Abstract
The adenohypophysis secretes multiple hormones that control vital physiological functions. A recent article in Nature (Suga et al., 2011) describes a 3D protocol for the derivation of several endocrine pituitary cell types from mouse ESCs.
The adenohypophysis is a remarkable endocrine organ that orchestrates the function of multiple targets via secretion of a set of regulatory hormones in charge of vital functions such as development growth, puberty, reproduction, lactation, and crucially, response to stress. Nested in a small bony cup at the base of the skull, it receives regulatory endocrine input from the adjacent hypothalamus through a portal circulation system, and communicates with the rest of the organism via an extensive network of vessels. Multitiered feedback is integrated by this master gland, leading to hormonal, and metabolic homeostasis. The development of the pituitary gland has been described in significant detail in mouse systems (Scully and Rosenfeld, 2002) but the full specification of the gland and its cellular derivatives from embryonic stem cells (ESCs) has not been reported. In an important advance, Yoshiki Sasai’s group at the RIKEN institute reports the complete specification from mouse ESCs of the pituitary primordium in a 3D culture system and the derivation of multiple endocrine lineages (Suga et al., 2011). The group also demonstrates in vivo function of ACTH-secreting cells via grafting of the ESC-derived corticotrophs into hypophysectomized mice leading to partial recovery of glucocorticoid hormone levels and improved survival.
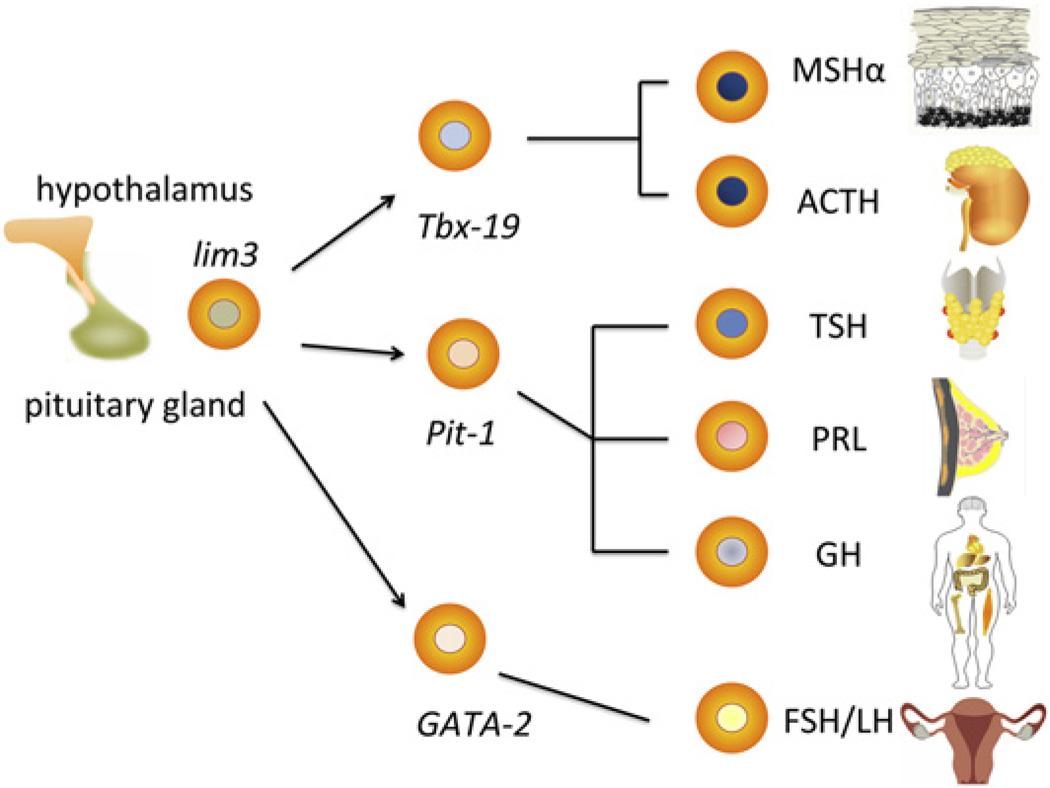
During development, the specification of the pituitary anlage occurs via a tour de force of exquisitely orchestrated temporo-spatial events. The pituitary placode develops in the midline area of the rostral most part of the oral ectoderm, adjacent to the neural plate. It is flanked by the paired olfactory, lens, and otic placodes. At E8.5 the pituitary placode invaginates, and develops into a hollowed “Rathke’s pouch” (RP) that detaches from the oral ectoderm but remains in immediate contact with the hypothalamic primordium in the ventral diencephalon. A gradient of signaling molecules, including FGF8/10 and Bmp4 is generated by the developing hypothalamus, which acts as an organizer and commits Rathke’s pouch to an adenohypophseal fate (Takuma et al., 1998). This is heralded by the upregulation of a set of tissue-specific transcription factors including lim3 (lhx3). Additional morphogen gradients (sonic hedgehog Wnt-2) lead to the specification of lineage precursors within the RP via expression of combinations of transcription factors along a dorso-ventral axis (Zhu et al., 2007). A proliferation phase is followed by differentiation into at least six different pituitary cell types that secrete hormones targeting multiple glands and organs (Figure 1). Recent data suggest the persistence into adulthood of pituitary stem cells that might enhance the capacity of the pituitary gland’s response to damage or stressful demand (Gleiberman et al., 2008).
Figure 1. A Simplified Diagram of the Multiple Endocrine Lineages Derived from the Pituitary Primordium under Hypothalamic Control.
A complex spatio-temporal gradient of morphogens results in the specification of early lim3+ progenitors and further commitment to precursors that will differentiate into the various endocrine cells as determined by combinations of transcription factors (Tbx19, Pit-1, GATA-2, and others). Major hormonal outputs include MSHα (melanocyte stimulating hormone alpha), ACTH (adrenocorticotropic hormone), TSH (thyroid stimulating hormone), PRL (prolactin), GH (growth hormone), and the gonadotropins (FSH, follicle-stimulating hormone; LH, luteinizing hormone).
In recapitulating these developmental events, Sasai and colleagues recognized the imperative need for the concomitant specification of nonneural ectoderm and rostral hypothalamic tissue in the culture dish in hopes of achieving the spatial requirement of juxtaposition. Interestingly, this was achieved via self-forming aggregates of embryoid body-derived cell cultures in serum-free media without any extrinsic molecules. The aggregates produced an ideal dose of endogenous Bmp2 /4 leading to the simultaneous specification of rostral ectoderm and hypothalamic tissue and the initiation of specification of RP. Treatment with sonic hedgehog increased the efficiency of derivation of lim3+ cells, initiating a beautiful sequence of developmental events that led to the formation of 3D vesicles that recapitulated the topography, morphology, marker, and transcriptional signature of Rathke’s pouch. Further specification of endocrine lineage was most successful in the case of Tbx19+ ACTH-secreting cells and possibly Pit-1+ somatotrophs (secreting growth hormone, prolactin, and rarely TSH). Gonadotropins were far less well specified. A set of in vivo experiments was performed on hypophysectomied mice that received ESC-derived pituitary cells grafted in the renal capsule. The treated animals exhibited elevations of ACTH and corticosterone blood levels under basal conditions, as well as after exogenous stimulation with the hypothalamic factor CRH (corticotropin releasing hormone). This was associated with improvements in motor activity and survival.
The role of regenerative approaches to the adenohypophysis has received very little attention despite the prevalence of pituitary disorders and the large number of patients requiring pituitary hormone replacement as a result of genetic, sporadic, or iatrogenic disease. Several syndromes of pituitary deficiencies are recognized in humans as the result of mutations of early transcription factors or cell cycle regulator proteins (Melmed, 2011). Tumors of the adenohypophysis itself (adenomas) or of remnants of Rathke’s pouch epithelium (craniopharyngiomas) are quite common and often pose significant clinical challenges. Treatment of large tumors is frequently associated with hypopituitarism due to surgery, and/or radiation. Craniopharyngiomas can be notoriously difficult to treat particularly in children. Despite their benign pathology, they tend to recur and curative approaches are frequently associated with hypothalamic damage, visual compromise, and panhypopituitarism. These factors result in significant growth impairment in children as well as cognitive and learning disabilities (Karavitaki et al., 2005).
An even larger group of patients suffers hypopituitarism as a consequence of brain radiation for a variety of disorders including hematological malignancies, head and neck cancers, brain tumors, and sellar lesions (Appelman-Dijkstra et al., 2011). In fact, a growing interest in cancer survivorship has led to the identification of hypopituitarism as a major contributor to poor quality of life indices (Darzy, 2009). Radiation damage to the hypothalamus and pituitary regions is progressive and irreversible. Current treatment consists of life-long multiple hormone replacement therapies, an expensive and suboptimal solution given that static delivery of these molecules is a poor substitute for normal pituitary gland functions such as the dynamic secretion of hormones in response to circadian patterns, feedback mechanisms, or stressful conditions. Regenerative approaches using ESC or iPSC-derived pituitary cells represent an exciting if challenging frontier. In their study, Suga et al. graft the pituitary cells ectopically under the renal capsule. However, the adenohypophysis is tightly controlled by hypothalamic releasing and inhibiting hormones delivered via portal vessels that bypass the systemic circulation. Modern surgical techniques, e.g., transnasal transphenoidal approaches in humans, allow the safe orthotopic transplantation of tissue into the sella turcica where the normal gland resides adjacent to the hypothalamus.
This would potentially permit the reconstitution of normal vascularization patterns and hypothalamic-adenohypophy-seal feedback loops. However, the hypothalamus is often affected by the same insults that result in pituitary damage, particularly in the case of radiation injury. The derivation of hypothalamic tissue has been reported (Wataya et al., 2008), but functional integration, and cografting with pituitary tissue remain largely unexplored. Many technical issues in the Sasai protocol remain to be resolved, such as applicability to the human ESC system, better definition of the 3D culture system requirements, and more precise specification of some pituitary lineages. Nonetheless, the ability to derive pituitary progenitors from pluripotent stem cells is an important step toward expanding our understanding of pituitary disorders and tumorigenesis, via in vitro disease modeling, and the development of regenerative approaches.
REFERENCES
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, Neelis KJ, Biermasz NR, Romijn JA, Smit JWA, Pereira AM. J. Clin. Endocrinol. Metab. 2011;96:2330–2340. doi: 10.1210/jc.2011-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzy KH. Nat. Clin. Pract. Endocrinol. Metab. 2009;5:88–99. doi: 10.1038/ncpendmet1051. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G. Proc. Natl. Acad. Sci. USA. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitaki N, Brufani C, Warner JT, Adams CBT, Richards P, Ansorge O, Shine B, Turner HE, Wass JAH. Horumon To Rinsho. 2005;62:397–409. doi: 10.1111/j.1365-2265.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- Melmed S. Nature Reviews Endocrinology. 2011;7:257–266. doi: 10.1038/nrendo.2011.40. [DOI] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, et al. Nature. 2011 doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BLM, Pfaff SL, Westphal H, Kimura S, Mahon KA. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y. Proc. Natl. Acad. Sci. USA. 2008;105:11796–11801. doi: 10.1073/pnas.0803078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang J, Ju B-G, Rosenfeld MG. Curr. Opin. Cell Biol. 2007;19:605–611. doi: 10.1016/j.ceb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]