Abstract
Calcium intake has been associated with promoting bone health in children and adolescents, thus, preventing osteoporosis later in life. Behavior change such as increased calcium intake, as well as physiological factors such as bone quality, may be facilitated by psychosocial and environmental factors. The purpose of this study was to identify pathways by which psychosocial factors influence calcium intake and bone quality in middle-school girls. The study design was cross-sectional. Baseline data from the Incorporating More Physical Activity and Calcium in Teens (IMPACT) study, collected in 2001–2003, were utilized. IMPACT, was a 1 ½ years nutrition and physical activity intervention study, designed to improve bone density in 717 middle-school girls in Texas. Main outcome measures were: 1) calcium intake determined using mean milligrams of calcium consumed per day and number of glasses of milk consumed per day, and 2) bone quality determined using calcaneal stiffness index. Confirmatory factor analysis and path analysis were performed to identify the direct and indirect pathways used by various psychosocial factors such as knowledge, self-efficacy, outcome expectations and milk availability at home, to influence calcium intake and bone quality. Results showed that knowledge of osteoporosis and calcium rich foods had an indirect effect on calcium intake, with outcome expectations as the mediating variable (β=0.035 and β=0.03 respectively, p < 0.05). Calcium self-efficacy had a significant indirect effect on calcium intake, with outcome expectations as the mediator (β=0.085, p < 0.05). None of the variables significantly influenced bone quality. Thus, several direct and indirect pathways used to influence calcium intake among adolescent girls, were identified. These findings are critical for the development of effective interventions to promote calcium intake in this population.
Keywords: adolescent girls, osteoporosis, dietary intake, bone quality, calcium-rich foods
INTRODUCTION
Osteoporosis is a disease characterized by low bone mass and deterioration of bone tissue, leading to increased risk of bone fractures (1). Dietary factors such as calcium and vitamin D intake during childhood, are major determinants of osteoporosis later in life.(2) Up to 90 percent of peak bone mass is acquired by age 18 in girls and 20 in boys, which makes childhood a critical time to promote behaviors that improve bone health (3). These behaviors include consuming the recommended amount of calcium and vitamin D, engaging in regular physical activity, and avoiding smoking and alcohol (2–5). These modifiable behaviors are influenced by psychosocial and environmental factors which, in turn, mediate the associations between behavior and disease (6–9). While there is some literature examining the psychosocial correlates of calcium intake (10), one of the strongest determinants of bone mass, none have examined these interrelationships with respect to bone quality in adolescent girls. Since females are at a higher risk for osteoporosis (11), it can be advantageous to examine these relationships in a gender-specific manner.
The purpose of this study was to identify the pathways by which psychosocial factors such as knowledge of osteoporosis and calcium-rich foods, self-efficacy towards consuming calcium-rich foods, calcium outcome expectations, and milk availability, influence calcium intake and bone quality using path analysis in a cohort of adolescent females. The hypothesis was that these psychosocial factors interact and use various direct and indirect pathways to influence calcium intake and bone quality.
METHODS
This study was secondary data analysis using baseline data from The Incorporating More Physical Activity and Calcium in Teens (IMPACT) study. IMPACT was a 1 ½ -year quasi-experimental, school-based nutrition and physical activity intervention, conducted from 2001–2003, to promote bone mineral density (BMD) among middle school girls in central Texas (12). For the purpose of this study, constructs from the Social Cognitive Theory (6) were chosen to evaluate the dietary behavior of adolescents. The study population for this analysis consisted of 6th grade girls (n=12 schools, 717 girls) (Table 1). Details regarding participant recruitment are presented elsewhere (12, 13). Parental consent and student assent were obtained from all participants. Study approval was obtained from the University of Texas Health Science Center, Committee for Protection of Human Subjects (HSC-SPH-02-046).
Table 1.
Descriptive characteristics of study participants, dependent and independent variables of interest, IMPACT study, 2001–2003.
| Variable | Number | Percent |
|---|---|---|
| BMI-for-age percentilesa < 5th percentile ≥5th to <85th percentile ≥≥85th to <95th percentile ≥≥ 95th percentile |
717 28 510 105 74 |
4 71 15 10 |
| Ethnicity White Hispanic Black Other |
717 515 83 39 80 |
72 12 5 11 |
| Onset of menses | 140 | 20 |
| Mean (SD) | Range | |
| Age (years) | 11.6 (±0.4) | 10–13 |
| Calcium intake Milligrams calcium consumed/day Glasses of milk consumed/day |
1321.3 (±754.8) 1.9 (±1.3) |
154.3–5268.8 0–5 |
| Bone quality Calcaneus stiffness index (SI) |
73.8 (±14.9) | 34.7–130.1 |
| Knowledge of: Osteoporosis (% correct) Calcium content of foods (% correct) |
26.4 (±16.6) 66.1 (±16.3) |
0–85.7 0–100 |
| Self-efficacy towards calcium-rich foodsb Calcium outcome expectationsc |
29.8 (±6.6) 17.9 (±2.9) |
9–45 7–35 |
| Milk availability at homed | 3.8 (±0.5) | 0–4 |
Body Mass Index- for-age percentile calculations and classifications are based on National Center for Health Statistics and National Center for Chronic Disease Prevention and Health Promotion growth charts with < 5th percentile = underweight, ≥5th to < 85th percentile = normal weight, ≥85th to <95th percentile = at overweight and ≥ 95th percentile = obese.
Self-efficacy towards calcium-rich foods was measured using nine items on the COPA, with scores ranging from 1 to 5 for each item. These scores were then summed to develop the scale for self-efficacy with the lowest possible score being 9 and highest possible being 45.
Calcium outcome expectations was measured using seven items on the COPA, with scores ranging from 1 to 5 for each item. These scores were then summed to develop the scale for outcome expectations with the lowest possible score being 7 and highest possible being 35.
Milk availability at home was measured using one item, “Milk is available at my house”, with scores ranging from 0 (never) to 4 (All the time). Mean scores are presented.
Data collection measures: Data collection of all baseline measures was conducted in the Fall of 2001, at school sites, by trained project staff. All measures and protocols are on the project website at: http://www.sph.uth.tmc.edu/dellhealthyliving/default.aspx?id=4011
Anthropometric measures
Digital scales (SECA 770 or Tanita BWB-800S, Perspectives Enterprises, Portage, MI) and stadiometers (Perspectives Enterprises, Portage, MI) were used for weight and height measurements, using a standardized protocol. Height (cm) and weight (kg) were used to calculate body mass index (BMI) percentiles in order to determine weight status (14).
Bone quality
Quantitative Ultrasound (QUS) has been shown to provide information on elasticity and trabecular connectivity of bone tissue in post-menopausal women (15) with good precision on children ages 6 to 13 (16). For IMPACT, QUS was measured at the calcaneus using the Achilles+ Lunar G/GE Electronics ultrasound unit (Lunar, Madison, WI). Each girl’s right foot was measured with the Achilles+ Food-Sizing System before using the ultrasound machine, and pediatric shims were used to properly situate the foot in the machine. Information about the participant’s age, gender, subject ID, shim size and foot was entered into the machine. Two ultrasound machines were used. A reliability assessment to assess the correlation of repeated measures among the two machines was 0.95. QUS parameters measured were broadband ultrasound attenuation (BUA), speed of sound (SOS) and a clinical index named Stiffness Index (SI). Stiffness Index was automatically calculated by the machine using the formula: SI = (0.67×BUA + 0.28×SOS) – 420. The Os calcis is a weight-bearing site with trabecular bone and has bone mineral density (BMD) values that follow a similar pattern to that of the proximal femur (15).
Food Frequency Questionnaire (FFQ)
The interviewer-administered FFQ was used to assess frequency of consumption of various foods and the mean portion size consumed during the previous week. Intake of 112 foods, including 52 nutrients, was calculated using the Food Frequency Data Entry and Analysis Program (17), a microcomputer program developed for the data entry and analysis of the FFQ using nutrient and gram weighted data from the Food Intake Analysis System (18). The FFQ was used to determine the mean milligrams of calcium consumed per day.
Calcium, Osteoporosis and Physical Activity (COPA)
Psychosocial variables were assessed using the self-administered 85-item Calcium, Osteoporosis and Physical Activity (COPA) questionnaire. COPA was developed by adapting questions from previous instruments (19, 20) and measured psychosocial constructs for calcium intake and physical activity behaviors. For the purpose of this study, the following items were included: a) knowledge of osteoporosis (7 items) and knowledge of calcium-rich foods (9 items), b) self-efficacy of consuming calcium-rich foods (9 items), c) outcome expectations (positive beliefs about the benefits) of consuming calcium-rich foods (7 items), d) milk availability at home (1 item) and, e) glasses of milk consumed per day (1 item). Internal consistency was determined using Cronbach’s alpha (α) for knowledge of osteoporosis (α=0.44) and calcium rich foods (α=0.24), calcium self-efficacy (α=0.65) and outcome expectations (α=0.65).
Statistical analysis
Descriptive statistics were calculated for demographic characteristics such as age, ethnicity, menarchal status and body mass index. The predictor variables of interest included; knowledge of calcium-rich foods, knowledge of osteoporosis, self-efficacy towards consuming calcium-rich foods, outcome expectations of consuming calcium-rich foods, and milk availability at home. The primary outcome variables were: 1) calcium intake: measured by mean milligrams of calcium consumed per day (FFQ) and glasses of milk consumed per day (COPA), which were combined to make calcium intake; and 2) bone quality, measured by the calcaneal SI (QUS).
Path analysis
Path analysis was used to determine the pathways by which the psychosocial and environmental variables interact to influence calcium intake and bone quality at baseline. A structural model with both latent and manifest variables was tested using a covariance matrix as input and maximum likelihood estimation. Confirmatory Factor Analysis (CFA) was conducted to test the validity of the measurement portion. Practical indices of fit for CFA include the chi-square, Root Mean Square Error of Approximation (RMSEA), normed and non-normed fit indices (NFI, NNFI) (21) and the non-normed comparative fit indices (CFI) (22). All CFA and path analysis were conducted using LISREL 8.7 statistical software (Scientific Software International Inc., Lincolnwood, IL). Missing values were handled via imputation. Less than 5% of the sample had missing values for the variables of interest and < 1% of the sample was eliminated after imputation. After multiple imputations, the final sample size for the CFA and path analysis was 715 participants. The adequacy of the model was tested using the chi-square goodness-of-fit test. Standardized parameter estimates were obtained and significance level was set at p < 0.05. Because mean milligrams of calcium intake per day was skewed to the right, a natural log transformation was done to achieve normality.
RESULTS AND DISCUSSION
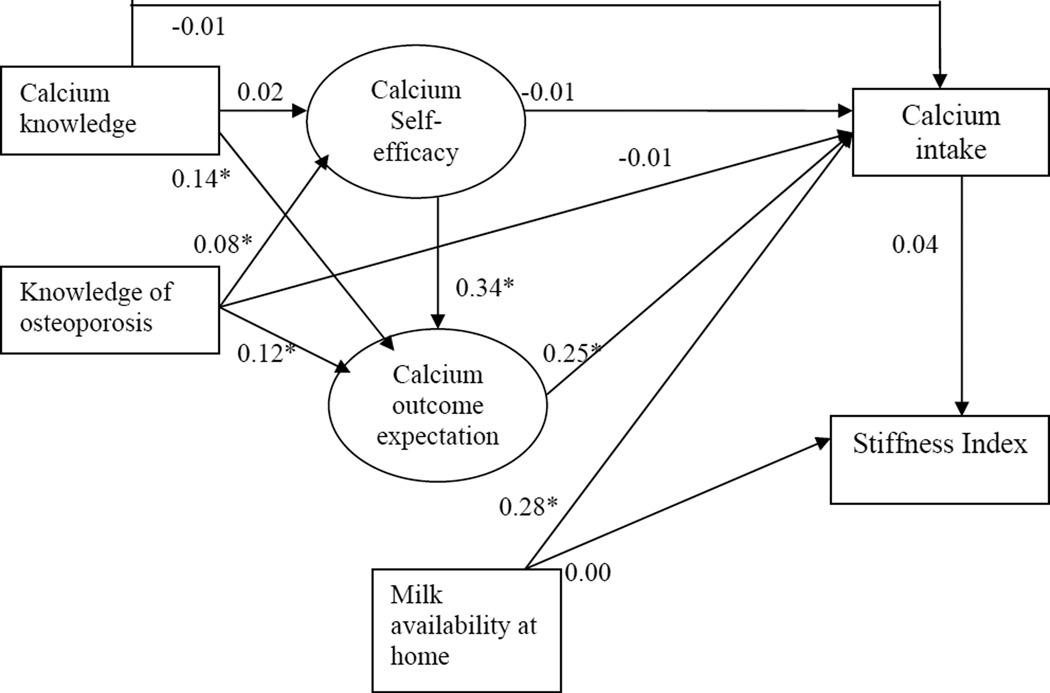
The results of the path analysis with the standardized regression coefficients are presented in Figure 1. This model showed good-fit indices with a chi-square of 80.20 (df=35, p-value= 0.00002), RMSEA=0.042, NFI=0.95, NNFI=0.96, CFI=0.97. The R-square indicates 14% of the variance in calcium intake, can be explained by this model.
Figure 1. Results of the path analysis for calcium intake and bone quality. Standardized coefficients are presented.
* significant at p < 0.05
Table 1 presents the Means ±Standard Deviations and frequency distributions for the various variables of interest. Of the 717 girls, 72% were non-Hispanic white and 25% were overweight or obese (BMI ≥ 85th percentile for age and gender) (Table 1). Most participants consumed more than the recommended amount of calcium/day (1321.3 ±754.8) and most consumed approximately 2 glasses of milk/day. This could be because milk availability at home was high (3.8 ±0.5 on a scale of 0 to 4). Additionally, milk availability was significantly correlated with both milligrams of calcium and glasses of milk consumed/day (r=0.15 and r=0.23 respectively, p < 0.01). Finally, path analysis showed that milk availability at home directly influenced calcium intake (β=0.28, p < 0.05). These findings are important because while it might seem intuitive, emphasizing the importance of milk availability to parents and schools is worthy. Recent studies state that calcium intakes of adolescents and pre-adolescents are strongly associated with availability of milk and/or calcium-rich beverages at meals (23, 24).
Only 26.4% of the participants had correct knowledge of osteoporosis. However, knowledge of calcium-rich foods was high with 66.1% responding correctly (Table 1). Pearson’s correlation coefficients showed that knowledge of osteoporosis and calcium-rich foods, calcium self-efficacy and outcome expectations, were all positively correlated with calcium intake (r=0.12-0.23; p < 0.01). Knowledge of calcium-rich foods was correlated with calcium self-efficacy and outcome expectations (r=0.12 and 0.14 respectively, p < 0.01). Path analysis showed that knowledge of osteoporosis and calcium-rich foods directly influenced outcome expectations (β=0.12 and 0.14 respectively, p < 0.05); but, only knowledge of osteoporosis directly influenced self-efficacy (β=0.08, p < 0.05). Neither of the knowledge variables directly influenced calcium intake, but, each had an indirect effect with outcome expectations as the mediator (knowledge of osteoporosis β=0.03, p < 0.05; knowledge of calcium-rich foods: β=0.035, p < 0.05). Since knowledge has been posited to be positively associated with intake (25–27), this could explain the relatively high calcium intake seen in this group. These results indicate that knowledge alone, without addressing the mediating variables, may not produce behavior change.
Calcium self-efficacy was significantly correlated with outcome expectations (r=0.24, p < 0.01). Also, self-efficacy had a direct influence on outcome expectations (β=0.34, p < 0.05) and a significant indirect effect on calcium intake, with outcome expectations as the mediator (β=0.085, p < 0.05). This indirect influence of self-efficacy on calcium intake could be because individuals with a higher confidence towards choosing calcium containing foods could have higher outcome expectations, which could lead to higher calcium intake. Studies in adults have demonstrated self-efficacy to be a better predictor of engaging in osteoporosis-preventive behaviors compared to other factors (28–30). This study is the first to demonstrate such relationship in adolescent girls.
None of the psychosocial variables or calcium intake had an influence on the SI. While some studies (4, 5) have examined the relationship between calcium consumption and bone health, a few (10, 29) have studied the relationship between psychosocial factors and calcium consumption in adolescents; and none have assessed their relationship with calcaneus SI. The lack of association between calcium intake and SI seen in the present study are not echoed by others that have demonstrated a positive effect of calcium supplementation among boys and girls (4, 5). However, in these studies, BMD was measured versus the present study where SI scores were used. Additionally, calcium supplementation was not measured in the present study. The lack of association could be attributed to using the QUS SI score versus the dual-energy x-ray absorptiometry (DXA) BMD value (30). While studies have shown a strong correlation between QUS and DXA in older women (31, 32), the relationship in adolescent girls has not yet been studied. However, Jaworski et al., reported in a study on 6 to 13 year old children, that the precision of QUS measurements in children was comparable to the precision in adults using a new positioning system (16). Finally, most study participants were meeting the recommendations for calcium. This suggests that the effects of calcium intake on bone quality might be most obvious when calcium intake is insufficient.
The strengths notwithstanding, this study has several limitations. Given the cross-sectional study design, causality cannot be determined. Other limitations include the relative ethnic homogeneity of the sample, which reduces generalizability and eliminates the ability to assess these relationships in different racial/ethnic groups. Finally, no covariates were adjusted for in the analysis. However, a recent article published by the investigative team assessed these relationships using a multivariate regression and found similar results (13).
CONCLUSION
The results of the path analysis identified some direct and indirect pathways which influence calcium intake but not bone quality in adolescent girls. The current study provides avenues for developing effective intervention strategies when addressing calcium intake in adolescents. Future studies need to evaluate the causal mechanisms influencing calcium intake and bone health in a diverse cohort of adolescent girls using a prospective design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shreela V. Sharma, Email: Shreela.V.Sharma@uth.tmc.edu, Michael and Susan Dell Center for the Advancement of Healthy Living, The University of Texas Health Science Center at Houston, School of Public Health, 1200 Herman Pressler, E RAS 603, Houston, TX 77030, 713.500.9344 (phone), 713.500.9264 (fax).
Deanna M. Hoelscher, Email: Deanna.M.Hoelscher@uth.tmc.edu, Michael and Susan Dell Center for the Advancement of Healthy Living, The University of Texas Health Science Center, School of Public Health, Austin Regional Campus, 313 E 12 St, Suite 220, Austin, TX 78701, 512.482.6168 (phone), 512.482.6185.
Steven H. Kelder, Email: Steven.H.Kelder@uth.tmc.edu, Michael and Susan Dell Center for the Advancement of Healthy Living, The University of Texas Health Science Center, School of Public Health, Austin Regional Campus, 313 E 12 St, Suite 220, Austin, TX 78701, 512.482.6170 (phone), 512.482.6185 (fax).
Pamela Diamond, Email: Pamela.M.Diamond@uth.tmc.edu, 7000 Fannin, UCT 2614, Houston, TX 77030, 713.500.9979 (phone).
R. Sue Day, Email: Rena.S.Day@uth.tmc.edu, The Michael and Susan Dell Center for the Advancement of Healthy Living, The University of Texas Health Science Center at Houston, School of Public Health, 1200 Herman Pressler, W RAS 916, Houston, TX 77030, 713.500.9317 (phone), 713.500.9329 (fax).
Albert Hergenroeder, Email: alberth@bcm.tmc.edu, Baylor College of Medicine, Chief, Adolescent Medicine Service and Sports Medicine Clinic, Texas Childrens' Hospital, 6621 Fannin Street CC610.01, Houston, Texas 77030-2399, 832-822-3660 (Office phone), 832-825-3689 (fax).
REFERENCES
- 1.Christiansen C. Consensus Development Conference - Prophylaxis and Treatment of Osteoporosis. Am J Med. 1991;90(1):107–110. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 2.Ondrak KS, Morgan DW. Physical activity, calcium intake and bone quality in children and adolescents. Sports Med. 2007;37(7):587–600. doi: 10.2165/00007256-200737070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of a meta-analysis. Bone. 2008;43(2):312–321. doi: 10.1016/j.bone.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Briganti EM, Hume C, Nowson C. Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res. 2007;22(3):458–464. doi: 10.1359/jbmr.061201. [DOI] [PubMed] [Google Scholar]
- 5.Lambert HL, Eastell R, Karnik K, Russell JM, Barker ME. Calcium supplementation and bone mineral accretion in adolescent girls: an 18-mo randomized controlled trial with 2-y follow-up. Am J Clin Nutr. 2008;87(2):455–462. doi: 10.1093/ajcn/87.2.455. [DOI] [PubMed] [Google Scholar]
- 6.Bandura A. Social Cognitive Theory of Self-Regulation. Organ Behav Hum Decis Process. 1991;50(2):248–287. [Google Scholar]
- 7.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev of Public Health. 2001;22:309–335. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 8.Lee SM, Reicks M. Environmental and behavioral factors are associated with the calcium intake of low-income adolescent girls. J Am Diet Assoc. 2003;103(11):1526–1529. doi: 10.1016/j.jada.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Glanz K, Rimer BK, Lewis FM. Health Behavior and Health Education. Third ed. San Francisco: Jossey Bass; 2002. [Google Scholar]
- 10.Magee JA, Stuberg WA, Schmutte GT. Bone quality knowledge, self-efficacy, and behaviors in adolescent females. Pediatr Phys Ther. 2008;20(2):160–166. doi: 10.1097/PEP.0b013e3181705814. [DOI] [PubMed] [Google Scholar]
- 11.Garcia BE, Spence JC, McGannon KR. Gender differences in perceived environmental correlates of physical activity. Int J Behav Nutr Phys Act. 2005;13(2):12. doi: 10.1186/1479-5868-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Springer AE, Kelder SH, Hoelscher DM. Social support, physical activity and sedentary behavior among 6th-grade girls: a cross-sectional study. Int J Behav Nutr Phys Act. 2006;3:8. doi: 10.1186/1479-5868-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SV, Hoelscher DM, Kelder SH, Day RS, Hergenroeder A. Psychosocial, environmental and behavioral factors associated with bone quality in middle-school girls. Health Educ Res. 2008;24(2):173–184. doi: 10.1093/her/cyn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. [Accessed October 3, 2008];BMI-Body Mass Index: About BMI for Children and Teens Web Site. http://www.cdc.gov/nccdphp/dnpa/bmi/childrens_BMI/about_childrens_BMI.htm.
- 15.Sahota O, San P, Cawte SA, Pearson D, Hosking DJ. A comparison of the longitudinal changes in quantitative ultrasound with dual-energy X-ray absorptiometry: The four-year effects of hormone replacement therapy. Osteoporos Int. 2000;11(1):52–58. doi: 10.1007/s001980050006. [DOI] [PubMed] [Google Scholar]
- 16.Jaworski M, Lebiedowski M, Lorenc RD, Trempe J. Ultrasound bone measurement in pediatric subjects. Calcified Tissue International. 1995;56:368–371. doi: 10.1007/BF00301604. [DOI] [PubMed] [Google Scholar]
- 17.Food frequency data entry and analysis program [computer program]. Version 2.0. Houston, TX: The University of Texas Health Science Center at Houston, School of Public Health; 1995. [Google Scholar]
- 18.Food intake analysis system [computer program]. Version 3.0. Houston, TX: The University of Texas Health Science Center at Houston, School of Public Health; 1996. [Google Scholar]
- 19.Edmundson E, Parcel GS, Feldman HA, Elder J, Perry CL, Johnson CC, Williston BJ, Stone EJ, Yang M, Lytle L, Webber L. The effects of the Child and Adolescent Trial for Cardiovascular Health upon psychosocial determinants of diet and physical activity behavior. Prev Med. 1996;25(4):442–454. doi: 10.1006/pmed.1996.0076. [DOI] [PubMed] [Google Scholar]
- 20.Saunders RP, Pate RR, Felton G, Dowda M, Weinrich MC, Ward DS, Parsons MA, Baranowski T. Development of questionnaires to measure psychosocial influences on children's physical activity. Prev Med. 1997;26(2):241–247. doi: 10.1006/pmed.1996.0134. [DOI] [PubMed] [Google Scholar]
- 21.Bentler PM, Bonett DG. Significance Tests and Goodness of Fit in the Analysis of Covariance-Structures. Psychol Bull. 1980;88(3):588–606. [Google Scholar]
- 22.Bentler PM. Comparative Fit Indexes in Structural Models. Psychol Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 23.Larson NI, Story M, Wall M, Neumark-Sztainer D. Calcium and dairy intakes of adolescents are associated with their home environment, taste preferences, personal health beliefs, and meal patterns. J Am Diet Assoc. 2006;106(11):1816–1824. doi: 10.1016/j.jada.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Cluskey M, Edlefsen M, Olson B, Reicks M, Auld G, Bock MA, Boushey CJ, Bruhn C, Goldberg D, Misner S, Wang CZ, Zaghloul S. At-home and away-from-home eating patterns influencing preadolescents' intake of calcium-rich food as perceived by Asian, Hispanic and non-Hispanic white parents. J Nutr Educ Behav. 2008;40(2):72–79. doi: 10.1016/j.jneb.2007.04.178. [DOI] [PubMed] [Google Scholar]
- 25.Krebs-Smith SM, Heimendinger J, Patterson BH, Subar AF, Kessler R, Pivonka E. Psychosocial Factors Associated with Fruit and Vegetable Consumption. Am J Health Promot. 1995;10(2):98–104. doi: 10.4278/0890-1171-10.2.98. [DOI] [PubMed] [Google Scholar]
- 26.Guthrie JF, Fulton LH. Relationship of knowledge of food group serving’s recommendations to food group consumption. Fam Econ Nutr Rev. 1995;8(4):2–17. [Google Scholar]
- 27.Harnack L, Block G, Subar A, Lane S, Brand R. Association of cancer prevention-related nutrition knowledge, beliefs, and attitudes to cancer prevention dietary behavior. J Am Diet Assoc. 1997;97(9):957–965. doi: 10.1016/S0002-8223(97)00231-9. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CH, Wang CY, McCubbin M, Zhang S, Inouye J. Factors influencing osteoporosis preventive behaviours: testing a path model. J Adv Nur. 2008;62(3):336–345. doi: 10.1111/j.1365-2648.2008.04603.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmiege SJ, Aiken LS, Sander JL, Gerend MA. Osteoporosis prevention among young women: Psychosocial models of calcium consumption and weight-bearing exercise. Health Psych. 2007;26(5):577–587. doi: 10.1037/0278-6133.26.5.577. [DOI] [PubMed] [Google Scholar]
- 30.Eddy DM, Johnston CC, Cummings SR. Osteoporosis: Review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(4):S1–S88. [PubMed] [Google Scholar]
- 31.Kang C, Speller R. Comparison of ultrasound and dual energy X-ray absorptiometry measurements in the calcaneus. Br J Radiol. 1998;71:861–867. doi: 10.1259/bjr.71.848.9828799. [DOI] [PubMed] [Google Scholar]
- 32.Trimpou P, Bosaeus I, Bengtsson BA, Landin-Wilhelmsen K. High correlation between quantitative ultrasound and DXA during 7 years of follow-up. [Accessed July 1, 2009];European J of Radiology. doi: 10.1016/j.ejrad.2008.11.024. Available online 8 January 2009. [DOI] [PubMed] [Google Scholar]