Abstract
Polybrominated diphenyl ethers (PBDEs) flame retardants (FRs) were phased-out in the mid-2000s (penta- and octaBDE) and 2013 (decaBDE); however, their hydroxylated metabolites (OH-BDEs) are still commonly detected in human serum. Today, novel FRs such as Firemaster® 550, a mixture that contains two brominated compounds, EH-TBB and BEH-TEBP are used as replacements for PBDEs in some applications, and there is a need to develop a comprehensive analytical method to assess exposure to both legacy PBDEs and novel FRs. This study developed a solid-phase extraction (SPE)-based method to analyze PBDEs, OH-BDEs, 2,4,6-tribromophenol (TBP), hexabromocylcododecane isomers (HBCDs), EH-TBB, and BEH-TEBP in human serum. Briefly, serum proteins were first denatured with formic acid, and then the target analytes were isolated using a SPE column. Finally, the extract was cleaned and fractioned using a silica SPE column. Method performance was assessed by spiking fetal bovine serum with 1–2 ng of the target analytes, and method accuracy was quantified by comparison to a serum Standard Reference Material (SRM). The developed method showed good recovery and accuracy for all target analytes with the exception of the very low and very high molecular weight PBDE congeners. Using this method, 43 serum samples collected from the Healthy Pregnancy, Healthy Baby Study (HPHB) cohort in Durham, NC, USA were analyzed for FRs. A novel finding was the ubiquitous detection of 2,4, 6-TBP, at levels greater than the individual PBDE congeners. Furthermore, 2,4,6-TBP was positively correlated with PBDEs, suggesting that they may have a similar source of exposure, or that 2,4,6-TBP may result from metabolism of PBDEs in vivo.
Keywords: Flame retardants, Serum analysis, Method development, Mass spectrometry
Introduction
Chemical flame retardants (FRs) are used in foams, polymers, and textiles to prevent combustion and suppress fire propagation. Due to strict fire safety regulations, FRs are broadly found in commercial products such as furniture, electronics, and construction material. Prior to the mid-2000s, one of the most common classes of FRs was the polybrominated diphenyl ethers (PBDEs). PBDEs were sold primarily as either penta-, octa-, or decaBDE commercial mixtures. Due to concerns regarding their persistence and human health effects, pentaBDE and octaBDE mixtures were banned in both Europe and the USA during the mid-2000s. Further, pentaBDE and octaBDE mixtures are now listed as persistent organic pollutants under Annex A of the Stockholm Convention [1]. In addition, the decaBDE mixture was phased-out by the end of 2013 [2]. Despite these phase-outs, PBDEs are still commonly detected in household furniture [3] and in house dust samples [4, 5]. PBDEs and their hydroxylated metabolites are also still widely detected in human serum [6–8]. For example, in the most recent NHANES data (2007–2008) BDE 28, 47, 99, 100, and 153 were detected in 100 % of the pooled samples.
In response to PBDE regulatory bans, the chemical flame retardant market has shifted to alternative, and sometimes novel, compound classes. Among these novel FRs is Firemaster 550® (FM550), which is a mixture of two brominated compounds [2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (BEH-TEBP)] and a suite of isopropylated aryl phosphates and triphenyl phosphate. Research from our laboratory, as well as other research groups, has shown that FM550 components are widely detected in foams from baby products [9] and sofas [3], as well as house dust [10–12]. Toxicological effects are relatively unknown, but in vitro studies have shown that EH-TBB and BEH-TEBP are potential endocrine disruptors [13]. Further, a recent rat study showed that perinatal exposure to FM550 resulted in obesity, anxiety, and early puberty [14].
Such market shifting of compounds, which can be expected to continue, suggests the need for comprehensive analytical methods to measure legacy PBDEs and their hydroxylated metabolites, as well as novel FRs in human tissues (i.e., serum). Most techniques for the analysis of PBDEs and OH-BDEs in human serum are based on those originally described by Hovander et al. [15]. This latter method involves protein denaturation with a strong acid, liquid-liquid extraction, separation of the OH-BDEs from the PBDEs by an alkaline wash, derivatization of the OH-BDEs by diazomethane (or another agent), silica or acid silica gel clean-up, and instrumental analysis by gas chromatography-mass spectrometry (GC-MS) [8, 16–19]. In recent years, liquid chromatography tandem mass spectrometry (LC-MS/MS) has been used for OH-BDE and bromophenol analysis [20–24], but with limited application to human serum [25].
Liquid-liquid extraction methods require large volumes of organic solvents and typically use diazomethane as the derivatizing agent, which is a potential hazard. Alternatively, solid-phase extraction (SPE) techniques have been developed to assist in the isolation of halogenated organic compounds (e.g., PCBs, PBDEs) and their hydroxylated metabolites, as well as halogenated phenols (e.g., brominated phenols, triclosan) [7, 26–30], in environmental matrices. However, these methods rely on derivatization for hydroxylated- and phenol-compound instrumental analysis by GC-MS. To our knowledge, no published method exists that can recover and quantify PBDEs, OH-BDEs, tribromophenols (TBPs), EH-TBB, BEH-TEBP, and HBCDs in serum samples.
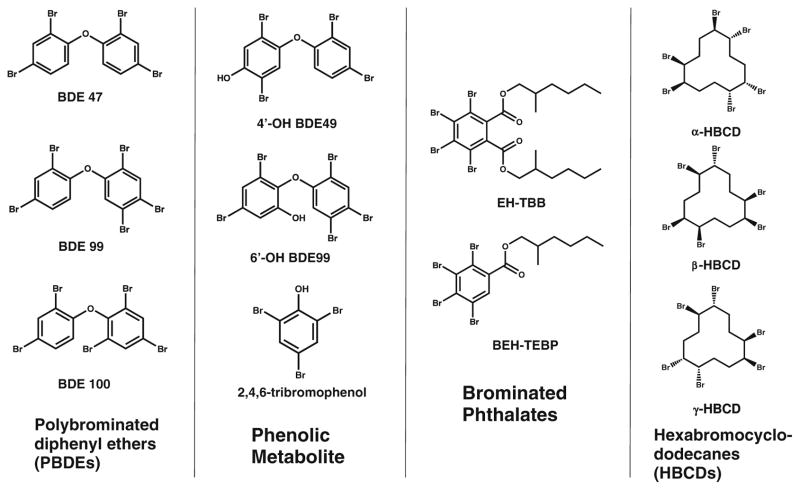
The present study developed a novel SPE extraction technique for the analysis of PBDEs, OH-BDEs, HBCDs, TBPs, EH-TBB, and BEH-TEBP in human serum. Specifically, our method uses dichloromethane (DCM): ethyl acetate to initially elute the analytes from the SPE cartridge, producing a cleaner extract. Additional purification of the extract and fractionation is performed using non-acidified silica, allowing for conservation of acid-labile compounds such as BEH-TEBP. Finally, our method is capable of analyzing TBPs, EH-TBB, and BEH-TEBP. Representative structures are shown in Fig. 1. PBDEs, TBPs, EH-TBB, and BEH-TEBP were analyzed by GC-MS, and OH-BDEs and HBCDs were analyzed by LC-MS/MS. The developed method was then applied to 43 serum samples collected in 2010–2011 from a pregnancy cohort in Durham County, NC, USA, to evaluate the detection frequency and distribution of these analytes.
Fig. 1.
Representative chemical structures for target analytes
Materials and methods
Chemicals
All solvents used were HPLC-grade or better (EMD Millipore Corporation, Billerica, MA). The PBDE calibration standard was prepared from 27 PBDE individual congeners (AccuStandard, New Haven, CT). Standard solutions of α-, β-, and γ-hexabromocyclodecane (HBCD, 99 %), 13C-α-,β-, and γ-HBCD, 13C-BDE 209 (2, 2′, 3, 3′, 4, 4′, 5, 5′, 6, 6′-BDE, 99 %), 13C-OH BDE 100 (3-OH 2,2′,4,4′,6-BDE, 99 %), 13C-6-OH BDE 47 (6-OH 2,2′,4,4′-BDE, 99 %), 2,4,5-TBP and 2, 4,6-TBP were purchased from Wellington Laboratories (Guelph, ON). 3-OH BDE 47 (3-OH 2,2′,4,4′-BDE, 97 %), 6-OH BDE 47 (100 %), 4′-OH BDE 49 (4′-OH 2,2′,4,5′-BDE, 97.8 %), 5′-OH BDE 99 (5′-OH 2,2′,4,4′,5-BDE, 99.0 %), 6′-OH BDE 99 (6′-OH 2,2′,4,4′,5-BDE, 99.3 %) and F-BDE 69 (4′-fluoro-2,3′4,6-tetrabromodiphenyl ether) were purchased from AccuStandard. 13C-CDE 141 (2,2′-3,4, 5,5′-hexachlorinated diphenyl ether, 99 %) was purchased from Cambridge Isotope Laboratories (Andover, MA). Formic acid (98 %) was purchased from Sigma-Aldrich.
Sample collection
Serum samples were collected as part of the Healthy Pregnancy, Healthy Baby Study (HPHB) cohort (n = 43) in Durham County, NC, USA. These samples all came from one clinic site: Lincoln Community Health Center, a federally qualified health center serving primarily low-income populations. A group of samples from the HPHB cohort were previously analyzed to investigate the association between PBDEs, OH-BDEs, and thyroid hormones [25]. However, the samples analyzed in the present study were from a separate group of individuals within the HPHB cohort. Serum samples were collected between September 2008 and June 2010 during a routine clinic visit in either the second (24–28 weeks of pregnancy) or third trimester (35–36 weeks). Full details regarding participant recruitment and sample collection have been described previously [25]. In brief, all participants were residents of Durham County in central NC, USA. In our cohort of 43 samples, their average age was 24.3 years (range, 18–36 years), and 55 % reported completing some college. Further, our cohort was predominately non-Hispanic black (60 %), followed by non-Hispanic white (17.5 %), and Hispanic (15 %) (demographic data was not available for 1 individual). Blood was collected in a clinical laboratory, allowed to sit on ice and clot, and then serum was isolated by centrifugation (5 min, 3500 rpm). Serum samples were kept frozen (−20 °C) until analysis. Sample collection and handling was performed according to the human subject research protocol approved by the Duke University Institutional Review Board.
Sample extraction and analysis
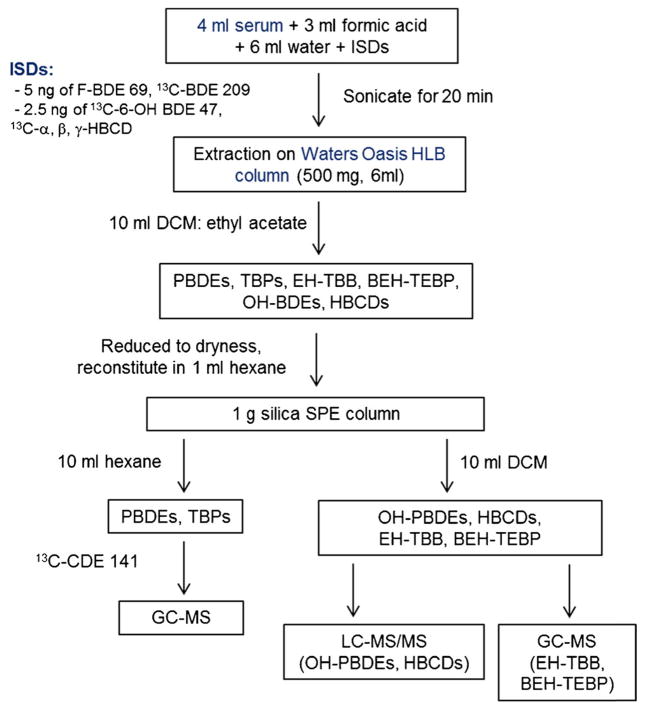
Serum was thawed and gravimetrically transferred (~3.0–4.0 g) to a glass test tube. All glassware was baked-out overnight (450 °C) and rinsed with DCM and hexane prior to use. Internal standards were spiked (2.5 ng of F-BDE 69 and 13C-BDE 209; 1 ng of 13C-6-OH BDE 47 and 13C-α-,β-, γ-HCBD) prior to the serum addition. Formic acid (2 ml) and water (6 ml) were added to denature the serum proteins and the mixture was sonicated for 20 min. The analytes were isolated on a Waters Oasis HLB column (500 mg, 6 ml). The column was conditioned with 5 ml each of dichloromethane (DCM), methanol (MeOH), and water; the sample was loaded, and the column washed with 5 ml water. The analytes were eluted with 10 ml of DCM:ethyl acetate, with the eluent blown to near dryness under a gentle nitrogen stream and reconstituted in 1 ml of hexane. The analytes were cleaned and fractionated using a silica column (1 g, Waters, Sep-Pak) that was initially conditioned with 10 ml of hexane. The first fraction was eluted with 10 ml of hexane, reduced in volume to ~100 μl, and analyzed by GC-MS for a suite of 27 PBDEs and 2,4,5- and 2,4,6-TBP. Prior to analysis, 5 ng of 13C-CDE 141 was spiked to assess F-BDE 69 recovery. The second fraction was eluted with 10 ml of DCM, blown to dryness, reconstituted in ~100 μl MeOH and analyzed by LC-MS/MS for the HBCDs and OH-PBDEs. After LC-MS/MS analysis, the MeOH fractions were solvent switched to hexane, 5 ng of 13C-TBB added, and analyzed for EH-TBB and BEH-TEBP by GC-MS.
Instrumental analysis was performed using methods previously developed by our laboratory [25, 31]. The GC-MS was operated in electron capture negative ionization mode. The LC-MS/MS conditions were altered slightly and are briefly described. Analytes were separated using a C18 column (Agilent Zorbax Eclipse XDB-C18, 4.6 × 50 mm, 1.8 μm particle size) under gradient conditions and mobile phases were water and acetonitrile (ACN), both modified with 5 mM acetic acid. Initial conditions were 40:60 water:ACN, held for 1 min, increased to 10:90 over 0.5 min, increased to 5:95 over 4.0 min, held for 0.5 min, returned to initial conditions over 0.25 min, and held for 4.25 min. The column temperature was 40 °C, the injection volume was 20 μl and flow rate was 400 μl/ml. The internal standards were 13C-α HCBD for the HBCDs and 13C-6-OH BDE 47 for the OH-BDEs. The multiple reaction monitoring transitions (MRMs) observed for the OH-tetraBDEs were m/z 500.7 > 418.8, m/z 500.7 > 265.9 and m/z 500.7 > 78.9. MRMs monitored for the OH-pentaBDEs were m/z 580.7 > 498.8, m/z 580.7 > 343.8, and m/z 580.7 > 78.9. We found that the position of the hydroxyl group gave diagnostic MRMs, as has been shown by other researchers [24]. For example, the m/z 500.7 > 418.8 and m/z 580.7 > 498.8 (formation of [M-Br]-) was unique to meta-OH BDEs. Also, the m/z 500.7 > 265.9 and m/z 580.7 > 343.7 (formation of the bromobenzoquinone) was indicative of para-OH BDEs. Finally, the ortho-OH BDEs showed only the m/z 500.7 > 78.9 and m/z 580.7 > 78.9 transitions (formation of Br-). In addition to retention time, these diagnostic MRMs were used to positively identify the OH-BDEs. The following OH-BDEs were quantified: 4′-OH BDE 49, 3-OH BDE 47, 6-OH BDE 47, 6′-OH BDE 99, and 5′-OH BDE 99. MRM chromatograms for a 0.1 ng standard (10 pg on-column) are shown in the Electronic Supplementary Material (ESM) (Fig. S1).
Lipid determination
Serum triglycerides (TG) and total cholesterol (CHOL) were determined using enzymatic techniques (Duke University Health System Clinical Laboratories, Durham, NC). The serum lipid content (TL) was estimated using the previously reported correlation equation, TL = 1.33 × TG + 1.12 × CHOL + 1.48 (g/l) [32].
Quality control
Fetal bovine serum (PAA Laboratories, Etobicoke, ON) and deionized water were both used as laboratory blanks. Method detection limits (MDLs) were calculated as three times the standard deviation of the laboratory blanks. Analytes levels were similar in both the fetal bovine serum and the deionized water and therefore both matrices were used for the MDL calculations. If analytes were not detected in the blanks, then the MDL was quantified as three times the standard deviation of the baseline noise. The only analytes detected in the laboratory blanks were BDE 47 (mean = 890 pg/g lw), BDE 99 (380 pg/g lw), BDE 100 (585 pg/g lw), and 6-OH BDE 47 (440 pg/g lw). For PBDEs, the accuracy of the developed method was tested by analyzing SRM 1958 [Fortified Human Serum; NIST (National Institute of Standards and Technology), Gaithersburg, MD].
Data analysis
Statistical analysis was performed using JMP® Pro (v. 11.0.0, 2013, SAS Institute Inc., Cary, NC). Values below MDL were assigned a value of half the detection limit for statistical analysis only. Summary statistics were only calculated for those variables with detection frequencies >50 %. Analyte data were assessed for normality using the Shapiro-Wilkes test and log-transformed as necessary. Correlations among analytes, and between serum lipid level and analyte concentrations, were examined using the Spearman rank-order correlation. Statistical significance was set at alpha < 0.05.
Results and discussion
General method development
Our SPE extraction methods were adapted from previous techniques using Oasis HLB SPE columns for the extraction of PBDEs, HBCDs, and OH-BDEs from serum [27–29, 33]. A flow chart of the developed method is shown in Fig. 2. Due to the range of analyte polarity, selection of an appropriate elution solvent was critical to elute the analytes only and avoid matrix co-elution. DCM was not sufficiently polar to extract the OH-BDEs from the HLB column, but was sufficient for PBDEs, HBCDs, EH-TBB, and BEH-TEBP (data not shown). Whereas other studies have used 1:1 DCM:MeOH as the elution solvent, we found that this solvent mixture eluted the serum matrix from the column. In particular, the matrix co-elution caused severe signal suppression and poor peak shape for the OH-BDEs and HBCDs during the LC-MS/MS analysis. Instead, we used 1:1 DCM:ethyl acetate to elute the target analytes. This elution mixture was sufficient to elute the polar analytes, while eluting a minimal amount of matrix.
Fig. 2.
Flow chart of extraction and analysis methods
Additional extract clean-up and analyte fractionation was performed using a silica gel SPE column. Previous studies have used sulfuric acid silica gel columns to clean the serum matrix, but in our experiments the use of acidic silica resulted in the near complete loss of BEH-TEBP (~5 % recovery, n = 3). Further, aggressive cleaning techniques were not required since very little matrix was eluted by the DCM:ethyl acetate. The first fraction (hexane) only contained the PBDEs; additional fractions with 1:1 DCM:hexane and DCM did not elute additional PBDEs. The OH-BDEs, HCBDs, EH-TBB, and BEH-TEBP were eluted in 1:1 DCM:hexane. Additional tests demonstrated equal extraction efficiency using DCM as the eluant, and thus DCM was selected due to the higher polarity.
Spike and recovery
Method performance was assessed by spiking 1–2 ng of all analytes (PBDEs, 2,4,6-TBP, α-HBCD, and OH-BDEs) into 3 ml of fetal bovine serum (Table 1). Overall, the method showed excellent recovery for the tri- to hexa-BDEs, with a mean recovery of 81 % (range, 61–106 %). The octa- and nona-BDE congeners showed lower recovery (mean, 55 %, range, 40–61 %), demonstrating that the method was not as efficient at capturing the higher brominated BDEs which is consistent with previous studies [34]. Further, BDE 209 was not recovered in the method. The 2,4,6-TBP, α-HBCD, EH-TBB, BEH-TEBP, and OH-BDEs generally showed >90 % recovery, but the tetra-OH BDE recovery was slightly lower, ranging from 59–75 %.
Table 1.
Analyte recovery (%) in fetal bovine serum matrix spikes
| Mean | Std Error | |
|---|---|---|
| BDE 30 | 87 | 3 |
| BDE 17 | 97 | 5 |
| BDE 25 | 106 | 4 |
| BDE 28,33 | 82 | 3 |
| BDE 75 | 74 | 6 |
| BDE 49 | n/a | n/a |
| BDE 71 | 85 | 1 |
| BDE 47 | 71 | 6 |
| BDE 66 | 90 | 6 |
| BDE 100 | 79 | 6 |
| BDE 119 | 81 | 5 |
| BDE 99 | 61 | 5 |
| BDE 116 | 83 | 4 |
| BDE 85,155 | 79 | 5 |
| BDE 154 | 75 | 5 |
| BDE 153 | 74 | 5 |
| BDE 138 | 81 | 5 |
| BDE 156 | 77 | 5 |
| BDE 183 | 60 | 4 |
| BDE 191 | 57 | 4 |
| BDE 181 | 59 | 3 |
| BDE 190 | 61 | 3 |
| BDE 203,200 | 53 | 9 |
| BDE 205 | 40 | 2 |
| EH-TBB | 111 | 4 |
| BEH-TEBP | 101 | 5 |
| 2,4,6-TBP | 93 | 5 |
| α-HBCD | 87 | 15 |
| 5′-OH BDE 99 | 94 | 7 |
| 6-OH BDE 99 | 89 | 4 |
| 3-OH BDE 47 | 64 | 8 |
| 4′-OH BDE 49 | 59 | 3 |
| 6-OH BDE 47 | 75 | 0.3 |
n = 5 for PBDEs; n = 3 for EH-TBB, BEH-TEBP, 2,4,6-TBP, α-HBCD and OH-BDEs
Spike levels: PBDEs, EH-TBB, BEH-TEBP = 1–2 ng; 2,4,6-TBP = 2 ng; α-HBCD and OH-BDEs = 1 ng
For the FBS matrix spikes only, BDE 49 and BDE 71 co-eluted and could be quantified separately
Standard reference material
Method accuracy for PBDEs was assessed by extracting NIST Standard Reference Material (SRM) 1958 (Fortified Human Serum). Most of the PBDEs showed good accuracy: ranging from 67 % (standard error = 2.9 %) for BDE 99 to 99 % (5.2 %) for BDE 154, with the exception of BDE 153 (mean recovery = 46 %, SE = 2.4 %) (Table 2). However, the method showed poor accuracy for the lower brominated (i.e., BDE-17) and higher brominated (i.e. BDE-183) congeners. Overall, these results suggest that the method developed was satisfactory for most tri- through hepta-BDE congeners.
Table 2.
Method performance (percent accuracy) for PBDEs in NIST SRM 1958
| SRM 1 | SRM 2 | SRM 3 | Mean (SE) | |
|---|---|---|---|---|
| BDE 17 | 51 | 51 | 60 | 54 (3.2) |
| BDE 28/33 | 80 | 80 | 88 | 82 (2.6) |
| BDE 47 | 76 | 77 | 85 | 80 (2.8) |
| BDE 66 | 74 | 79 | 86 | 79 (3.5) |
| BDE 100 | 71 | 74 | 85 | 77 (4.1) |
| BDE 99 | 64 | 64 | 73 | 67 (2.9) |
| BDE 85/155 | 69 | 75 | 82 | 75 (3.8) |
| BDE 154 | 90 | 100 | 107 | 99 (5.2) |
| BDE 153 | 42 | 45 | 50 | 46 (2.4) |
| BDE 183 | 20 | 25 | 25 | 23 (1.4) |
Method application to maternal serum
Associations between serum lipid and flame retardant levels
Hydrophobic organic compounds tend to partition into the lipid component of tissues. As a result, normalizing the serum chemical concentrations by the total serum lipid level [35] has been suggested, and this approach is typically used in the literature [6, 36]. To our knowledge, though, very few studies actually report lipid content-organic contaminant correlations [37]. In our cohort, the lipid content was not correlated with the predominate BDE congeners or 2,4,6-TBP (BDE-47: p = 0.60; BDE-99: p = 0.64; BDE-100: p = 0.43; BDE-153: p = 0.75; BDE-154: p = 0.69; sum-BDE: p = 0.58; 2,4,6-TBP: p = 0.27). To be consistent with the literature, we are here reporting values in ng/g lipid weight (ng/g lw). However, the non-lipid-normalized values are presented in the supporting information.
PBDEs
Seven of the 27 PBDE congeners were detected above the MDL in the 43 maternal serum samples, and at least 1 PBDE was detected in every sample (Table 3). The congeners frequently detected included: BDE 28/33, 47, 99, 100, 153, 154, and 183. Total PBDEs ranged from 0.71–252 ng/g lipid in the serum samples. Total PBDEs, BDE-47, BDE-100, and BDE-99 were log-normally distributed, but BDE-154 and BDE-153 were neither normally nor log-normally distributed.
Table 3.
Serum concentration (pg/g lw)
|
|
Percentile
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | MDL (pg/g lw) | Detection frequency (%) | Geometric mean (95 % CI) | Min | Max | 25th | 50th | 75th | 95th |
| PBDEs | |||||||||
| BDE 28/33 | 360 | 49 | NA | <360 | 2600 | NA | NA | NA | NA |
| BDE 47 | 1100 | 98 | 13,400 (1000–18,100) | <1100 | 122,000 | 7600 | 13,700 | 25,300 | 48,400 |
| BDE 66 | 1300 | 0 | NA | <1300 | <1300 | NA | NA | NA | NA |
| BDE 99 | 750 | 93 | 3600 (2600–5100) | <750 | 78,300 | 2100 | 3500 | 7000 | 31,700 |
| BDE 100 | 490 | 93 | 2500 (1800–3500) | <490 | 27,800 | 700 | 3000 | 5800 | 14,300 |
| BDE 153 | 240 | 98 | 3800 (2800–5200) | <240 | 22,100 | 2300 | 4400 | 6900 | 15,100 |
| BDE 154 | 140 | 81 | 430 (310–620) | <140 | 7500 | 200 | 600 | 900 | 1700 |
| BDE 183 | 310 | 5 | NA | <310 | 1800 | NA | NA | NA | NA |
| ΣPBDEs | 27,500 (21,000–35,800) | 710 | 252,000 | 19,000 | 27,200 | 46,300 | 97,800 | ||
| Phenolic metabolites | |||||||||
| 2,4,5-TBP | 1200 | 0 | NA | <1200 | <1200 | NA | NA | NA | NA |
| 2,4,6-TBP | 1800 | 100 | 19,200 (15,700–23,400) | 5100 | 63,400 | 11,800 | 21,500 | 28,200 | 45,600 |
| 5′-OH BDE 99 | 2.1 | 53 | 62 (34–110) | <2.1 | 1800 | 10 | 30 | 330 | 1200 |
| 6-OH BDE 99 | 5.6 | 0 | NA | <5.6 | <5.6 | NA | NA | NA | NA |
| 3-OH BDE 47 | 20 | 5 | NA | <20 | 610 | NA | NA | NA | NA |
| 4′-OH BDE 49 | 1.4 | 58 | 29 (19–46) | <1.4 | 370 | 10 | 50 | 120 | 220 |
| 6-OH BDE 47 | 340 | 0 | NA | <340 | <340 | NA | NA | NA | NA |
| Brominated phthalates | |||||||||
| EH-TBB | 56,000 | 0 | NA | <56,000 | <56,000 | NA | NA | NA | NA |
| BEH-TEBP | 24,000 | 0 | NA | <56,000 | <56,000 | NA | NA | NA | NA |
| Hexabromocyclodecanes | |||||||||
| α-HBCD | 84 | 7 | NA | <84 | 3000 | NA | NA | NA | NA |
| β-HBCD | 84 | 0 | NA | <84 | <84 | NA | NA | NA | NA |
| γ-HBCD | 84 | 0 | NA | <84 | <84 | NA | NA | NA | NA |
BDE-47 was the most abundant congener, contributing approximately 50 % of the total BDE burden in the serum samples. BDE-47 was detected in 98 % of samples with a geometric mean of 13.4 ng/g lipid (95 % confidence interval, 10.0–18.1). The second most abundant congeners were BDE-153 and BDE-99, with geometric mean levels of 3.8 ng/g lipid (95 % CI, 2.8–5.2) and 3.6 ng/g lipid (95 % CI, 2.6–5.1), respectively. These PBDE levels and patterns were similar to those previously reported for this cohort, with the exception of BDE-153, which was lower in the current study [25]. The lower BDE-153 levels are presumably due to the low method accuracy as reflected in the SRM 1958 sample analysis. In addition, the PBDE congener patterns in the current study are similar to those from other regions of North America [6, 7].
PBDE levels from the current North Carolina cohort were lower than those reported in the 2003–2004 National Health and Nutrition Examination Survey (NHANES) from the USA [36]. The NHANES 2003–2004 dataset is the most recent national representative dataset for which samples were analyzed on the individual level. For example, the 2003–2004 NHANES 20–39 year age group levels were 1.6-fold and 1.4-fold lower in the more recent North Carolina cohort for BDE-47 and BDE-99, respectively. The NHANES cohort includes individuals from diverse race/ethnicity groups, whereas our cohort was predominantly African-American. However, PBDE levels in the NHANES non-Hispanic Black, female sub-group were higher than those of the overall pooled mean for the full NHANES sample, suggesting that racial/ethnic differences were not responsible for the higher NHANES levels. The North Carolina cohort was collected at a significantly later time period (2008–2010), and PBDE serum levels have generally shown decreasing trends in the USA since the early 2000s [7, 38], which may explain some of the variability between the NHANES and NC samples. In addition, differences in the geographic scope of study areas and the representation of low-income individuals between the cohorts may be responsible for the observed differences. Similarly, PBDE serum levels from the 2007–2009 Canadian Health Measures Survey (20–39 year group) were 1.6-fold higher for both BDE-47 and BDE-99, respectively [6]. Again, these differences may be driven by geographic, temporal, or socioeconomic differences.
OH-BDEs and TBPs
Five OH-BDE congeners were monitored in the present study, but only 5′-OH BDE 99 and 4′-OH BDE 49 were detected in >50 % of the samples. Geometric mean levels were 0.062 ng/g lw and 0.029 ng/g lw for 5′-OH BDE 99 and 4′-OH BDE 49, respectively. These levels were 1–2 orders of magnitude lower than the PBDEs; this finding is consistent with previous studies in human serum [8, 19, 25, 38]. The 4′-OH BDE 49 levels in our cohort (geometric mean = 29 pg/g lw) were somewhat lower than those reported from a different subset of the study cohort (geometric mean = 110 pg/g lw) [25]. It is difficult to directly compare the OH-BDE levels in our study to those from other regions since most report levels on ng (or pg) per ml basis. However, comparing wet weight concentrations (ESM Table S1) shows that the 4′-OH BDE 49 and 5′-OH BDE 99 levels in our cohort were ~10- to 60-fold lower than from other regions in the USA [8, 38, 39]. The higher concentrations in the previously published studies [38, 39] may be due to the fact that the cohorts were from California, which is known to have higher PBDE exposure due to California Technical Bulletin 117.
2,4,6-TBP was detected in 100 % of samples, but 2,4, 5-TBP was not detected in any sample. Interestingly, the levels of 2,4,6-TBP were greater than individual PBDE congeners. For example, the 2,4,6-TBP geometric mean was 19.2 ng/g lw and the BDE-47 geometric mean was 13.4 ng/ g lw. Although studies are limited, 2,4,6-TBP has also been reported in serum from Japan [40], Hong Kong [19, 41], Norway [42], Pakistan [27], northern Quebec [43], and the USA [16]. However, reported 2,4,6-TBP concentrations vary greatly among studies. For example, Qui et al. [16] reported mean 2,4,6-TBP levels of 0.8 ng/g lw in maternal serum from Indiana in 2003–2004, ~25-times lower than our study. 2,4, 6-TBP exposure is not well understood, but can originate from multiple sources. 2,4,6-TBP is used as a flame retardant and is an intermediate in the production of other flame retardants chemicals [44]. For example, 2,4,6-TBP was recently detected in a novel brominated triazine-based flame retardant—2,4, 6-tris(2,4,6-tribromophenoxy-1,3,5-triazine (TTBP-TAZ), a compound used in various electronics plastics [45]. As such, 2,4,6-TBP has been detected near e-waste sites [46], as well as in indoor air [47]. In addition, 2,4,6-TBP is formed naturally in marine algae [48] and sponges [49] and can bioaccumulate in higher trophic level marine mammals [49]. Finally, 2,4, 6-TBP could also be formed through the metabolism of certain PBDEs, in a mechanism similar to the formation of 2,4, 5-TBP from BDE-99 [50, 51].
HBCDs
HBCDs were infrequently detected (7 % of individuals), with only the α-HBCD isomer observed (range, <0.02–3.0 ng/g lw). Dominance of the α-HBCD isomer in serum is consistent with previous studies with the exception of a recent study from South Korea that showed the γ-HBCD as the dominant isomer. HBCDs are widely detected in house dust, at levels comparable to PBDEs [5], suggesting that the low serum levels may be due to poor uptake or rapid metabolism. Overall, there are few literature reports of HBCDs in human serum, but levels are generally within the range observed in the present study [6, 52–54]. In contrast to our findings, a recent Canadian study observed 100 % detection of α-HBCD and 23 and 35 % detection of β- and γ-HBCD, respectively (geometric mean ΣHBCD = 0.85 ng/g lipid) [6].
EH-TBB and BEH-TEBP
EH-TBB and BEH-TEBP were not detected in any serum sample, but these analytes had relatively high MDLs (56, 000 pg/g lw for EH-TBB and 24,000 pg/g lw for BEH-TEBP). To our knowledge, only one other study has investigated EH-TBB and BEH-TEBP in human serum [55] . The previously reported cohort comprised nursing women from Quebec, Canada and collected samples in 2008–2009 (n = 102). The detection frequencies for the Quebec cohort were 57 % for EH - TBB (geometric mean concentration = 1300 pg/g lw) and 17 % for BEH-TEBP (geometric mean not reported) [55]. Although exposure to EH-TBB via house dust appears to be ubiquitous [3, 5], EH-TBB is rapidly metabolized to TBBA [56] which may explain the non-detect serum levels in our cohort.
Associations between PBDEs, OH-BDEs, and 2,4,6-TBP
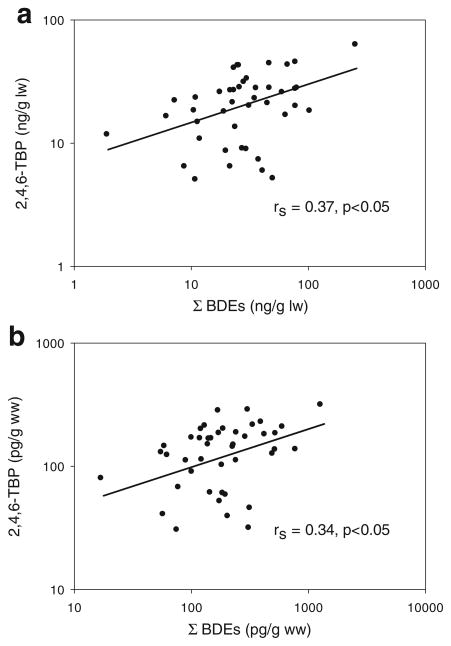
Considering the lipid normalized concentrations, all PBDEs and ΣPBDE were highly correlated with Spearman rank correlation coefficients ranging from 0.40–0.95 (p < 0.05 for all relationships) (Fig. 3 and Table 4). Since the lipid levels were not associated with the PBDE and 2,4,6-TBP levels in our cohort, Spearman coefficients were also calculated using non-lipid normalized concentrations and negligible differences were observed. These trends suggest a common exposure source such as house dust [57]. Further, 4′-OH BDE 49 and 5′-OH BDE 99 were correlated with most BDEs. A notable exception was that 5′-OH BDE 99 was not correlated with BDE 99 (p = 0.14). This was unexpected considering that 5′-OH BDE 99 is metabolite of BDE 99 [50]. As well, 2,4, 6-TBP and the PBDE/ ΣPBDE congeners were correlated with the exception of BDE-47. These relationships were not as strong as among the individual PBDE congeners. This may indicate multiple exposure sources or that some of the PBDEs are metabolizing to 2,4,6-TBP. To our knowledge, this is the first report describing the relationships between 2,4,6-TBP and PBDE congeners in blood. However, a recent study from a Hong Kong cohort showed a positive relationship between Σ 2,4,6-TBP-glucuronide and sulfate conjugates in urine and Σ 2,4,6-tribromo-BDEs in human serum [41].
Fig. 3.
Scatter plot 2,4,6-TBP versus ΣBDEs in serum from the HPHB cohort in North Carolina using a lipid normalized and b non-lipid normalized concentrations
Table 4.
Spearman rank correlation matrix for PBDEs (ng/g lw) with frequency of detection >50 %
| Variable | BDE-47 | BDE-100 | BDE-99 | BDE-153 | BDE-154 | ΣBDEs | 2,4,6-TBP | 4′-OH BDE49 |
|---|---|---|---|---|---|---|---|---|
| PBDEs | ||||||||
| BDE-47 | ||||||||
| BDE-100 | 0.94## | |||||||
| BDE-99 | 0.82## | 0.80## | ||||||
| BDE-153 | 0.45** | 0.60## | 0.48# | |||||
| BDE-154 | 0.66## | 0.63## | 0.65## | 0.40** | ||||
| ΣBDEs | 0.95## | 0.95## | 0.89## | 0.71## | 0.63## | |||
| Phenolic metabolites | ||||||||
| 2,4,6-TBP | 0.29 | 0.37** | 0.30* | 0.43** | 0.30* | 0.37* | ||
| 4′-OH BDE 49 | 0.47** | 0.43** | 0.25* | 0.19 | 0.17 | 0.41** | 0.01 | |
| 5′-OH BDE 99 | 0.36* | 0.39** | 0.23 | 0.38* | 0.11 | 0.34* | −0.03 | 0.57# |
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001
Conclusions
A comprehensive analytical method was developed to measure legacy PBDEs and their hydroxylated metabolites, as well as HBCDs, EH-TBB, and BEH-TEBP in human serum. The nearly ubiquitous detection of PBDEs in serum indicates that, despite their phase-out in the mid-2000s and 2013, PBDEs continue to be present in human tissues. Future monitoring is essential. In addition, two metabolites of PBDEs—5′-OH BDE 99 and 4′-OH BDE 47—were broadly detected. 2,4,6-TBP was ubiquitously detected at levels that were generally greater than individual PBDE congeners. The detection of OH-BDEs and 2,4,6-TBP is important because these compounds have been shown to inhibit thyroid hormone regulating enzymes, with potencies greater than the PBDEs [58, 59]. There are very few reports of 2,4,6-TBP in human serum; future monitoring should be expanded to include this compound. Finally, as newer and alternative flame retardants are introduced into the marketplace, existing analytical techniques will need to be modified to expand monitoring programs.
Supplementary Material
Acknowledgments
We would like to thank all of our participants in this study. We also would like to thank Dr. Pamela Maxson for managing the recruitment effort and to Claire Osgood for data management support. This work was supported by a grant from the National Institute of Environmental Health Sciences, R01 ES020430, and a grant from the USEPA (RD-83329301).
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00216-016-9340-3) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflicts of interest with this research.
References
- 1.Nations S-GotU. [Accessed April 25, 2014];C.N.524.2009.Treaties-4. 2009 Aug 26; http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-TREATY-NOTIF-CN524-2009.En.pdf.
- 2.Agency USEP DecaBDE Phase-out Initiative. [Accessed October 6, 2014]; http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html.
- 3.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46(24):13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromme H, Hilger B, Kopp E, Miserok M, Völkel W. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int. 2014;64:61–8. doi: 10.1016/j.envint.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawn DFK, Ryan JJ, Sadler AR, Sun WF, Weber D, Laffey P, et al. Brominated flame retardant concentrations in sera from the Canadian Health Measures Survey (CHMS) from 2007 to 2009. Environ Int. 2014;63:26–34. doi: 10.1016/j.envint.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey: 2003–2008. Environ Sci Technol. 2014;48(1):753–60. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen A, Park JS, Linderholm L, Rhee A, Petreas M, Defranco EA, et al. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol. 2013;47(8):3902–8. doi: 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, Van Bergen S, Cooper E, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodson RE, Perovich LJ, Covaci A, Van Den Eede N, Ionas AC, Dirtu AC, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46(24):13056–66. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoeib M, Harner T, Webster GM, Sverko E, Cheng Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ Pollut. 2012;169:175–82. doi: 10.1016/j.envpol.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 13.Saunders DMV, Higley EB, Hecker M, Mankidy R, Giesy JP. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol Lett. 2013;223(2):252–9. doi: 10.1016/j.toxlet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster® 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124–36. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J Anal Toxicol. 2000;24(8):696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- 16.Qiu XH, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the united states. Environ Health Perspect. 2009;117(1):93–8. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athanasiadou M, Cuadra SN, Marsh G, Bergman A, Jakobsson K. Polybrominated diphenyl ethers (PBDEs) and bioaccumulative hydroxylated PBDE metabolites in young humans from Managua, Nicaragua. Environ Health Perspect. 2008;116(3):400–8. doi: 10.1289/ehp.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45(18):7896–905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HS, Chen ZJ, Ho KL, Ge LC, Du J, Lam MHW, et al. Hydroxylated and methoxylated polybrominated diphenyl ethers in blood plasma of humans in Hong Kong. Environ Int. 2012;47:66–72. doi: 10.1016/j.envint.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Chang H, Wan Y, Naile J, Zhang X, Wiseman S, Hecker M, et al. Simultaneous quantification of multiple classes of phenolic compounds in blood plasma by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2010;1217(4):506–13. doi: 10.1016/j.chroma.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 21.Chu S, Letcher RJ. Halogenated phenolic compound determination in plasma and serum by solid phase extraction, dansylation derivatization and liquid chromatography-positive electrospray ionization-tandem quadrupole mass spectrometry. J Chromatogr A. 2013;1320:111–7. doi: 10.1016/j.chroma.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 22.Feo ML, Barón E, Aga DS, Eljarrat E, Barceló D. Development of a liquid chromatography-electrospray chemical ionization tandem mass spectrometry analytical method for analysis of eleven hydroxylated polybrominated diphenyl ethers. J Chromatogr A. 2013;1301:80–7. doi: 10.1016/j.chroma.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y, Chen X, Lam MHW, Cai Z. Analysis of hydroxylated polybrominated diphenyl ethers in rat plasma by using ultra performance liquid chromatography-tandem mass spectrometry. J Chromatogr, B: Anal Technol Biomed Life Sci. 2011;879(15–16):1086–90. doi: 10.1016/j.jchromb.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Mas S, Jauregui O, Rubio F, de Juan A, Tauler R, Lacorte S. Comprehensive liquid chromatography-ion-spray tandem mass spectrometry method for the identification and quantification of eight hydroxylated brominated diphenyl ethers in environmental matrices. J Mass Spectrom. 2007;42(7):890–9. doi: 10.1002/jms.1224. [DOI] [PubMed] [Google Scholar]
- 25.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119(10):1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dirtu AC, Jaspers VLB, Cernat R, Neels H, Covaci A. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ Sci Technol. 2010;44(8):2876–83. doi: 10.1021/es902149b. [DOI] [PubMed] [Google Scholar]
- 27.Ali N, Eqani SAMAS, Malik RN, Neels H, Covaci A. Organohalogenated contaminants (OHCs) in human serum of mothers and children from Pakistan with urban and rural residential settings. Sci Total Environ. 2013;461–462:655–62. doi: 10.1016/j.scitotenv.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 28.Dirtu AC, Roosens L, Geens T, Gheorghe A, Neels H, Covaci A. Simultaneous determination of bisphenol A, triclosan, and tetrabromobisphenol A in human serum using solid-phase extraction and gas chromatography-electron capture negative-ionization mass spectrometry. Anal Bioanal Chem. 2008;391(4):1175–81. doi: 10.1007/s00216-007-1807-9. [DOI] [PubMed] [Google Scholar]
- 29.Covaci A, Voorspoels S. Optimization of the determination of polybrominated diphenyl ethers in human serum using solid-phase extraction and gas chromatography-electron capture negative ionization mass spectrometry. J Chromatography B Anal Technol Biomed Life Sci. 2005;827(2):216–23. doi: 10.1016/j.jchromb.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen C, Liane VH, Becher G. Automated solid-phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum-application on archived Norwegian samples from 1977 to 2003. J Chromatogr, B: Anal Technol Biomed Life Sci. 2007;846(1–2):252–63. doi: 10.1016/j.jchromb.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, et al. Alternate and new brominated flame retardants detected in US house dust. Environ Sci Technol. 2008;42(18):6910–6. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 32.Covaci A, Voorspoels S, Thomsen C, van Bavel B, Neels H. Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci Total Environ. 2006;366(1):361–6. doi: 10.1016/j.scitotenv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Kim UJ, Oh JE. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ Pollut. 2014;184:193–200. doi: 10.1016/j.envpol.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Cequier E, Marcé RM, Becher G, Thomsen C. The lipid content of serum affects the extraction efficiencies of highly lipophilic flame retardants. Environ Sci Technol Lett. 2014;1(1):82–6. [Google Scholar]
- 35.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 36.Sjödin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol. 2008;42(4):1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 37.Makey CM, McClean MD, Braverman LE, Pearce EN, He XM, Sjödin A, Weinberg JM, Webster TF. Polybrominated diphenyl ether exposure and thyroid function tests in North American Adults. Environ Health Perspect EHP Advance Publication. 2015 doi: 10.1289/ehp.1509755. http://dx.doi.org/10.1289/ehp.1509755. [DOI] [PMC free article] [PubMed]
- 38.Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, et al. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ Sci Technol. 2013;47(20):11776–84. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petropoulou SSE, Duong W, Petreas M, Park JS. Fast liquid chromatographic-tandem mass spectrometric method using mixed-mode phase chromatography and solid phase extraction for the determination of 12 mono-hydroxylated brominated diphenyl ethers in human serum. J Chromatogr A. 2014;1356:138–47. doi: 10.1016/j.chroma.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 40.Fujii Y, Harada KH, Hitomi T, Kobayashi H, Koizumi A, Haraguchi K. Temporal trend and age-dependent serum concentration of phenolic organohalogen contaminants in Japanese men during 1989–2010. Environ Pollut. 2014;185:228–33. doi: 10.1016/j.envpol.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Ho KL, Yau MS, Murphy MB, Wan Y, Fong BMW, Tam S, et al. Urinary bromophenol glucuronide and sulfate conjugates: potential human exposure molecular markers for polybrominated diphenyl ethers. Chemosphere. 2015;133:6–12. doi: 10.1016/j.chemosphere.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ Sci Technol. 2002;36(7):1414–8. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- 43.Dallaire R, Ayotte P, Pereg D, Dery S, Dumas P, Langlois E, et al. Determinants of plasma concentrations of perfluorooctanesulfonate and brominated organic compounds in Nunavik Inuit adults (Canada) Environ Sci Technol. 2009;43(13):5130–6. doi: 10.1021/es9001604. [DOI] [PubMed] [Google Scholar]
- 44.Howe PD, Dobson S, Malcolm HM. IPCS Concise International Chemical Assessment Documents. 2005. 2,4,6-Tribromophenol and other simple brominated phenols. [Google Scholar]
- 45.Ballesteros-Gömez A, De Boer J, Leonards PEG. A novel brominated triazine-based flame retardant (TTBP-TAZ) in plastic consumer products and indoor dust. Environ Sci Technol. 2014;48(8):4468–74. doi: 10.1021/es4057032. [DOI] [PubMed] [Google Scholar]
- 46.Eguchi A, Nomiyama K, Minh Tue N, Trang PTK, Hung Viet P, Takahashi S, et al. Residue profiles of organohalogen compounds in human serum from e-waste recycling sites in North Vietnam: association with thyroid hormone levels. Environ Res. 2015;137:440–9. doi: 10.1016/j.envres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi S, Kojima H, Saito I, Jin K, Kobayashi S, Tanaka-Kagawa T, et al. Detection of 34 plasticizers and 25 flame retardants in indoor air from houses in Sapporo, Japan. Sci Total Environ. 2014;491–492:28–33. doi: 10.1016/j.scitotenv.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D. Distribution of bromophenols in species of marine algae from eastern Australia. J Agric Food Chem. 1999;47(6):2367–73. doi: 10.1021/jf981080h. [DOI] [PubMed] [Google Scholar]
- 49.Vetter W, Janussen D. Halogenated natural products in five species of antarctic sponges: compounds with POP-like properties? Environ Sci Technol. 2005;39(11):3889–95. doi: 10.1021/es0484597. [DOI] [PubMed] [Google Scholar]
- 50.Erratico CA, Szeitz A, Bandiera SM. Oxidative metabolism of BDE-99 by human liver microsomes: predominant role of CYP2B6. Toxicol Sci. 2012;129(2):280–92. doi: 10.1093/toxsci/kfs215. [DOI] [PubMed] [Google Scholar]
- 51.Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ Health Perspect. 2009;117(2):197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss J, Wallin E, Axmon A, Jönsson BAG, Åkesson H, Janák K, et al. Hydroxy-PCBs, PBDEs, and HBCDDs in serum from an elderly population of Swedish fishermen’s wives and associations with bone density. Environ Sci Technol. 2006;40(20):6282–9. doi: 10.1021/es0610941. [DOI] [PubMed] [Google Scholar]
- 53.Roosens L, Abdallah MAE, Harrad S, Neels H, Covaci A. Exposure to hexabromocyclododecanes (HBCDs) via dust ingestion, but not diet, correlates with concentrations in human serum: preliminary results. Environ Health Perspect. 2009;117(11):1707–12. doi: 10.1289/ehp.0900869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meijer L, Weiss J, Van Velzen M, Beouwer A, Bergman ÅKE, Sauer PJJ. Serum concentrations of neutral and phenolic organohalogens in pregnant women and some of their infants in the Netherlands. Environ Sci Technol. 2008;42(9):3428–33. doi: 10.1021/es702446p. [DOI] [PubMed] [Google Scholar]
- 55.Zhou SN, Buchar A, Siddique S, Takser L, Abdelouahab N, Zhu J. Measurements of selected brominated flame retardants in nursing women: implications for human exposure. Environ Sci Technol. 2014;48(15):8873–80. doi: 10.1021/es5016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts SC, Macaulay LJ, Stapleton HM. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem Res Toxicol. 2012;25(7):1435–41. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120(7):1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid regulating deiodinases in human liver. Toxicol Sci. 2011;124(2):339–47. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butt CM, Stapleton HM. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem Res Toxicol. 2013;26(11):1692–702. doi: 10.1021/tx400342k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.