: 10.1242/jcs.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11:187–191. doi: 10.1177/1073858404268768. [DOI] [PubMed] [Google Scholar]
- 21.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the Rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 22.Van Aelst L, Cline HT. Rho GTPases and activity-dependent dendrite development. Curr Opin Neurobiol. 2004;14:297–304. doi: 10.1016/j.conb.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 24.Strong LC, Hollander WF. Hereditary loop-tail in the house mouse. J Hered. 1947;40:329–334. [Google Scholar]
- 25.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin H, Copley CO, Goodrich LV, Deans MR. Comparison of phenotypes between different vangl2 mutants demonstrates dominant effects of the Looptail mutation during hair cell development. PLoS One. 2012;7:e31988. doi: 10.1371/journal.pone.0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivimae S, Yang XY, Cheyette BNR. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33. doi: 10.1186/1471-2091-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okerlund ND, Kivimae S, Tong CK, Peng I-F, Ullian EM, Cheyette BNR. Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. J Neurosci. 2010;30:4362–4368. doi: 10.1523/JNEUROSCI.0354-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banker G, Goslin K, editors. Culturing Nerve Cells. ed 2. Cambridge: MIT Press; 1998. [Google Scholar]
- 32.Lake BB, Sokol SY. Strabismus regulates asymmetric cell divisions and cell fate determination in the mouse brain. J Cell Biol. 2009;185:59–66. doi: 10.1083/jcb.200807073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagiwara A, Yasumura M, Hida Y, Inoue E, Ohtsuka T. The planar cell polarity protein Vangl2 bidirectionally regulates dendritic branching in cultured hippocampal neurons. Mol Brain. 2014;7:79. doi: 10.1186/s13041-014-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gravel M, Iliescu A, Horth C, Apuzzo S, Gros P. Molecular and cellular mechanisms underlying neural tube defects in the loop-tail mutant mouse. Biochemistry. 2010;49:3445–3455. doi: 10.1021/bi902180m. [DOI] [PubMed] [Google Scholar]
- 35.Rosso SB, Sussman DJ, Wynshaw-Boris A, Salinas PC. Wnt signalling through Dishevelled, Rac and Jnk regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 36.Arguello A, Yang X, Vogt D, Stanco A, Rubenstein JL, Cheyette BN. Dapper antagonist of catenin-1 cooperates with Dishevelled-1 during postsynaptic development in mouse forebrain GABAergic interneurons. PLoS One. 2013;8:e67679. doi: 10.1371/journal.pone.0067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arguello A, Cheyette BN. Dapper Antagonist of Catenin-1 (Dact1) contributes to dendrite arborization in forebrain cortical interneurons. Commun Integr Biol. 2014;6:e26656. doi: 10.4161/cib.26656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glasco DM, Sittaramane V, Bryant W, Fritzsch B, Sawant A, Paudyal A, Stewart M, Andre P, Cadete Vilhais-Neto G, Yang Y, Song MR, Murdoch JN, Chandrasekhar A. The mouse Wnt/PCP protein Vangl2 is necessary for migration of facial branchiomotor neurons, and functions independently of Dishevelled. Dev Biol. 2012;369:211–222. doi: 10.1016/j.ydbio.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu Y, Huang Y, Feng J, Alvarez-Bolado G, Grove EA, Yang Y, Tissir F, Zhou L, Goffinet AM. Genetic evidence that Celsr3 and Celsr2, together with Fzd3, regulate forebrain wiring in a Vangl-independent manner. Proc Natl Acad Sci USA. 2014;111:E2996–E3004. doi: 10.1073/pnas.1402105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krumm N, O'Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha KE, Cicek AE, Kou Y, Liu L, Fromer M, Walker EM, Singh T, Klei L, Kosmicki J, Fu S-C. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry. 2013;73:1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Yi F, Danko T, Botelho SC, Patzke C, Pak C, Wernig M, Sudhof TC. Autism-associated SHANK3 haploinsufficiency causes Ih channelopathy in human neurons. Science. 2016;352:aaf2669. doi: 10.1126/science.aaf2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 45.Sowers LP, Loo L, Wu Y, Campbell E, Ulrich JD, Wu S, Paemka L, Wassink T, Meyer K, Bing X, El-Shanti H, Usachev YM, Ueno N, Manak JR, Shepherd AJ, Ferguson PJ, Darbro BW, Richerson GB, Mohapatra DP, Wemmie JA, Bassuk AG. Disruption of the non-canonical Wnt gene PRICKLE2 leads to autism-like behaviors with evidence for hippocampal synaptic dysfunction. Mol Psychiatry. 2013;18:1077–1089. doi: 10.1038/mp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park E, Kim GH, Choi SC, Han JK. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev Biol. 2006;292:344–357. doi: 10.1016/j.ydbio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 48.Syrbe S, Hedrich UB, Riesch E, Djémié T, Müller S, Møller RS, Maher B, Hernandez-Hernandez L, Synofzik M, Caglayan HS, Arslan M, Serratosa JM, Nothnagel M, May P, Krause R, Löffler H, Detert K, Dorn T, Vogt H, Krämer G, Schöls L, Mullis PE, Linnankivi T, Lehesjoki AE, Sterbova K, Craiu DC, Hoffman-Zacharska D, Korff CM, Weber YG, Steinlin M, et al. De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat Genet. 2015;47:393–399. doi: 10.1038/ng.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, Alliey-Rodriguez N, Cooper J, Romanos B, Liu C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop SL, Dong S, Goldberg AP, Jinlu C, Keaney JF, 3rd, Klei L, Mandell JD, Moreno-De-Luca D, Poultney CS, Robinson EB, Smith L, Solli-Nowlan T, Su MY, Teran NA, Walker MF, Werling DM, Beaudet AL, Cantor RM, Fombonne E, Geschwind DH, Grice DE, Lord C, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]