Abstract
Neuregulin 3 (NRG3) is a paralog of NRG1. Genetic studies in schizophrenia demonstrate that risk variants in NRG3 are associated with cognitive and psychotic symptom severity, and several intronic single nucleotide polymorphisms in NRG3 are associated with delusions in patients with schizophrenia. In order to gain insights into the biological function of the gene, we generated a novel Nrg3 knockout (KO) mouse model and tested for neurobehavioral phenotypes relevant to psychotic disorders. KO mice displayed novelty-induced hyperactivity, impaired prepulse inhibition of the acoustic startle response, and deficient fear conditioning. No gross cytoarchitectonic or layer abnormalities were noted in the brain of KO mice. Our findings suggest that deletion of the Nrg3 gene leads to alterations consistent with aspects of schizophrenia. We propose that KO mice will provide a valuable animal model to determine the role of the NRG3 in the molecular pathogenesis of schizophrenia and other psychotic disorders.
Key Words: Neuregulin 3, Psychotic disorders, Prepulse inhibition, Hyperactivity, Fear conditioning
Introduction
Schizophrenia is a severe and debilitating mental disorder with poorly understood neurodevelopmental etiology [1,2,3]. Many genetic loci associated with schizophrenia only impart a small genetic risk [4]. Genetic studies in schizophrenia demonstrate that risk variants in Neuregulin 3 (NRG3) are associated with cognitive and psychotic symptom severity, accompanied by increased expression of prefrontal cortical NRG3 [5,6,7]. Our studies found a linkage signal in chromosome 10q22, containing NRG3, in an Ashkenazi Jewish population, and a Han Chinese population that showed association with schizophrenia [8,2]. A follow-up association study across the linkage peak identified a strong association of several intronic single nucleotide polymorphisms (SNPs) in NRG3 with the ‘Delusion’ factor as a measure of various delusions exhibited by patients [9,10]. Similar results linking NRG3 and various types of delusions were obtained in other populations [7,11], suggesting that genetic variants of NRG3 could contribute to the risk of schizophrenia.
NRG3 is one of three paralogs of Neuregulin 1 (NRG1), which is a schizophrenia susceptibility gene [12,13]. The NRG3 protein is a single-pass membrane protein with an extracellular N-terminal EGF-like domain that is cleaved to bind ErbB4 receptors [14,15,16], another schizophrenia susceptibility gene [17,18,19]. NRG3 and NRG1 are chemorepellant signals that guide GABAergic interneuron progenitors from their origin in the medial ganglionic eminence (MGE) through tangential migration to their final location in the developing cerebral cortex [20,21,22]. However, the long-term neurobehavioral effects of abolishing or diminishing levels of NRG3 in the brain have not been tested. In order to address this question, we generated a mouse model with a deletion of the Nrg3 gene and tested for neurobehavioral phenotypes [22]. We found that Nrg3 knockout (KO) mice displayed novelty-induced hyperactivity, impaired prepulse inhibition (PPI) of the acoustic startle response, and deficient fear conditioning. No gross cytoarchitectonic or layering abnormalities were noted in the brain of KO mice. Our findings suggest that deletion of the Nrg3 gene leads to neurobehavioral alterations consistent with aspects of schizophrenia. We propose that Nrg3 KO mice provide a valuable animal model to determine the role of this gene in the molecular pathogenesis of schizophrenia and other psychotic disorders.
Materials and Methods
Generation of Nrg3-/- KO Mice
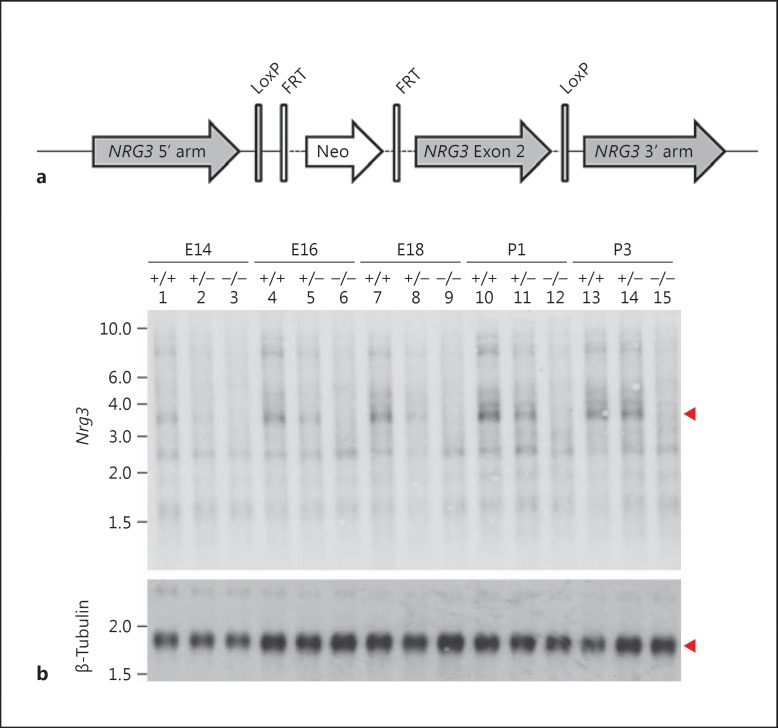
To generate the Nrg3-/- KO mouse model, we used homologous recombination to knock in a construct that contained a loxP-flanked exon 2 with an inserted frt-flanked neomycin cassette for selection (fig. 1a). The Johns Hopkins Transgenic Core Facility generated chimeric mice. We confirmed recombination efficiency using Southern blot and bred to CAG-cre transgenic mice with the Cre gene under control of the CMV immediate early enhancer-chicken β-actin hybrid (CAG) promoter [23].
Fig. 1.
Schematic of the knockdown strategy to generate Nrg3 KO mice. a Schematic of the knockdown strategy. Exon 2 of murine Nrg3 (GRCm38/mm10 Chr14: 39,011,969-39,012,099) was targeted for deletion using a neomycin cassette (Neo) flanked by both FRT and LoxP sites as well as genomic regions flanking Exon 2 (5′ and 3′ arms of murine Nrg3). b Northern blots. RNA extracted from whole brains of E14 (lanes 1-3), E16 (lanes 4-6), E18 (lanes 7-9), P1 (lanes 10-12) and P3 (lanes 13-15) Nrg3+/+ (lanes 1, 4, 7, 10, and 13), Nrg3+/- (lanes 2, 5, 8, 11, and 14) and Nrg3-/- (lanes 3, 6, 9, 12, and 15) was probed with Nrg3 and β-tubulin radiolabeled probe. Arrowheads point to the Nrg3 and β-tubulin bands.
The animals were housed in an animal facility with food and water ad libitum. The Johns Hopkins University Animal Care and Use Committee approved all rodent work. The KO mice used in this study were then backcrossed with C57/BL6 mice for 4 generations. We used a heterozygous breeding scheme and utilized littermates for neurobehavioral testing.
Northern Blot
To confirm loss of Nrg3, Northern blots were prepared with 2 μg poly-A RNA extracted from murine brains at embryonic day (E) 14, E16, E18, postnatal day (P) 1 and P3, as described previously [24,25].
Behavioral Studies
We performed a series of behavioral tests using male mice at 3-6 months of age. Different cohorts of wild-type (WT) and homozygous KO mice were used for no more than 2-3 behavioral tests to avoid confounding effects of overtesting. We performed the following behavioral tests: novelty-induced hyperactivity (cohort No. 1); 24-hour spontaneous activity test and elevated plus maze (cohort No. 2); PPI of the acoustic startle response; fear conditioning, and hot plate test (cohort No. 3), and psychostimulant-induced locomotion test (MK-801, 0.3 mg/kg, i.p.) (cohort No. 4). The behavioral protocols used were previously described [26,27,28]. For hot plate tests [29], mice were placed on a Columbus Instruments (Columbus, Ohio, USA) hot plate analgesia meter set at 55°C and the time to paw-lick response was recorded in seconds. The time interval between each behavioral test was at least 1 week. The numbers of animals used in each test are indicated in the figure legends.
Histopathology
A separate group of control and KO mice was sacrificed at 6 weeks of age to evaluate potential gross brain abnormalities. Mice were perfused with ice-cold phosphate-buffered saline followed by 4% paraformaldehyde; the brains were removed, cryoprotected in 30% sucrose, and frozen at −80°C. 40-µm sections were prepared and stained with Nissl for histopathological evaluation.
Statistics
The data from behavioral tests were analyzed using ANOVA with the genotype or time as the independent variables. p < 0.05 was chosen as the level of significance.
Results
Generation of the Nrg3 KO Model
Efficiency of the Nrg3 KO was tested by Northern blot comparing Nrg3-/-, Nrg3+/-, and Nrg3+/+ littermates. The KO animals showed greatly reduced levels of Nrg3 mRNA at E14, E16, E18, P1, and P3 (fig. 1b). Nrg1 and ErbB4 mRNA were similar across all genotypes (data not shown). No group differences were noted in viability, gross body morphology, or breeding capabilities.
Behavioral Phenotyping
We performed a series of tests to evaluate behavioral phenotypes resulting from deletion of the Nrg3 gene.
Novelty-Induced Locomotor Activity
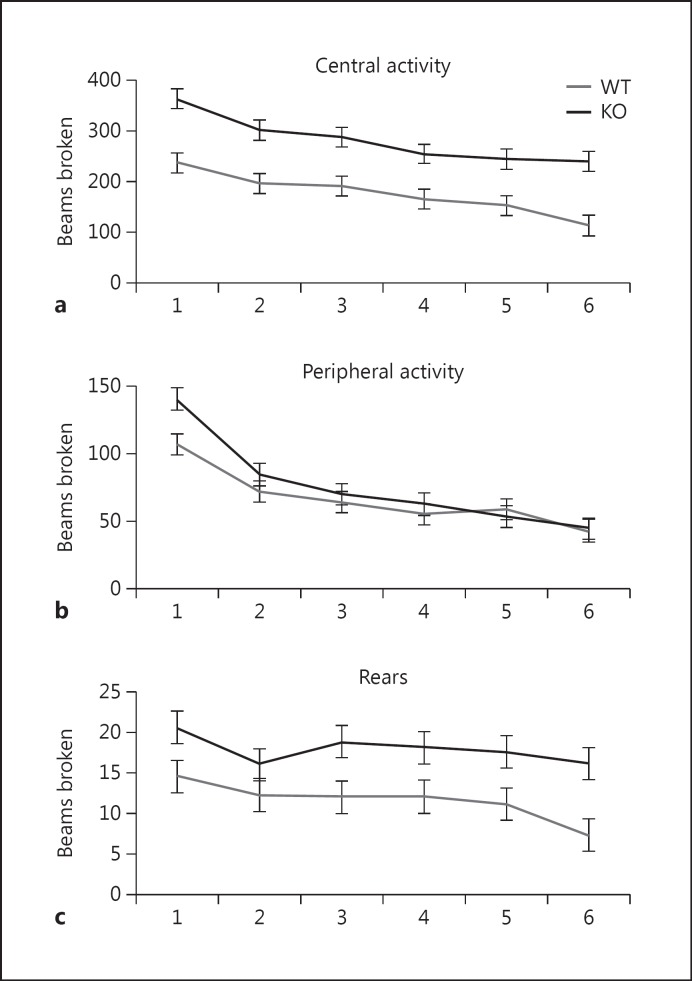
Novelty-induced activity in an open field is a test that evaluates several, often conflicting, behavioral responses to an unfamiliar environment including reactivity to novelty, exploratory activity, anxiety, and general locomotion [30]. Compared to WT mice, KO mice exhibited greater activity in the central area of the open field (fig. 2a). Two-way repeated measures ANOVA detected a significant effect of genotype [F(1,191) = 6.04, p = 0.002] and time [F(5,191) = 9.7, p < 0.001]. Post hoc tests showed significantly greater central activity in KO mice compared to WT mice (p < 0.05). KO mice did not differ from WT animals in peripheral activity or rearing activity (fig. 2b, c). Two-way repeated measures ANOVA detected only a significant effect of time for peripheral [F(5,191) = 24.0, p < 0.001] and rearing activity [F(5,191) = 4.0, p = 0.002]. The results suggest increased exploratory activity and/or attenuated anxiety in KO mice.
Fig. 2.
Novelty-induced hyperactivity in Nrg3 KO mice. a Central activity (y-axis: beam breaks, x-axis: 5-min intervals). Two-way repeated measures ANOVA detected a significant effect of genotype [F(1,191) = 6.04, p = 0.002] and time [F(5,191) = 9.7, p < 0.001]. Post hoc tests showed significantly greater novelty-induced activity in KO mice compared to WT mice (p < 0.05). b Peripheral activity (y-axis: beam breaks, x-axis: 5-min intervals). Two-way repeated measures ANOVA detected only a significant effect of time [F(5,191) = 24.0, p < 0.001]. c Rearing activity (y-axis: beam breaks, x-axis: 5-min intervals). Two-way repeated measures ANOVA detected only a significant effect of time [F(5,191) = 4.0, p = 0.002]. n = 6 per group.
24-Hour Spontaneous Locomotor Activity
As increased activity in the open field may relate to elevated spontaneous activity, we performed a 24-hour circadian rhythm test (online suppl. fig. S1; see www.karger.com/doi/10.1159/000445836 for all online suppl. material). Both groups exhibited the expected pattern of higher activity during the dark period and attenuated activity during the light period. Two-way repeated measures ANOVA detected a significant effect of time [F(23,287) = 15.4, p < 0.001]. Compared to control animals, KO mice exhibit slightly higher locomotive activity, but the results did not reach statistical significance. Together these results suggest that KO mice do not have increased baseline locomotor activity further strengthening the increased exploratory and/or attenuated anxiety findings in the open field test.
Elevated Plus Maze
In order to further assess the anxiolytic phenotype in KO mice, we tested their behaviors in the elevated plus maze, a classical test for anxiety [30]. In this test, mice that are more anxious prefer the closed arms, whereas less anxious mice tend to explore the open arms. No significant group-related differences in the time spent in the open arms or stretches (a risk-assessment behavior) were found (online suppl. fig. S2), suggesting that decreased anxiety might not explain enhanced activity in KO mice.
PPI of the Acoustic Startle
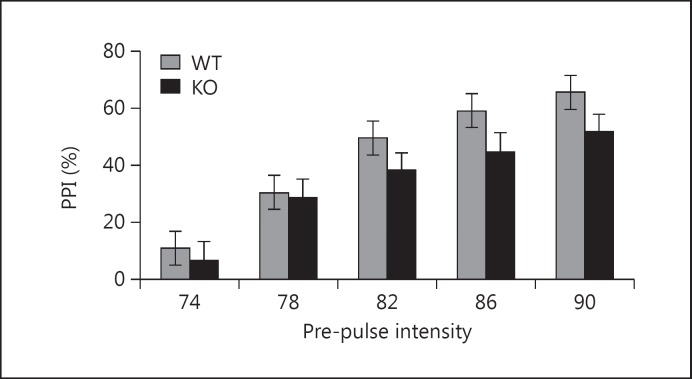
In addition to hyperactivity, positive symptoms of psychoses are modeled using PPI of the acoustic startle response, a test for sensorimotor gating impaired in patients with schizophrenia and other neuropsychiatric disorders [31,32,33]. Male KO mice exhibited diminished PPI at all prepulse intensities (fig. 3). Two-way repeated measures ANOVA detected a significant effect of genotype [F(1,169) = 5.5, p = 0.02] and prepulse intensity [F(4,169) = 21.51, p < 0.001] but no interaction between these factors, suggesting that KO mice had an overall decrease in PPI. This impairment is unlikely related to altered startle responsiveness because we found no group-dependent differences in startle magnitude (online suppl. fig. S3).
Fig. 3.
Impaired PPI in Nrg3 KO mice (y-axis: % of PPI, x-axis: different intensities of prepulse in dB). Two-way repeated measures ANOVA detected a significant effect of genotype [F(1,169) = 5.5, p = 0.02] and intensity of prepulse [F(4,169) = 21.51, p < 0.001], but no interaction between these factors. KO: n = 16, WT: n = 17.
MK-801-Induced Hyperactivity
Patients with schizophrenia display exaggerated responses to psychostimulants [34]. Acute treatments with stimulants are widely used to mimic aspects of positive symptoms in different rodent models [31,32]. Thus, we sought to evaluate behavioral responses to MK-801, an NMDA receptor antagonist, to assess glutamate synaptic transmission in WT and KO mice. Consistent with the open field test results, KO mice displayed greater activity in the habituation phase of the test and continued to be more active following MK-801 administration (online suppl. fig. S4). Two-way repeated measures ANOVA detected a significant effect of genotype [F(1,1259) = 5.9, p = 0.021] and time [F(35, 1259) = 26.8, p < 0.001] but no interaction between the two factors, suggesting that at this dose (0.3 mg/kg, i.p.) KO mice did not seem to exhibit greater responses to MK-801.
Fear Conditioning
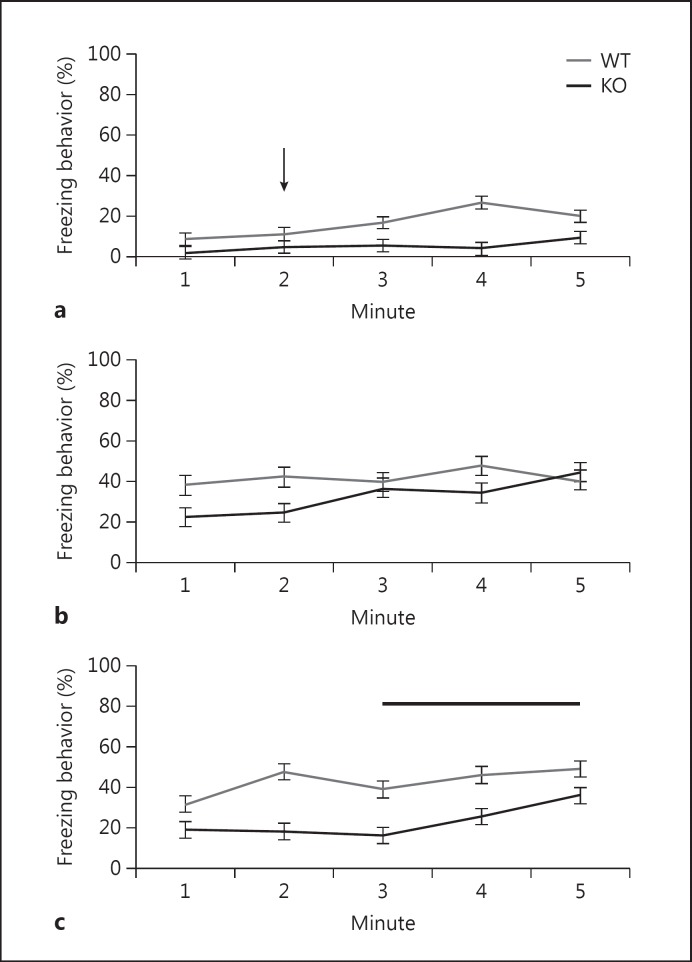
Cognitive impairment is another cardinal feature of schizophrenia [35]. Fear conditioning is a popular learning and memory test in which mice learn to associate a context (e.g., an experimental chamber) or a cue (e.g., a sound) with foot shock and is affected in patients with psychoses [36,37]. The test assesses context-dependent and cue-dependent memories that are reliant on distinct brain circuitries [38,39,40]. On day 1 (training), KO males showed decreased freezing behavior compared to control littermates (fig. 4a). Two-way repeated measures ANOVA showed a significant effect of genotype [F(1,89) = 27.1, p < 0.001] and time [F(4,89) = 3.7, p = 0.01]. No group differences were observed in context-dependent freezing on day 2 (p > 0.05) (fig. 4b). In contrast, on day 3 (cue-dependent conditioning), KO mice demonstrated significantly less freezing in response to the sound (cue) compared to control littermates (fig. 4c). Two-way repeated measures ANOVA showed a significant effect of genotype [F(1,89) = 9.8, p = 0.006] and time [(4,89) = 5.8, p < 0.001]. Post hoc test revealed significantly more freezing behavior in WT mice at time interval 5 (the end of the cue's presentation) compared to time interval 3 (the onset of the cue's presentation) (p < 0.05). In addition, there was significantly more freezing in WT mice compared to KO over the entire period of the cue's presentation (p < 0.05), suggesting attenuated associative conditioning in KO mice.
Fig. 4.
Attenuated cue-dependent fear conditioning in Nrg3 KO mice. a Training (y-axis: % of freezing behavior, x-axis: 1-min sampling intervals). Two-way repeated measures ANOVA showed a significant effect of genotype [F(1,89) = 27.1, p < 0.001] and time [F(4,89) = 3.7, p = 0.01]. Post hoc test revealed more freezing in WT mice compared to KO (p < 0.05). Arrow points to the time of presentation of sound followed by shock (one pairing). b Context-dependent fear conditioning (y-axis: % of freezing behavior, x-axis: 1-min sampling intervals). Two-way repeated measures ANOVA showed no effect of genotype (p > 0.05) and a borderline effect of time [F(4,89) = 2.4, p = 0.06]. c Cue-dependent fear conditioning (y-axis: % of freezing behavior, x-axis: 1-min sampling intervals). Two-way repeated measures ANOVA showed a significant effect of genotype [F(1,89) = 9.8, p = 0.006] and time [F(4,89) = 5.8, p < 0.001]. Post hoc test revealed significantly more freezing behavior in WT mice compared to KO (p < 0.05). KO: n = 11, WT: n = 10. Thick black line denotes the time of cue presentation (sound).
As KO mice displayed decreased freezing behavior during training, we sought to evaluate possible alterations in pain sensitivity. We used a hot plate test and found no differences between genotypes in latency to first paw lick (online suppl. fig. S5), suggesting that the observed genotype-related difference in cue-dependent fear conditioning was not due to changes in pain threshold.
Histopathological Evaluation
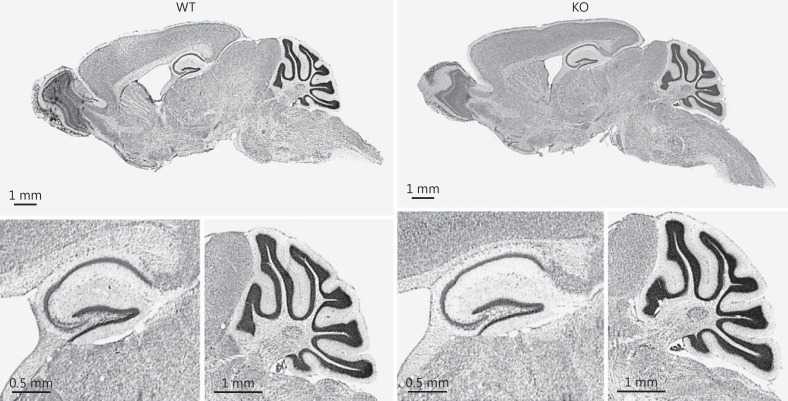
Nissl staining did not reveal any gross abnormalities in the brain cytoarchitecture or anomalies in layering of the cortex, hippocampus or cerebellum (fig. 5).
Fig. 5.
No gross brain abnormalities Nrg3 KO mice. Upper row: representative sagittal images of the brain from a WT (left) and KO (right) mouse. Bottom row: representative sagittal images of the hippocampus and the cerebellum of a WT (left) and KO (right) mouse. Nissl staining. n = 3-4 per group.
Discussion
NRG3 is an ErbB4 ligand associated with the increased risk for neuropsychiatric disorders, including schizophrenia [5,6,7,10]. However, the role of NRG3 in the neurobiological mechanisms of abnormal behaviors consistent with major psychiatric disorders remains poorly understood. The present study describes the neurobehavioral abnormalities in mice with deletion of the Nrg3 gene. We found that Nrg3 KO mice demonstrated behavioral phenotypes consistent with aspects of schizophrenia and related psychotic disorders, including increased novelty-induced activity, abnormal sensorimotor gating, and attenuated fear conditioning.
Locomotor hyperactivity and hyperreactivity in rodents are behavioral analogs of aspects of positive symptoms observed in patients with schizophrenia [31,32]. Our findings are consistent with numerous reports on novelty-induced hyperactivity in genetic mouse models of the NRG pathway. For example, Gerlai et al. [41] reported ‘a consistent hyperactivity across tests’ in a heterozygous mutant Nrg1 mice. Nrg1 hypomorph mice also exhibit hyperactivity in open field and Y-maze [13]. ErbB4 conditional KO mice with selective deletion of ErbB4 in neurons and glia or parvalbumin+ (PV) neurons had a hyperactive phenotype [42,43]. Similar behavioral alterations were found in Bace1 KO mice with deletion of BACE1, the enzyme that cleavages full-length NRG3 to release of the extracellular EGF domain [44]. Interestingly, although no group differences were observed in the elevated plus maze, KO mice tended to demonstrate less anxiety-like behavior in this test (online suppl. fig. S2). This phenotype appears opposite to that exhibited by mice exposed to exogenous NRG3 during early neonatal development as the latter treatment led to life-long anxiety and impaired social development [6]. Thus, one can suggest that hyperactivity in our Nrg3 KO model is a behavioral feature consistent with aspects of schizophrenia also observed in multiple genetic models targeting NRG signaling.
We found that Nrg3 KO mice showed decreased PPI compared to control littermates suggesting abnormal sensorimotor gating. Deficient attention and sensory information processing can be assessed by PPI of the acoustic startle, another behavioral alteration in genetic mouse models relevant to schizophrenia and other psychotic disorders [32]. As with locomotor hyperactivity, this phenotype has been reported for mice deficient in other genes of the NRG/ErbB4 pathway. Specifically, Bace1 KO mice [44], male mice with deletion of both the ErbB2 and ErbB4 genes in the brain [45], mice with conditional deletion of the ErbB4 gene in PV [43], and Nrg1 hypomorphs all exhibited deficient PPI [13]. Although impaired sensorimotor gating is not a specific sign of psychotic conditions and is observed in other neuropsychiatric disorders [33], this behavioral test has strong face and construct validity and is widely used for testing new pharmacological treatments [31,33], suggesting the potential value of our genetic mouse model for future search of new therapeutic compounds.
Cognitive impairment is an important feature and serious problem in patients with schizophrenia. While cognition encompasses many mental processes, seven domains are consistently impaired in schizophrenia: speed of processing, attention, working memory, verbal learning and memory, visual learning and memory, reasoning and problem solving, and social cognition. The three types of learning and memory that are severely affected are spatial, olfactory, and associative [46]. We found that Nrg3 KO mice demonstrated attenuated cue-dependent associative conditioning compared to control WT mice. Notably, prior reports have identified variable cognitive phenotypes among different genetic models of the NRG signaling, suggesting that targeting different components of this complex signaling pathway might result in varying behavioral outcomes. For example, mice with targeted deletion of the Egf-like domain showed deficits in contextual fear conditioning [47], while Nrg1 KO mice that lack the transmembrane domain had no cognitive abnormalities or anxiety [48]. Surprisingly, overexpression of NRG1 also attenuated context-dependent fear conditioning [49] and hippocampus-dependent spatial working memory in old transgenic mice [50]. Similarly, conditional deletion of the ERB4 receptor in PV neurons impaired contextual and working memory [43,51]. As the underlying mechanisms of cognitive impairment in patients with schizophrenia are not completely understood, the development of better therapies is impeded. Relevant genetic mouse models could help identify new targets for pharmacological interventions.
Our initial histopatholoigcal evaluation did not reveal any gross abnormalities in the brain of KO mice, including the overall cytoarchitecture and cortical layer formation. The results are congruent with multiple postmortem and imaging data that consistently show no major brain pathology in patients with psychoses [52,53,54]. Still, future studies should attempt to identify more subtle neuronal deficiencies likely present in Nrg3 KO mice as it has been demonstrated in various Nrg1 and Erb4 genetic murine models [55,56]. Another limitation of the present study is using male mice only. Future experiments will evaluate the brain and behavioral effects of deletion in female mice as well.
The future studies will also address the underlying mechanisms of the behavioral alterations produced by deletion of the Nrg3 gene. The plethora of experimental evidence suggests the role of NRG1/ErbB signaling in neuron and glial cell proliferation and differentiation, neurotransmitter function, and synaptic plasticity [19]. It is tempting to speculate that perturbations in these signaling pathways and those involving other interacting partners and genetic risk factors (e.g., DISC1) [57] likely influence the phenotypes in Nrg3 KO mice.
In conclusion, the present study describes a novel mouse model of the Nrg3 genetic deficiency relevant to aspects of schizophrenia. Future research will utilize this model to elucidate the molecular pathogenesis and underlying pathophysiology of the observed behavioral abnormalities that are relevant to psychotic conditions. In addition, our novel mouse model may help identify new targets of the NRG3/ErbB4 pathway to be useful for treatment of psychotic symptoms in patients.
Statement of Ethics
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Disclosure Statement
The authors declare no conflicts of interest. The funding agencies mentioned above did not have any involvement in study design, collection, analysis, or interpretation of the results. The writing and decision to submit is the sole decision of the authors.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
We thank the following funding agencies for the support: MH-083728 (M.V. Pletnikov), MH-094268 Silvo O. Conte Center, the Brain and Behavior Research Foundation and the Stanley Medical Research Institute (A. Sawa and M.V. Pletnikov), MH-084018 (A. Sawa), MH-069853 (A. Sawa), MH-085226 (A. Sawa), MH-088753 (A. Sawa), MH-092443 (A. Sawa), DA-040127 (A. Sawa), RUSK (A. Sawa), and S-R foundations (A. Sawa).
References
- 1.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 2.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016 doi: 10.1016/S0140-6736(15)01121-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tost H, Callicott JH, Rasetti R, Vakkalanka R, Mattay VS, Weinberger DR, et al. Effects of neuregulin 3 genotype on human prefrontal cortex physiology. J Neurosci. 2014;34:1051–1056. doi: 10.1523/JNEUROSCI.3496-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson C, Law AJ. Transient overexposure of neuregulin 3 during early postnatal development impacts selective behaviors in adulthood. PLoS One. 2014;9:e104172. doi: 10.1371/journal.pone.0104172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, et al. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc Natl Acad Sci USA. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, Liang KY, Pulver AE. Genomewide linkage scan for schizophrenia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 10q22. Am J Hum Genet. 2003;73:601–611. doi: 10.1086/378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGrath JA, Avramopoulos D, Lasseter VK, Wolyniec PS, Fallin MD, Liang K, Nestadt G, Thornquist MH, Luke JR, Chen PL, Valle D, Pulver AE. Familiality of novel factorial dimensions of schizophrenia. Arch Gen Psychiatry. 2009;66:591–600. doi: 10.1001/archgenpsychiatry.2009.56. [DOI] [PubMed] [Google Scholar]
- 10.Chen PL, Avramopoulos D, Lasseter VK, McGrath JA, Fallin DM, Liang KY, Nestadt G, Feng N, Steel G, Cutting AS, Wolyniec P, Pulver AE, Valle D. Fine mapping on chromosome 10q22-q23 implicates Neuregulin 3 in schizophrenia. Am J Hum Genet. 2009;84:21–34. doi: 10.1016/j.ajhg.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morar B, Dragović M, Waters FA, Chandler D, Kalaydjieva L, Jablensky A. Neuregulin 3 (NRG3) as a susceptibility gene in a schizophrenia subtype with florid delusions and relatively spared cognition. Mol Psychiatry. 2011;16:860–866. doi: 10.1038/mp.2010.70. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski PJ. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 17.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 18.Hatzimanolis A, McGrath JA, Wang R, Li T, Wong PC, Nestadt G, et al. Multiple variants aggregate in the neuregulin signaling pathway in a subset of schizophrenia patients. Transl Psychiatry. 2013;3:e264. doi: 10.1038/tp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 20.Assimacopoulos S, Grove EA, Ragsdale CW. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Chou SJ, Hamasaki T, Perez-Garcia CG, O'Leary DD. Neuregulin repellent signaling via ErbB4 restricts GABAergic interneurons to migratory paths from ganglionic eminence to cortical destinations. Neural Dev. 2012;7:10. doi: 10.1186/1749-8104-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Zhang L, Jie C, Obie C, Abidi F, Schwartz CE, Stevenson RE, Valle D, Wang T. X chromosome cDNA microarray screening identifies a functional PLP2 promoter polymorphism enriched in patients with X-linked mental retardation. Genome Res. 2007;17:641–648. doi: 10.1101/gr.5336307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- 27.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 30.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 31.Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 34.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 36.Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 39.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 40.Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Gerlai R, Pisacane F, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 42.Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific ErbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Jr, Vazdarjanova A, Xiong WC, Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Muller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci USA. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 47.Ehrlichman RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, et al. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res. 2009;1294:116–127. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Tuathaigh CM, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 49.Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, et al. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS One. 2010;5:e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deakin IH, Nissen W, Law AJ, Lane T, Kanso R, Schwab MH, et al. Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb Cortex. 2012;22:1520–1529. doi: 10.1093/cercor/bhr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32:2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakhshi K, Chance SA. The neuropathology of schizophrenia: a selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience. 2015;303:82–102. doi: 10.1016/j.neuroscience.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Heckers S. Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull. 1997;23:403–421. doi: 10.1093/schbul/23.3.403. [DOI] [PubMed] [Google Scholar]
- 54.Stevens JR. Neuropathology of schizophrenia. Arch Gen Psychiatry. 1982;39:1131–1139. doi: 10.1001/archpsyc.1982.04290100011003. [DOI] [PubMed] [Google Scholar]
- 55.O'Tuathaigh CM, Desbonnet L, Waddington JL. Mutant mouse models in evaluating novel approaches to antipsychotic treatment. Handb Exp Pharmacol. 2012;213:113–145. doi: 10.1007/978-3-642-25758-2_5. [DOI] [PubMed] [Google Scholar]
- 56.O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL. Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2007;31:60–78. doi: 10.1016/j.neubiorev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Seshadri S, Faust T, Ishizuka K, Delevich K, Chung Y, Kim SH, et al. Interneuronal DISC1 regulates NRG1-ErbB4 signalling and excitatory-inhibitory synapse formation in the mature cortex. Nat Commun. 2015;6:10118. doi: 10.1038/ncomms10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data