Abstract
Objective
To establish an algorithm that incorporates sentinel lymph node (SLN) mapping to the surgical treatment of early cervical cancer, ensuring that lymph node (LN) metastases are accurately detected but minimizing the need for complete lymphadenectomy (LND).
Methods
A prospectively maintained database of all patients who underwent SLN procedure followed by a complete bilateral pelvic LND for cervical cancer (FIGO stages IA1 with LVI to IIA) from 03/2003 to 09/2010 was analyzed. The surgical algorithm we evaluated included the following: 1. SLN are removed and submitted to ultrastaging; 2. Any suspicious LN is removed regardless of mapping; 3. If only unilateral mapping is noted, a contralateral side-specific pelvic LND is performed (including inter-iliac nodes); 4. Parametrectomy en bloc with primary tumor resection is done in all cases. We retrospectively applied the algorithm to determine how it would have performed.
Results
One hundred twenty-two patients were included. Median SLN count was 3 and median total LN count was 20. At least one SLN was identified in 93% of cases (114/122), while optimal (bilateral) mapping was achieved in 75% (91/122). SLN correctly diagnosed 21 of 25 patients with nodal spread. When the algorithm was applied, all pts with LN metastasis were detected and bilateral pelvic LND could have been spared in the 75% of cases with optimal mapping.
Conclusions
In the surgical treatment of early cervical cancer, the algorithm we propose allows for comprehensive detection of all patients with nodal disease and spares complete LND in the majority of cases.
Introduction
Cervical cancer is the tenth most common cancer affecting women in developed countries (1). The International Federation of Gynecology and Obstetrics (FIGO) staging system does not include lymph node status (2), but in surgically treated patients with early cervical cancer, lymph node (LN) metastasis is the most important risk factor for recurrence and death (3–6). Accurate knowledge of LN status is essential to tailor adjuvant treatment.
Traditionally, to obtain histological diagnosis of nodal spread, the entire lymphatic basin draining a tumor is removed. Complete retroperitoneal lymphadenectomy (LND) is associated with short- and long-term morbidities such as prolonged duration of surgery, increased blood loss, infection, nerve injury, lymphocyst formation, vascular injury, venous thromboembolism, and lower extremity lymphedema (7–9). In operable stage Ib1 cervical cancer, prevalence of LN metastasis is estimated to be about 15% (10,11). In addition, the majority of patients are young and expected to live many years since the disease is amenable to cure in the majority of cases. This suggests that up to 85% of these patients may undergo LND for no direct benefit while being subjected to the potential risk of associated morbidities (8).
In an effort to decrease complications associated with LND, improve detection of micrometastatic disease, and fine tune our lymphadenectomy anatomic templates, sentinel lymph node (SLN) techniques have been developed and extensively studied in many oncologic fields. As a result, SLN technique is now part of the standard treatment guidelines for the management of breast cancer (12), melanoma (13), and more recently, it is being recognized as a safe and reasonable approach in select cases of vulvar cancer (14).
Since the first report in 1999 (15), the use of SLN mapping procedure in cervical cancer has been studied by multiple groups with encouraging data from hundreds of patients (16–18); nevertheless, it has not yet been adopted as a standard of care. Our objective was to develop a simple and systematic algorithm for the treatment of early cervical cancer that incorporates SLN mapping, ensuring that all cases with lymph node metastases are detected while minimizing the need for complete lymphadenectomy.
Methods
After obtaining approval from the Memorial Sloan-Kettering Cancer Center Institutional Review Board, the authors analyzed a prospectively maintained database of all consecutive patients who underwent SLN mapping for early cervical cancer at our institution between March 2003 and September 2010. This database included patients with stage IA1 and lymphovascular invasion (LVI) to stage IIA cervical cancer. Informed consent was obtained in all cases.
Our SLN mapping protocol includes intracervical injection of 1% isosulfan blue dye immediately after induction of anesthesia. This was performed for all cases. Our current practice is to slowly inject the cervix at both the 3 o’clock and 9 o’clock positions; 1mL is injected superficially (2–3mm) and 1mL deep (1–2cm), for a total of 4mL. Some patients in the earlier part of the study additionally underwent preoperative intracervical injection with technicium-99 (99m Tc) sulfur colloid and lymphoscintigraphy. At time of surgery, after accessing the retroperitoneum, the blue lymphatic channels were identified and followed to their designated SLN. In cases when radioisotope injection had been performed, a gamma probe was then used to identify any other hot nodes and these were also considered SLN. A thorough description of this mapping protocol can be found in a previous publication (19). Retroperitoneal exploration is performed and grossly enlarged LN are removed. These are sent for frozen-section at the discretion of the surgeon. Grossly enlarged nodes were properly documented on the surgical specimen sheet and in the surgeon’s operative report. For the purpose of statistical analysis in this manuscript, these enlarged nodes were not considered SLN.
Following SLN procedure, complete bilateral pelvic LND was then performed on all patients. Paraaortic LND was performed at the discretion of the surgeon if no mapping had occurred in that region. The uterus or cervix was then removed along with the parametria unless evidence of metastatic spread was present at time of surgery, in which case completion of the surgery was at the discretion of the surgeon. In a few cases of microinvasive (IA1) cancer, parametrectomy was omitted.
Surgical specimens were all examined by specialized gynecologic pathologists, and SLN were submitted to the institutional ultrastaging protocol, described in a previous publication (20). We considered lymph nodes positive for metastasis if they showed macrometastasis (>2mm tumor cells), micrometastasis (≥0.2mm to 2mm), or isolated tumor cells (ITC), which are defined as microscopic clusters and single cells of carcinoma measuring <0.2mm (21). Cytokeratin immunohistochemical stains (AE1:AE3) were performed as part of the protocol. Nodes containing only single isolated cytokeratin-positive cells were not compiled as metastatic; they were considered of indeterminate clinical significance and patients did not receive adjuvant treatment based upon this finding only.
We defined “optimal mapping” as the identification of at least one SLN on each side. Results of two distinct analyses were obtained using different sets of definitions. For the “patient-specific” analysis: a true negative (TN) was defined as no nodal metastasis; a true positive (TP) as at least one positive SLN; a false negative (FN) as a patient having at least one metastatic node but no positive SLN; failed mapping was the bilateral absence of SLN in that patient. A “side-specific” analysis was also performed, considering each hemi-pelvis as a distinct unit: a TN was assigned when a SLN is negative in a hemi-pelvis that does not contain nodal metastasis; a TP was defined as at least one positive SLN in one hemi-pelvis; a FN as the absence of a positive SLN on a side where there was nodal metastasis; failed mapping was the absence of any SLN on that side.
Descriptive statistics were performed on the entire cohort. Cases with failed mapping were considered non-evaluable and were further excluded from the statistical analyses according to the appropriate definitions cited above. Sensitivities, negative predictive values (NPV), and false negative (FN) rates were calculated. Categorical variables were compared using Chi square or Fisher’s exact test. Sensitivities among the two groups were compared using Fisher’s exact test assuming binomial proportions. For the side-specific analysis, correlation within subjects was taken into account. All tests were two-sided, and statistical significance was set at P <0.05. The SAS System (version 9) was used for this analysis.
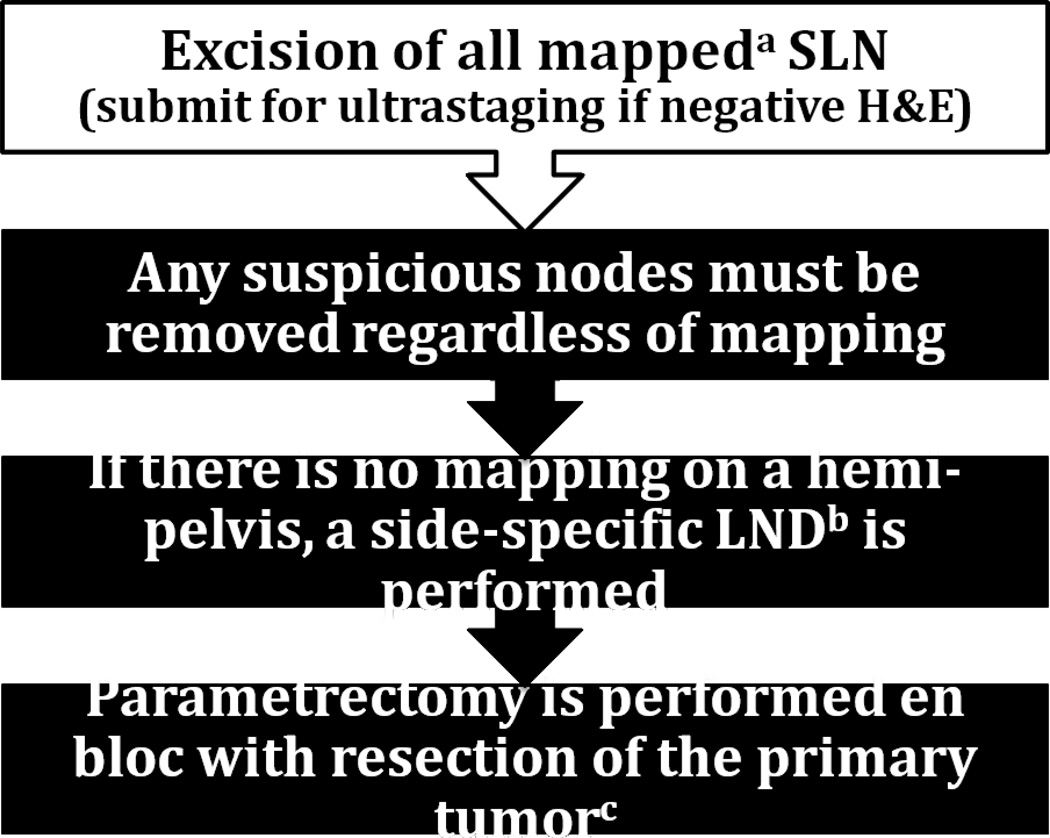
The surgical algorithm we evaluated includes the following (Fig 1): 1. SLN are removed and, if negative on routine hematoxylin and eosin (H&E) staining, they are submitted to ultrastaging (if the initial nodal section detects metastasis by H&E staining, no further pathologic ultrastaging is needed); 2. Any suspicious LN are removed regardless of mapping; 3. If there is no mapping on a hemi-pelvis, then a side-specific LND including inter-iliac or subaortic nodes is performed; 4. Parametrectomy is performed en bloc with resection of the cervical tumor (20). We applied this algorithm to our patients in a retrospective theoretical manner in order to determine how it would have performed in our population. We examined the surgeon’s intraoperative findings based on the dictated intraoperative report, and accounted for all individual metastatic nodes included in the pathology report.
Figure 1.
Surgical algorithm for early cervical cancer.
Abbreviations: “SLN” Sentinel lymph node; “H&E” hematoxylin and eosin staining; “LND” lymphadenectomy
aIntracervical injection with isosulfan blue dye, 99m Technicium, or both; bIncluding interiliac/subaortic nodes; cExceptions made for select cases, see text.
Results
A total of 122 patients were included in our study; patient and tumor characteristics are presented in Table 1. Median SLN count was 3 (range, 0–13) and median total LN count was 20 (range, 2–74). Parametrectomy en bloc with resection of cervical lesion was performed at the time of radical hysterectomy in 70 (57.4%) patients and during radical trachelectomy for 42 (34.4%) patients. In our cohort, 10 (8.2%) patients did not undergo parametrectomy: 2 because of aborted hysterectomy for grossly metastatic lymph nodes, and 8 for stage Ia1 lesions with LVI undergoing cervical conization or extrafascial hysterectomy combined with SLN mapping. Median age was 35 (range, 15–68), 66% of patients were less than 40 years old and 60% were nulliparous. Median follow-up (FU) was 21 months (range, 1–87; mean 26). Eleven patients were lost to FU (median loss of FU occurred after 1 year); 1 patient died of unrelated causes; 3 patients developed evidence of persistent high-grade dysplasia and required subsequent interventions; all other patients remained without evidence of invasive disease at time of writing.
Table 1.
Patient and tumor characteristics, N=122
| Variable | N (%) |
|---|---|
| Age | |
| Median (range) | 35 (15–68) |
| Body Mass Index (in kg/m2) | |
| Median (range) | 24 (18–45) |
| Tumor diameter in cma | |
| Median (range) | 1.1 (0–6.0) |
| History of cone biopsyb | 72 (59) |
| Surgical approach | |
| Minimally invasivec | 37 (30.3) |
| Laparotomy | 85 (69.7) |
| Surgery for cervical tumor | |
| Radical hysterectomy | 70 (57.4) |
| Radical trachelectomy | 42 (34.4) |
| Cervical conization | 6 (4.9) |
| Hysterectomy | 2 (1.6) |
| Aborted hysterectomy | 2 (1.6) |
| Histology | |
| Squamous carcinoma | 40 (32.8) |
| Adenocarcinomad | 80 (65.6) |
| Neuroendocrine | 2 (1.6) |
| FIGO stage | |
| IA1 with LVI | 14 (11.5) |
| IA2 | 8 (6.6) |
| IB1 | 93 (76.2) |
| IB2 | 6 (4.9) |
| IIA | 1 (0.8) |
| Length of follow up in months | |
| Median (range) | 21 (1–87) |
LVI, lymphovascular invasion
Evaluated pre-operatively by physical exam or after excisional biopsy pathology report;
Includes conization or Loop Electrosurgical Excision Procedure
Includes laparoscopy or robotic surgery
Including adenosquamous
At least one SLN was identified in 93% (114/122) of patients. Of the patients who mapped, 105/114 (92.1%) mapped only to the pelvic nodes, 8 (7%) to both pelvic and paraaortic regions, and 1 (0.9%) patient mapped only to the paraaortic region. Unilateral mapping (11 left hemi-pelvis, 12 right hemi-pelvis) was obtained in 19% (23/122) of patients, while optimal mapping (bilateral) was achieved 75% (91/122). When we consider there were 244 possible hemi-pelvises, 205 (84%) sides mapped while 39 (16%) had failed mapping (Table 2). A total of 469 individual SLN were retrieved, with the following distribution: 179 (38%) external iliac region, 137 (29%) hypogastric, 88 (19%) obturator, 36 (13%) common iliac, 16 (3%) paraaortic, and 13 (3%) parametrial.
Table 2.
Sentinel lymph node mapping by definition: patient vs. side-specific.
| Variable | Patient-specific N = 122, N (%) |
Side-specific N = 244, N (%) |
|---|---|---|
| Failed mapping | 8 (6.6) | 39 (16.0) |
| Unilateral mapping | 23 (18.9) | 23 (9.4) |
| Optimal mappinga | 91 (74.6) | 182 (74.6) |
| At least one SLNb | 114 (93.4) | 205 (84.0) |
bilateral identification of at least one sentinel lymph node
sentinel lymph node
Patient-specific analysis
Metastatic disease to the lymph nodes was documented in 25/122 (20.5%) patients; this number includes one case with bilateral grossly enlarged nodes which was not counted as a FN because of failed mapping. Excluding that patient, there were therefore 21/24 metastatic patients with a positive SLN, resulting in a sensitivity of 87.5%. There were 3 FN cases: two patients had positive parametrial nodes on final pathology (nodes removed en bloc with cervix) after pelvic SLN yielded negative for metastasis; the third patient had unilaterally mapped to the right hemi-pelvis but had a positive pelvic non-SLN on the left side-specific LND (side that had not mapped). This resulted in a FN rate (among the cases that mapped) of 12.5% and a NPV of 96.8%.
Side-specific analysis
Of the 205 sides that mapped, SLN correctly predicted the presence or absence of ipsilateral involvement in 203 cases. A total of 33 individual hemi-pelvises were positive for LN metastasis and 27 of these had mapped. Of the sides that mapped, 25/27 (92.6%) had a positive SLN. Only 2 cases were FN when the side-specific definition was applied. Both cases were stage Ib1 with a single non-SLN parametrial node as their only positive finding. Side-specific sensitivity was 92.6%, FN rate 7.4%, and NPV 98.9%.
Analysis by type of intracervical injection marker
There appears to be a trend towards increased rates of optimal mapping (bilateral) in patients who underwent a combined approach injection (both blue dye and technetium) when compared to those who only received blue dye (85.5% vs. 73.1%, P=0.112). However, when analyzed in both patient-specific and side-specific models, there was no significant difference in the diagnostic performance of SLN procedure (by measure of sensitivity, NPV, or FN rate) for patients mapped with blue dye injection only, compared to those with a combined (blue dye and technetium) injection (Table 3).
Table 3.
Sentinel lymph node performance according to injection method used
| Analysis according to injection methoda | ||||
|---|---|---|---|---|
| Variable | All patientsa N=114, N (%) |
Isosulfan blue only N=52, N (%) |
99m Technitium + Isosulfan blue, N=62, N (%) |
p |
| Mappinga | 0.1002 | |||
| Optimal mapping | 91 (79.8) | 38 (73.1) | 53 (85.5) | |
| Unilateral mapping | 23 (20.2) | 14 (26.9) | 9 (14.5) | |
| Any positive nodea | 0.3691 | |||
| Yes | 24 (21.1) | 9 (17.3) | 15 (24.2) | |
| No | 90 (78.9) | 43 (82.7) | 47 (75.8) | |
| Any positive SLNa | 0.4437 | |||
| Yes | 21 (18.4) | 8 (15.4) | 13 (21.0) | |
| No | 93 (81.6) | 44 (84.6) | 49 (79.0) | |
| False Negativea | NA | |||
| Yes | 3 (2.1) | 1 (1.9) | 2 (3.2) | |
| Patient-specifica | ||||
| Sensitivity | 21/24 (87.5) | 8/9 (88.9) | 13/15 (86.7) | 1 |
| NPV | 90/93 (96.8) | 43/44 (97.7) | 47/49 (95.9) | |
| FN rate | 3/24 (12.5) | 1/9 (11.1) | 2/15 (13.3) | |
| Side-specificb | ||||
| Sensitivity | 25/27 (92.6) | 10/11 (90.9) | 15/16 (93.7) | 1 |
| NPV | 178/180 (98.9) | 79/80 (98.7) | 99/100 (99.0) | |
| FN rate | 2/27 (7.4) | 1/11 (9.1) | 1/16 (6.3) | |
SLN sentinel lymph node, NPV negative predictive value, FN false negative
excludes patient-specific failed mapping,
bexcludes side-specific failed mapping
Ultrastaging was performed in 96 of 122 patients (Table 4). A majority of patients with nodal spread were diagnosed on routine H&E staining, but 4/25 patients (16%) were diagnosed as being micrometastatic based only on the ultrastaging findings. On sides that did not map, a total of 6 LN were metastatic. Upon review, 5/6 of these nodes had been described by the surgeon as grossly enlarged and suspicious during laparotomy for radical hysterectomy.
Table 4.
Pathology findings on lymph nodes (when ultrastaging performed) or indication why ultrastaging not performed (N = 122)
| Variable | N (%) |
|---|---|
| Ultrastaging performed | |
| No disease | 77 (63.1) |
| Isolated cytokeratin-staining cells | 8 (6.6) |
| Positive on H&E | |
| Macrometastasis | 6 (4.9) |
| Micrometastasis | 1 (0.8) |
| Positive on ultrastaging only | |
| Macrometastasis | 1 (0.8) |
| Micrometastasis | 2 (1.6) |
| Isolated tumor cells | 1 (0.8) |
| Ultrastaging not performed | |
| Failed mapping, no disease | 7 (5.7) |
| SLN positive on H&E | 14 (11.5) |
| No reason why not performed | 5 (4.1) |
If the algorithm described above had been applied in this cohort, 8/122 (6.6%) patients with failed mapping would have required complete bilateral pelvic LND, 23/122 (18.9%) patients would have undergone a unilateral SLN with contralateral pelvic LND, and 91/122 (74.6%) patients with optimal mapping would have avoided complete bilateral pelvic LND. The FN rate for the algorithm seems to be 0 in this cohort and the sensitivity and NPV are 100%. The performance of the algorithm when compared to SLN procedure (from a side-specific and a patient-specific perspective) is summarized in Table 5.
Table 5.
Performance of sentinel lymph node procedure per different criteria, compared to proposed surgical algorithma
| Values | SLN Procedure | Algorithm | |
|---|---|---|---|
| Patient-specific | Side-specific | ||
| Sensitivity | 87.5 | 92.6 | 100 |
| Negative predictive value | 96.8 | 98.9 | 100 |
| False negative rate | 12.5 | 7.4 | 0 |
| Lymphadenectomy | |||
| Bilateral | 100 | 6.6 | 6.6 |
| Unilateral | 0 | 18.9 | 18.9 |
| Avoided | 0 | 74.6 | 74.6 |
SLN sentinel lymph node
see Figure 1
Discussion
The present study demonstrates that our proposed algorithm for the surgical treatment of early cervical cancer could accurately detect all patients with LN metastasis, while potentially eliminating the need for complete bilateral lymphadenectomy in 75% of this group. We established this algorithm based on data from 122 patients having undergone SLN procedure for cervical cancer, one of the largest single institution retrospective cohorts published to date.
The overall sensitivity of SLN procedure in our patients was 87.5%. A large and important multicenter validation study by Altgassen et al. (22) had found a lower overall sensitivity (77%), and only 213 of the 504 patients evaluated (42%) had mapped bilaterally. These findings likely represent a learning curve effect as it was a pioneer study that started accruing in 1998, and also may reflect the lack of uniformity as far as mapping method utilized (the surgeons were free to chose between using blue dye only, technicium only, or a combined approach). In the recent SENTICOL study (23), SLN procedure was uniformly performed by experienced surgeons using a combined blue dye + technicium approach. In this paper, the sensitivity was found to be 92% and optimal mapping was reached in 75% of patients. These authors concluded that when combined labeling is used, the presence of negative SLN on both sides indicates absence of metastatic disease.
It has been shown that SLN status on one side of the pelvis does not predict the presence or absence of metastasis on the contralateral side (18). Many authors have noted that SLN should be evaluated per side and not per patient (24–26). For this reason, we conducted 2 separate analyses: the first, patient-specific, considers the group as a whole where each individual patient represents a diagnostic entity; the second, side-specific, considers each hemi-pelvis as a distinct unit. We found that diagnostic performance was higher when analyzed from a side-specific compared to a patient-specific perspective (sensitivity 92.6 % vs. 87.5%, NPV 98.9% vs. 96.8%, and FN rate 7.4% vs. 12.5%). On the sides that had not successfully mapped for SLN but contained LN metastases, we found that 5/6 (83%) of these nodes were identified at time of surgery as grossly abnormal. A possible explanation to why grossly enlarged lymph nodes fail to map is that when filled with tumor, the lymphatic drainage is blocked or altered and may prevent the tracer or dye from reaching the node (24,27,28). For the purpose of analysis in this manuscript, which includes assessment of mapping, we did not consider grossly enlarged nodes to be SLN unless they had mapped for blue or technetium. However, we do believe that from a biological standpoint, these enlarged nodes represent the principal drainage lymph node and as such are de facto SLN.
With SLN mapping, there is an increased likelihood of detecting potentially positive LN in unusual locations (29,30). We found 3% of SLN were parametrial and 3% were paraaortic. These nodes could have gone unnoticed had it not been for mapping. We did not find any SLN in the presacral area, but other studies have noted this occurrence in up to 5% of cases and it can be a source of false negatives (31–33). Presacral nodes are midline and receive afferent lymphatics from deep lymphatic trunks draining the parametria from either side of the pelvis (27,34). Because presacral nodes can be a site of metastasis, we believe they should be included in a side-specific lymphadenectomy when ipsilateral mapping has failed.
If a SLN is found in a hemi-pelvis, it almost always correctly predicts that side’s involvement (35). Exceptions to this in our study were parametrial nodes diagnosed at time of final pathology. Parametrial LN are challenging to identify as SLN because they are in close proximity with the cervix: with blue dye, the parametria are sometimes stained, making isolation of a node more difficult; with a radiocolloid tracer, proximity to the injection site makes it almost impossible to evaluate for a hot node (8,24). Parametrectomy is usually not necessary in microinvasive (IA1) tumors because of a documented low rate of parametrial metastases (10), but for stage IB1 or greater, parametrectomy en bloc with the primary tumor remains the gold standard surgical treatment. For stage IA2 and selected stage IB1 patients, some teams have proposed it may be safe to omit parametrectomy in patients with favorable prognostic factors such as size <2cm, absence of LVI and absence of pelvic LN metastasis or if the SLN reveals absence of metastasis (36–39). Our data reveal that for 3/25 metastatic patients (12%), the parametrium was the only site of metastasis (1 was SLN, 2 were non-SLN), and parametrial nodes accounted for 9/40 (22.5%) of all metastatic nodes. Most associated primary tumors in these patients measured between 2 and 3 cm but for one case it measured 1cm; all of these cases presented LVI. We recommend exercising caution when considering abbreviation of parametrectomy and, based on our data, we continue to include this procedure in our surgical management of most early cervical cancers.
A documented advantage of SLN technique is the increased detection rate due to the performance of serial step sectioning and IHCS (40). Ultrastaging allows for easier identification of micrometastasis and these have been associated with increase risk of recurrence (41–44). In our study, 4/25 (16%) metastatic patients were detected by ultrastaging only and would have been misdiagnosed if only routine H&E had been performed; therefore, ultrastaging increased the detection rate of SLN procedure by 3.5% (4/114 patients who mapped). In addition, 8 patients had cells staining positive for cytokeratin as their only abnormal finding. These findings do not warrant adjuvant treatment, but the documentation of their presence can be a pathway to future research. It is not known whether performing ultrastaging on the non-SLN could reveal other sites of micrometastasis. However, because we believe that lymphatic spread follows an anatomic course, if micrometastases are present in non-SLN there most likely will be tumor in the SLN as well.
A previous algorithm has been described by a team from Toronto (26). Their protocol mandates preoperative technetium injection and lymphoscintiography, adding intraoperative blue dye injection in cases where no bilateral mapping has occurred. We did not find any significant difference in the diagnostic performance of SLN technique when a combined (blue dye + technetium) approach was used for mapping compared to blue dye alone (P=1). However, it is preferred to use both modalities of dye injection in the learning phase of applying SLN in cervical cancer surgery, and once experience is gained it may be possible to eliminate one of the two injectable substances at the physician’s discretion. Importantly, the algorithm corrects for all failures of mapping and results in a 100% detection rate for both blue only and combined approaches. The side-specific LND proposed by the Toronto team is similar to ours but does not include presacral or subaortic nodes. Finally, they suggest a full pelvic LND when macroscopically enlarged LN are noted intraoperatively. Patients with nodal metastasis require adjuvant treatment, usually chemoradiation, and may encounter more complications if complete pelvic LND has been performed (45). A recent study showed that in cases of cervical cancer with nodal metastasis, there was no impact on survival when the LN count increased (46). If frozen-section confirms metastatic disease, paraaortic dissection may be done in order to determine necessary fields of radiation but there exists controversy as to the therapeutic value of completing pelvic LND. This question is beyond the scope of this paper and further research is needed on the subject.
In accordance with the recent literature, we have found that in the setting of surgical treatment of early cervical cancer, SLN procedure with the addition of ultrastaging is a safe and accurate technique that increases metastatic nodal detection rates by 3.5%. In experienced hands, when SLN mapping is approached with an algorithm where side-specific evaluation is combined with removal of clinically suspicious LN and parametrectomy, this could spare approximately 75% of patients a complete bilateral pelvic lymphadenectomy while maintaining the diagnostic performance of this surgical procedure.
The concept of SLN mapping in cervical cancer should go beyond just identifying blue or hot nodes. By utilizing this algorithm, our templates for pelvic lymphadenectomy will change in favor of less radical lymphadenectomy in the majority of cases, and the classic operation for early cervical cancer will be fine tuned to each individual patient’s anatomy. This approach honors many of the traditions of pelvic surgery for cervical cancer and allows for modern individualized patient-specific surgery to improve the detection of metastatic disease and limit unnecessary removal of normal appearing tissue.
Research Highlights.
-
*
An algorithm that incorporates SLN mapping into surgical treatment of early cervical cancer
-
*
This algorithm ensures that LN mets are detected but minimizes need for complete lymphadenectomy
Acknowledgments
Alexia Iasonos, Ph.D., Department of Biostatistics, Memorial Sloan-Kettering Cancer Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Creasman WT, Kohler MF. Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol Oncol. 2004;92:525–529. doi: 10.1016/j.ygyno.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C, Wang P, Lai C, Tsu E, Yen M, Ng H. Recurrence and survival analyses of 1,115 cervical cancer patients treated with radical hysterectomy. Gynecol Obstet Invest. 1999;47:127–132. doi: 10.1159/000010076. [DOI] [PubMed] [Google Scholar]
- 6.Biewenga P, Van der Velden J, Mol BW, et al. Prognostic model for survival in patients with early stage cervical cancer. Cancer. 2011;117:768–776. doi: 10.1002/cncr.25658. [DOI] [PubMed] [Google Scholar]
- 7.Franchi M, Ghezzi F, Riva C, Miglierina M, Buttarelli M, Bolis P. Postoperative complications after pelvic lymphadenectomy for the surgical staging of endometrial cancer. J Surg Oncol. 2001;78:232–237. doi: 10.1002/jso.1158. [DOI] [PubMed] [Google Scholar]
- 8.Levenback C, Coleman RL, Burke TW, et al. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J Clin Oncol. 2002;20:688–693. doi: 10.1200/JCO.2002.20.3.688. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura Y, Kawagoe T, Toki N, Tanaka M, Kashimura M. Long-standing complications after treatment for cancer of the uterine cervix-clinical significance of medical examination at 5 years after treatment. Int J Gynecol Cancer. 2006;16:294–297. doi: 10.1111/j.1525-1438.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 10.Delgado G, Bundy BN, Fowler WC, Jr, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989;35:314–320. doi: 10.1016/0090-8258(89)90070-x. [DOI] [PubMed] [Google Scholar]
- 11.Look KY, Brunetto VL, Clarke-Pearson DL, et al. An analysis of cell type in patients with surgically staged stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;63:304–311. doi: 10.1006/gyno.1996.0327. [DOI] [PubMed] [Google Scholar]
- 12.Carlson RW. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer version 2. NCCN Guidelines Panel Members Breast Cancer; 2011. [Google Scholar]
- 13.Coit DG. Cinical Practice Guidelines in Oncology, Melanoma version 1. NCCN Guidelines Panel Members Melanoma; 2011. [Google Scholar]
- 14.Van der zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 15.Echt ML, Finan MA, Hoffman MS, Kline RC, Roberts WS, Fiorica JV. Detection of sentinel lymph nodes with lymphazurin in cervical, uterine, and vulvar malignancies. South Med J. 1999;92:204–208. doi: 10.1097/00007611-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Frumovitz M, Ramirez PT, Levenback CF. Lymphatic mapping and sentinel lymph node detection in women with cervical cancer. Gynecol Oncol. 2008;110:529–536. doi: 10.1016/j.ygyno.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Oonk MH, Van de Nieuwenhof HP, De Hullu JA, Van der Zee AG. The role of sentinel node biopsy in gynecological cancer: a review. Curr Opin Oncol. 2009;21:425–432. doi: 10.1097/CCO.0b013e32832f3d53. [DOI] [PubMed] [Google Scholar]
- 18.Van de Lande J, Torrenga B, Raijmakers PG, et al. Sentinel lymph node detection in early stage uterine cervix carcinoma: a systematic review. Gynecol Oncol. 2007;106:604–613. doi: 10.1016/j.ygyno.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Rustum NR, Khoury-Collado F, Gemignani ML. Techniques of sentinel lymph node identification for early-stage cervical and uterine cancer. Gynecol Oncol. 2008;111:S44–S50. doi: 10.1016/j.ygyno.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Diaz JP, Gemignani ML, Pandit-Taskar N, et al. Sentinel lymph node biopsy in the management of early-stage cervical carcinoma. Gynecol Oncol. 2011;120:347–352. doi: 10.1016/j.ygyno.2010.12.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 22.Altgassen C, Hertel H, Brandstadt A, Kohler C, Durst M, Schneider A. Multicenter validation study of the sentinel lymph node concept in cervical cancer: AGO Study Group. J Clin Oncol. 2008;26:2943–2951. doi: 10.1200/JCO.2007.13.8933. [DOI] [PubMed] [Google Scholar]
- 23.LeCuru F, Mathevet P, Querleu D, et al. Bilateral Negative Sentinel Nodes Accurately Predict Absence of Lymph Node Metastasis in Early Cervical Cancer: Results of the SENTICOL Study. J Clin Oncol. 2011 Mar 28; doi: 10.1200/JCO.2010.32.0432. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Plante M, Renaud MC, Tetu B, Harel F, Roy M. Laparoscopic sentinel node mapping in early-stage cervical cancer. Gynecol Oncol. 2003;91:494–503. doi: 10.1016/j.ygyno.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Vicus D, Covens A. Role of sentinel lymph node biopsy in cervical cancer: pro. Int J Gynecol Cancer. 2010;20:S34–S36. doi: 10.1111/IGC.0b013e3181f60d60. [DOI] [PubMed] [Google Scholar]
- 26.Hauspy J, Beiner M, Harley I, Ehrlich L, Rasty G, Covens A. Sentinel lymph nodes in early stage cervical cancer. Gynecol Oncol. 2007;105:285–290. doi: 10.1016/j.ygyno.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Dargent D, Martin X, Mathevet P. Laparoscopic assessment of the sentinel lymph node in early stage cervical cancer. Gynecol Oncol. 2000;79:411–415. doi: 10.1006/gyno.2000.5999. [DOI] [PubMed] [Google Scholar]
- 28.Yuan SH, Xiong Y, Wei M, et al. Sentinel lymph node detection using methylene blue in patients with early stage cervical cancer. Gynecol Oncol. 2007;106:147–152. doi: 10.1016/j.ygyno.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Hauspy J, Verkinderen L, De Pooter C, Dirix LY, Van Dam PA. Sentinel node metastasis in the groin detected by technetium-labeled nannocolloid in a patient with cervical cancer. Gynecol Oncol. 2002;86:358–360. doi: 10.1006/gyno.2002.6770. [DOI] [PubMed] [Google Scholar]
- 30.Mathevet P. Surgical lymph-node evaluation in cervical cancer. Cancer Radiother. 2009;13:499–502. doi: 10.1016/j.canrad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Rob L, Strnad P, Robova H, et al. Study of lymphatic mapping and sentinel node identification in early stage cervical cancer. Gynecol Oncol. 2005;98:281–288. doi: 10.1016/j.ygyno.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Bader AA, Winter R, Haas J, Tamussino KF. Where to look for the sentinel lymph node in cervical cancer. Am J Obstet Gynecol. 2007;197:678.e1–678.e7. doi: 10.1016/j.ajog.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 33.Kushner DM, Connor JP, Wilson MA, et al. Laparoscopic sentinel lymph node mapping for cervix cancer--a detailed evaluation and time analysis. Gynecol Oncol. 2007;106:507–512. doi: 10.1016/j.ygyno.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Cibula D, Abu-Rustum NR. Pelvic lymphadenectomy in cervical cancer--surgical anatomy and proposal for a new classification system. Gynecol Oncol. 2010;116:33–37. doi: 10.1016/j.ygyno.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Cibula D, Kuzel D, Slama J, et al. Sentinel node (SLN) biopsy in the management of locally advanced cervical cancer. Gynecol Oncol. 2009;115:46–50. doi: 10.1016/j.ygyno.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Coutant C, Cordier AG, Guillo E, Ballester M, Rouzier R, Darai E. Clues pointing to simple hysterectomy to treat early-stage cervical cancer. Oncol Rep. 2009;22:927–934. doi: 10.3892/or_00000519. [DOI] [PubMed] [Google Scholar]
- 37.Pluta M, Rob L, Charvat M, et al. Less radical surgery than radical hysterectomy in early stage cervical cancer: a pilot study. Gynecol Oncol. 2009;113:181–184. doi: 10.1016/j.ygyno.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Strnad P, Robova H, Skapa P, et al. A prospective study of sentinel lymph node status and parametrial involvement in patients with small tumour volume cervical cancer. Gynecol Oncol. 2008;109:280–284. doi: 10.1016/j.ygyno.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Covens A, Rosen B, Murphy J, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol. 2002;84:145–149. doi: 10.1006/gyno.2001.6493. [DOI] [PubMed] [Google Scholar]
- 40.Euscher ED, Malpica A, Atkinson EN, Levenback CF, Frumovitz M, Deavers MT. Ultrastaging improves detection of metastases in sentinel lymph nodes of uterine cervix squamous cell carcinoma. Am J Surg Pathol. 2008;32:1336–1343. doi: 10.1097/PAS.0b013e31816ecfe4. [DOI] [PubMed] [Google Scholar]
- 41.Horn LC, Hentschel B, Fischer U, Peter D, Bilek K. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: Frequency, topographic distribution and prognostic impact. Gynecol Oncol. 2008;111:276–281. doi: 10.1016/j.ygyno.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Juretzka MM, Jensen KC, Longacre TA, Teng NN, Husain A. Detection of pelvic lymph node micrometastasis in stage IA2-IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol. 2004;93:107–111. doi: 10.1016/j.ygyno.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Fregnani JH, Latorre MR, Novik PR, Lopes A, Soares FA. Assessment of pelvic lymph node micrometastatic disease in stages IB and IIA of carcinoma of the uterine cervix. Int J Gynecol Cancer. 2006;16:1188–1194. doi: 10.1111/j.1525-1438.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 44.Marchiole P, Buenerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol. 2005;97:727–732. doi: 10.1016/j.ygyno.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment : prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 46.Shah M, Lewin SN, Deutsch I, et al. Therapeutic role of lymphadenectomy for cervical cancer. Cancer. 2011;117:310–317. doi: 10.1002/cncr.25408. [DOI] [PubMed] [Google Scholar]