There is an urgent need for new strategies to reduce carbapenem consumption. Ceftazidime-avibactam was highly effective for empiric treatment of complicated urinary tract infection, including in patients with ceftazidime-nonsusceptible pathogens, and may offer an alternative to carbapenems in this setting.
Keywords: ceftazidime-avibactam, complicated urinary tract infection, acute pyelonephritis
Abstract
Background. The global emergence of carbapenem-resistant Enterobacteriaceae highlights the urgent need to reduce carbapenem dependence. The phase 3 RECAPTURE program compared the efficacy and safety of ceftazidime-avibactam and doripenem in patients with complicated urinary tract infection (cUTI), including acute pyelonephritis.
Methods. Hospitalized adults with suspected or microbiologically confirmed cUTI/acute pyelonephritis were randomized 1:1 to ceftazidime-avibactam 2000 mg/500 mg every 8 hours or doripenem 500 mg every 8 hours (doses adjusted for renal function), with possible oral antibiotic switch after ≥5 days (total treatment duration up to 10 days or 14 days for patients with bacteremia).
Results. Of 1033 randomized patients, 393 and 417 treated with ceftazidime-avibactam and doripenem, respectively, were eligible for the primary efficacy analyses; 19.6% had ceftazidime-nonsusceptible baseline pathogens. Noninferiority of ceftazidime-avibactam vs doripenem was demonstrated for the US Food and Drug Administration co-primary endpoints of (1) patient-reported symptomatic resolution at day 5: 276 of 393 (70.2%) vs 276 of 417 (66.2%) patients (difference, 4.0% [95% confidence interval {CI}, −2.39% to 10.42%]); and (2) combined symptomatic resolution/microbiological eradication at test of cure (TOC): 280 of 393 (71.2%) vs 269 of 417 (64.5%) patients (difference, 6.7% [95% CI, .30% to 13.12%]). Microbiological eradication at TOC (European Medicines Agency primary endpoint) occurred in 304 of 393 (77.4%) ceftazidime-avibactam vs 296 of 417 (71.0%) doripenem patients (difference, 6.4% [95% CI, .33% to 12.36%]), demonstrating superiority at the 5% significance level. Both treatments showed similar efficacy against ceftazidime-nonsusceptible pathogens. Ceftazidime-avibactam had a safety profile consistent with that of ceftazidime alone.
Conclusions. Ceftazidime-avibactam was highly effective for the empiric treatment of cUTI (including acute pyelonephritis), and may offer an alternative to carbapenems in this setting.
Clinical Trials Registration. NCT01595438; NCT01599806.
Urinary tract infections (UTIs) are a substantial cause of global morbidity, mortality, and healthcare expenditure [1, 2]. UTIs are considered complicated (cUTI) when associated with acute pyelonephritis, chronic urinary retention in men, or obstruction, urinary catheters, recent urinary instrumentation, or urologic abnormalities [3, 4]. The gram-negative bacteria frequently implicated in cUTI, including various Enterobacteriaceae (particularly Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis), and Pseudomonas aeruginosa [5–7], often harbor multidrug resistance mechanisms (particularly extended-spectrum β-lactamases [ESBLs]) that limit the effectiveness of antibiotics previously considered first-line treatments [5, 8–10]. The risks of initial antimicrobial therapy failure, which include increased mortality [11–13], are thus driving ever-increasing reliance on carbapenems. The global emergence of carbapenem-resistant Enterobacteriaceae and P. aeruginosa [14–17] is undermining the effectiveness of the carbapenems, and highlights the urgent need for new antimicrobial treatments [18, 19].
Ceftazidime-avibactam combines ceftazidime and avibactam, a first-in-class non–β-lactam β-lactamase inhibitor which restores the in vitro activity of ceftazidime against Ambler class A (eg, ESBL and K. pneumoniae carbapenemase), class C (eg, AmpC), and some class D β-lactamase–producing bacteria [20, 21]; it is not active against metallo-β-lactamases. Ceftazidime-avibactam (2000 mg/500 mg every 8 hours) was approved by the US Food and Drug Administration (FDA) [22] based on phase 2 data (including a phase 2 cUTI trial [NCT00690378] evaluating efficacy and safety of ceftazidime-avibactam 500 mg/125 mg every 8 hours [23]) for the treatment of cUTI, including acute pyelonephritis, and complicated intra-abdominal infections (in combination with metronidazole) in adults with limited/no alternative treatment options. Approval of ceftazidime-avibactam has also been granted by the European Medicines Agency (EMA) based on additional data from the phase 3 RECAPTURE program, which evaluated the efficacy and safety of ceftazidime-avibactam (2000 mg/500 mg every 8 hours) in patients with cUTI including acute pyelonephritis.
METHODS
Study Design and Participants
RECAPTURE 1 and 2 comprised 2 identical phase 3, randomized, multicenter, double-blind, double-dummy, parallel-group trials designed incorporating FDA and EMA guidance [24, 25]. All patients (or their legal representatives) provided written informed consent. The studies were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Protocols (available at: www.astrazenecaclinicaltrials.com) were approved by study sites' independent ethics committees and/or institutional review boards.
The Supplementary Appendix lists full inclusion/exclusion criteria. In brief, eligible patients were aged 18–90 years, and had cUTI or acute pyelonephritis considered by the investigator to be serious and requiring hospitalization for intravenous (IV) antibiotic therapy. Diagnosis was based on positive urine cultures obtained within 48 hours of enrollment showing 1–2 gram-negative uropathogens at ≥105 colony-forming units (CFU)/mL, and pyuria. Complicated UTI without pyelonephritis was defined as presence of ≥2 symptoms, including ≥1 UTI-specific symptom (dysuria, urgency, frequency, and suprapubic pain with onset/worsening within the previous 7 days) as well as ≥1 complicating factor. Acute pyelonephritis was indicated by flank pain with onset/worsening within the previous 7 days, and/or costovertebral angle tenderness, with fever and/or nausea/vomiting. Patients could be enrolled before cultures were available, providing that positive results were expected, the study drugs were considered appropriate empiric therapy, and a urine Gram stain showed gram-negative bacilli and no gram-positive bacteria. Indwelling bladder catheters in place for >24 hours had to be removed or replaced (unless considered unsafe or contraindicated) before the baseline urine collection. Standardized catheter management guidelines (Supplementary Appendix) were followed.
Key exclusion criteria included complete obstruction of any portion of the urinary tract, perinephric or intrarenal abscess, or prostatitis; UTI symptoms potentially attributable to another process; urinary diversion or vesicoureteral reflux; creatinine clearance (CrCl) ≤30 mL/minute (including patients on dialysis).
Randomization and Masking
Eligible patients were randomized 1:1 to ceftazidime-avibactam 2000 mg/500 mg every 8 hours or doripenem 500 mg every 8 hours (see Supplementary Appendix for renal dose adjustment protocol) using a computer-generated central randomization code and an interactive voice/web response system. Randomization was stratified by baseline infection type (acute pyelonephritis or cUTI) and region (North America and Western Europe, Eastern Europe, and rest of world); randomization codes were assigned sequentially within each stratum using a block size of 4. Ceftazidime-avibactam was administered as 2 concurrent 1-hour IV infusions and doripenem as a 1-hour IV infusion followed by a 1-hour matching dummy (placebo) infusion to maintain blinding.
Patients meeting prespecified clinical improvement criteria (Supplementary Appendix) after ≥5 days of IV therapy could be switched to oral ciprofloxacin (500 mg every 12 hours) or sulfamethoxazole-trimethoprim (800 mg/160 mg every 12 hours) for those with a fluoroquinolone-resistant pathogen, administered approximately 8 hours after the last dose of IV treatment. Total study treatment duration (IV plus optional oral therapy) was 10 days, or up to 14 days for patients with bacteremia at baseline.
Study Procedures
The schedule of procedures (Supplementary Appendix) included urine collections for quantitative culture as well as blood cultures at baseline and as clinically indicated. Routine pathogen identification and susceptibility testing were performed at local laboratories, including study drug susceptibility assessed by Clinical and Laboratory Standards Institute (CLSI) disk diffusion methodology; all isolates were shipped to a central reference laboratory (Covance Central Laboratory Services Inc., Indianapolis, Indiana) for identification confirmation and CLSI broth microdilution susceptibility testing [26].
Assessments (Supplementary Appendix) included a patient symptom assessment questionnaire (PSAQ). Patient-reported symptomatic responses were derived programmatically from the PSAQ as resolution, persistence, or indeterminate. Microbiological outcomes were classified as eradication, persistence, persistence with increasing minimum inhibitory concentration (MIC), or indeterminate. Per-patient and per-pathogen microbiological responses were assessed as favorable (ie, eradication), unfavorable (ie, persistence or persistence with increasing MIC), or indeterminate. Investigator-determined clinical responses were assessed as cure, failure, or indeterminate.
Primary Endpoints
Separate primary efficacy endpoints were defined for the FDA and the EMA with guidance from the respective authorities. The FDA co-primary endpoints were (1) the proportion of patients with symptomatic resolution (or return to premorbid state) of UTI-specific symptoms, except flank pain, with resolution or improvement in flank pain from baseline at the day 5 visit (based on the PSAQ); (2) the proportion of patients with both microbiological eradication and symptomatic resolution (or return to premorbid state) of all UTI-specific symptoms at test of cure (TOC) (21–25 days post-randomization) in the microbiological modified intent-to-treat (mMITT) population. The EMA primary endpoint was the proportion of patients with a favorable per-patient microbiological response (ie, eradication) at TOC in the mMITT population.
Secondary and Exploratory Endpoints
Secondary endpoints included per-patient microbiological response at end of IV study treatment (EOT [IV]) and late follow-up (LFU; 45–52 days post-randomization); per-patient and per-pathogen microbiological response at TOC and LFU in patients with ≥1 ceftazidime-nonsusceptible (based on CLSI breakpoints [26]) or only ceftazidime-susceptible pathogens isolated at baseline; investigator-determined clinical cure at EOT (IV), TOC, and LFU; sustained clinical cure at LFU; and safety. Exploratory endpoints included per-patient and per-pathogen microbiological response using a cutoff of 103 CFU/mL.
Statistical Analysis
Data from the 2 studies were analyzed as a single dataset. The safety population included all patients who received any IV study therapy. The mMITT population comprised all randomized patients with minimum disease criteria and eligible baseline pathogen(s). The microbiologically evaluable (ME), extended ME (eME), and clinically evaluable populations (Supplementary Appendix) were used to verify the primary analyses, and perform secondary and exploratory analyses. Sample size was calculated using nQuery version 7 (Statistical Solutions Ltd, Cork, Ireland) using the Newcombe-Wilson score method (uncorrected) [27]. The sample size across the combined study database ensured 90% power for a 10.0% noninferiority margin and 95% power for a 12.5% noninferiority margin. Assuming both treatments had an underlying true response of >73.5% for each co-primary endpoint, and that the mMITT population included 85% of randomized patients, a sample size of 964 patients was required. Between-group treatment differences and 2-sided 95% confidence intervals (CIs) were determined using the Miettinen and Nurminen unstratified method [28]. For each primary endpoint, noninferiority of ceftazidime-avibactam vs doripenem was considered demonstrated if the lower limit of the 2-sided 95% CI around the treatment difference was greater than −12.5% (EMA) or greater than −10.0% (FDA).
Sensitivity analyses for the primary efficacy variables included (1) adjusting for prespecified stratification factors (type of infection, region, and protocol [ie, RECAPTURE 1 or 2]) using the Miettinen and Nurminen stratified method with Cochran-Mantel-Haenszel weights for the stratum weights; (2) treating indeterminate response as favorable response; and (3) considering symptomatic response at day 5 using a last post-baseline completed questionnaire carried-forward approach. Subgroup analyses assessed the impact of protocol, age group, sex, race, infection type (acute pyelonephritis or cUTI without pyelonephritis), region, baseline CrCl, bacteremia, and prior antibiotic usage on the primary efficacy variables. All analyses were performed using SAS version 9.1 or higher (SAS Institute, Cary, North Carolina).
RESULTS
Patients
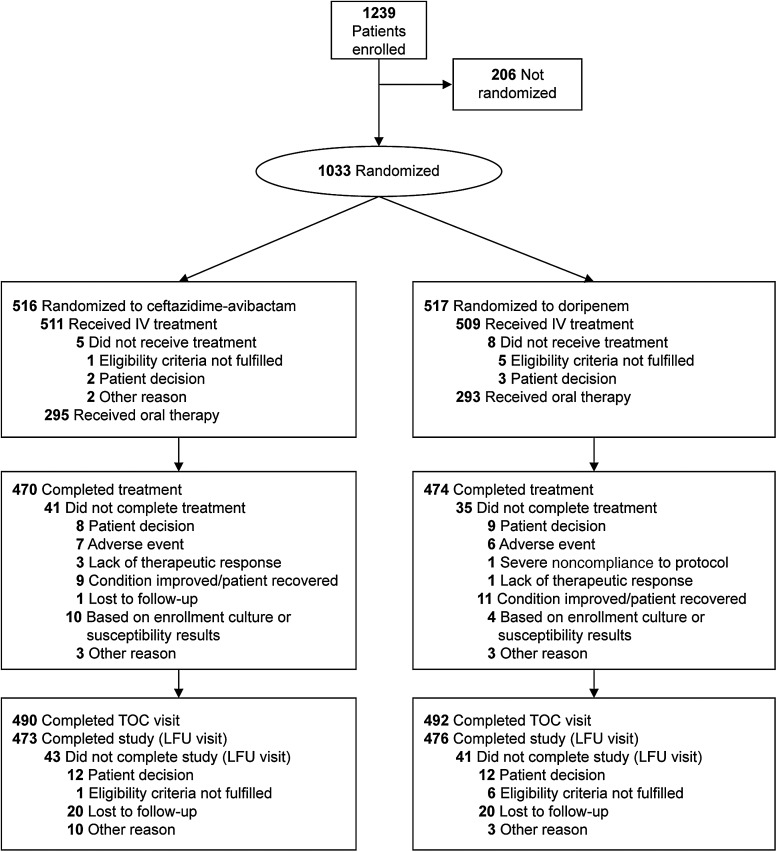
Between October 2012 and August 2014, 1033 patients at 160 centers in 25 countries were randomized (Supplementary Table 1), and 1020 received ≥1 dose of IV study drug (Figure 1). The mMITT population comprised 810 patients; 227 (28.0%) had cUTI without pyelonephritis and 583 (72.0%) had acute pyelonephritis, of whom 64 (7.9%) had ≥1 complicating factor and ≥2 symptoms, thus also meeting the cUTI enrollment criteria (Table 1). Premorbid and baseline PSAQ scores were similar between groups (data not shown).
Figure 1.
Flow of patients in the RECAPTURE trials. Abbreviations: IV, intravenous; LFU, late follow-up (45–52 days after randomization); TOC, test of cure (21–25 days after randomization).
Table 1.
Baseline Patient Characteristics (Microbiological Modified Intent-to-Treat Population)
| Characteristic | Ceftazidime- Avibactam (n = 393) | Doripenem (n = 417) |
|---|---|---|
| Age, y, mean (SD) | 51.4 (20.2) | 53.3 (18.6) |
| Male | 121 (30.8) | 124 (29.7) |
| Race | ||
| White | 321 (81.7) | 351 (84.2) |
| Black or African American | 1 (0.3) | 4 (1.0) |
| Asian | 35 (8.9) | 28 (6.7) |
| American Indian or Alaska Native | 1 (0.3) | 3 (0.7) |
| Other | 35 (8.9) | 31 (7.4) |
| Body mass index, kg/m2, mean (SD) | 26.2 (5.9) | 26.3 (5.6) |
| Diagnosis | ||
| cUTI without pyelonephritis | 106 (27.0) | 121 (29.0) |
| Pyelonephritis | 287 (73.0) | 296 (71.0) |
| With ≥1 complicating factor | 41 (10.4) | 39 (9.4) |
| Meeting symptom criteria for cUTI | 33 (8.4) | 31 (7.4) |
| Bacteremia | 38 (9.7) | 33 (7.9) |
| Fever | 157 (39.9) | 150 (36.0) |
| White blood cell count, 109/mL, median (range) | 8.5 (3.3–27.8) | 7.9 (3.1–35.4) |
| CrCl, mL/min, mean (SD)a | 87.6 (34.5) | 85.9 (34.5) |
| Renal status | ||
| Normal renal function/mild impairment (CrCl >50 mL/min) | 350 (89.1) | 379 (90.9) |
| Moderate impairment (CrCl 31–50 mL/min) | 42 (10.7) | 35 (8.4) |
| Severe impairment (CrCl <31 mL/min) | 1 (0.3) | 3 (0.7) |
| Baseline pathogen in urineb | ||
| Enterobacteriaceae | 376 (95.7) | 396 (95.0) |
| Escherichia coli | 292 (74.3) | 306 (73.4) |
| Klebsiella pneumoniae | 44 (11.2) | 56 (13.4) |
| Proteus mirabilis | 17 (4.3) | 13 (3.1) |
| Enterobacter cloacae | 11 (2.8) | 13 (3.1) |
| ESBL-positive Enterobacteriaceae | 73 (18.6) | 82 (19.7) |
| Other gram-negative bacteria | 18 (4.6) | 21 (5.0) |
| Pseudomonas aeruginosa | 18 (4.6) | 20 (4.8) |
| Prior systemic antibiotic use | 28 (7.1) | 27 (6.5) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CrCl, creatinine clearance; cUTI, complicated urinary tract infection; ESBL, extended-spectrum β-lactamase; SD, standard deviation.
a As reported by the site using the Cockcroft-Gault method based on local laboratory data.
b Includes pathogens reported with a combined frequency of ≥10 patients. Patients could have >1 pathogen. Multiple isolates of the same species from the same patient are counted only once. Other pathogens isolated in <10 patients were Citrobacter freundii complex (n = 6 overall), Citrobacter koseri (n = 2), Enterobacter aerogenes (n = 2), Klebsiella oxytoca (n = 7), Morganella morganii (n = 4), Serratia marcescens (n = 3), Proteus vulgaris group (n = 2), Providencia rettgeri (n = 2), and Burkholderia cepacia complex (n = 1).
Most patients (801/810 [98.9%]) had a single baseline uropathogen; 9 patients each had 2 pathogens. Uropathogens identified in ≥10 patients in either group are shown in Table 1. E. coli was the most frequently isolated pathogen from urine and blood. In the mMITT population, ceftazidime-nonsusceptible pathogens were identified in 159 (19.6%) patients (ceftazidime-avibactam, n = 75; doripenem, n = 84); most were E. coli or K. pneumoniae. Twenty-seven patients had baseline pathogens potentially nonsusceptible to ceftazidime-avibactam (breakpoints were provisional at time of study) or doripenem (19 isolates nonsusceptible to doripenem, 5 to doripenem and ceftazidime-avibactam, 1 to ceftazidime-avibactam only, and 7 had missing susceptibility data/breakpoint undefined).
Treatments
Median durations of IV therapy (safety population) were 7 and 8 days for ceftazidime-avibactam and doripenem, respectively; 992 of 1020 (97.3%) patients received 5‒14 days of IV treatment. In the mMITT population, 468 (57.7%) patients switched to oral antibiotics after day 5; 188 and 207 ceftazidime-avibactam and doripenem patients, respectively, switched to ciprofloxacin, 23 and 33 switched to sulfamethoxazole-trimethoprim, and 10 and 7 received alternative antibiotics.
Primary Endpoints
The efficacy analyses are summarized in Table 2. Noninferiority of ceftazidime-avibactam vs doripenem was demonstrated for the FDA co-primary endpoints (prespecified margin of −10%), as well for the EMA primary endpoint (prespecified margin of −12.5%). The lower limit of the 2-sided 95% CI for the treatment difference for the EMA primary endpoint was also >0%, showing superiority of ceftazidime-avibactam at the 5% significance level. The primary efficacy results were generally consistent across the mMITT, eME, and ME populations, and sensitivity analyses confirmed the robustness of the results (data not shown). Subgroup analyses of the primary endpoints (Supplementary Figures 1–3) were largely consistent across various baseline patient characteristics, with point estimates of treatment difference generally favoring ceftazidime-avibactam; exceptions were bacteremia for FDA co-primary endpoint 1, and moderate renal impairment (CrCl 30–50 mL/minute) for FDA co-primary endpoint 2 and the EMA primary endpoint. Across treatment groups, fewer patients with cUTI without pyelonephritis achieved the primary endpoints compared to those with acute pyelonephritis.
Table 2.
Summary of Primary and Secondary Efficacy Endpoints (Microbiological Modified Intent-to-Treat Population)
| Endpoint | Patients, No. (%) |
Difference, % (95% CI) | |
|---|---|---|---|
| Ceftazidime-Avibactam (n = 393) | Doripenem (n = 417) | ||
| FDA co-primary endpoints | |||
| Patient-assessed symptomatic resolutiona at day 5b | 276 (70.2) | 276 (66.2) | 4.0 (−2.39 to 10.42) |
| Combined patient-assessed symptomatic resolutionc and favorable per-patient microbiological response at TOCb | 280 (71.2) | 269 (64.5) | 6.7 (.30 to 13.12) |
| Per-patient favorable microbiological response at TOC | 304 (77.4) | 296 (71.0) | 6.4 (.33 to 12.36) |
| Patient-reported symptomatic resolution at TOC | 332 (84.5) | 360 (86.3) | −1.9 (−6.78 to 3.02) |
| EMA primary endpoint | |||
| Per-patient favorable microbiological response at TOCd | 304 (77.4) | 296 (71.0) | 6.4 (.33 to 12.36) |
| Secondary endpoints | |||
| Microbiological | |||
| Per-patient favorable microbiological response at EOT (IV) | 374 (95.2) | 395 (94.7) | 0.4 (−2.7 to 3.56) |
| Per-patient favorable microbiological response at LFU | 268 (68.2) | 254 (60.9) | 7.3 (.68 to 13.81) |
| Per-patient favorable microbiological response at TOC in patients with a ceftazidime-nonsusceptible pathogene | 47/75 (62.7) | 51/84f (60.7) | 2.0 (−13.18 to 16.89) |
| Per-patient favorable microbiological response at LFU in patients with a ceftazidime-nonsusceptible pathogene | 46/75 (61.3) | 38/84 (45.2) | 16.1 (.50 to 30.89) |
| Per-patient favorable microbiological response at TOC in patients with a ceftazidime-susceptible pathogene | 256/316 (81.0) | 238/326 (73.0) | 8.0 (1.50 to 14.48) |
| Per-patient favorable microbiological response at LFU in patients with a ceftazidime-susceptible pathogene | 221/316 (69.9) | 209/326 (64.1) | 5.8 (−1.46 to 13.05) |
| Clinical | |||
| Investigator-determined clinical cure | |||
| EOT (IV) | 378 (96.2) | 407 (97.6) | −1.4 (−4.07 to 1.02) |
| TOC | 355 (90.3) | 377 (90.4) | −0.1 (−4.23 to 4.03) |
| LFU | 335 (85.2) | 350 (83.9) | 1.3 (−3.71 to 6.30) |
| Sustained clinical cure at LFU in patients who were cured at TOC | 330/355 (93.0) | 345/377 (91.5) | 1.4 (−2.5 to 5.4) |
| Investigator-determined clinical cure at TOC in patients with a ceftazidime-susceptible pathogene | 287/316 (90.8) | 295/326 (90.5) | 0.3 (−4.3 to 4.9) |
| Investigator-determined clinical cure at TOC in patients with a ceftazidime-nonsusceptible pathogene | 67/75 (89.3) | 75/84f (89.3) | 0.0 (−10.4 to 10.1) |
Denominators are the total numbers in each group unless shown otherwise.
Abbreviations: CI, confidence interval; EMA, European Medicines Agency; EOT (IV), end of intravenous therapy; FDA, US Food and Drug Administration; LFU, late follow--up (45–52 days after randomization); TOC, test of cure (21–25 days after randomization).
a Symptomatic resolution of symptoms of frequency, urgency, dysuria, and suprapubic pain with resolution or improvement in flank pain, based on the patient-reported symptom assessment questionnaire (PSAQ).
b Co-primary endpoints for the FDA. The sponsor concluded noninferiority if the lower limit of the 95% CI at TOC was greater than −12.5%. The FDA noninferiority margin was a lower limit of the 95% CI greater than −10.0%.
c Symptomatic resolution of all symptoms (frequency, urgency, dysuria, suprapubic pain, and flank pain) based on the PSAQ.
d Primary endpoint for the EMA. The sponsor concluded noninferiority if the lower limit of the 95% CI at TOC was greater than −12.5%.
e Ceftazidime nonsusceptibility was defined as a central microbiology reference laboratory minimum inhibitory concentration ≥8 µg/mL for Enterobacteriaceae or ≥16 µg/mL for Pseudomonas aeruginosa, or local laboratory disk diffusion diameter (from a 30 μg ceftazidime disk) of ≤20 mm for Enterobacteriaceae and ≤17 mm for P. aeruginosa. Nine patients were not included in either subset (ceftazidime-nonsusceptible or ceftazidime-susceptible) because no susceptibility tests were performed (6 patients) or baseline blood or urine susceptibility results were missing (3 bacteremic patients).
f One patient in the doripenem group had 2 ceftazidime-nonsusceptible pathogens isolated at baseline.
Secondary and Exploratory Efficacy Endpoints
The higher microbiological eradication rate at TOC with ceftazidime-avibactam vs doripenem (EMA primary endpoint) was maintained at LFU (Table 2). Among patients with ceftazidime-nonsusceptible pathogens, eradication rates were similar for ceftazidime-avibactam and doripenem at TOC, and numerically higher for ceftazidime-avibactam at LFU; for ceftazidime-susceptible pathogens, eradication rates at TOC and LFU were numerically higher with ceftazidime-avibactam (Table 2).
Per-pathogen eradication rates at TOC were numerically higher for ceftazidime-avibactam vs doripenem for all baseline pathogens, for ceftazidime-nonsusceptible and ceftazidime-susceptible Enterobacteriaceae (Table 3), and for E. coli, K. pneumoniae, and P. mirabilis across all analysis populations (data not shown). P. aeruginosa eradication rates at TOC were higher for doripenem in the mMITT and eME populations, and higher for ceftazidime-avibactam in the ME population (in all cases 95% CI included 0). Persistence with increasing MIC at TOC (≥4-fold to study drug received from baseline) was documented for 11 pathogens (6 E. coli, 2 K. pneumoniae, 2 Enterobacter cloacae, 1 P. aeruginosa) in 11 patients (8 ceftazidime-avibactam, 3 doripenem). Apart from 1 P. aeruginosa isolate (doripenem group), MICs remained within the susceptible ranges of the respective study drugs. Multilocus sequence typing (MLST) indicated that 5 cases (4 ceftazidime-avibactam, 1 doripenem) were actually new infections. Of the remaining 4 cases (2 E. coli, 1 K. pneumoniae, 1 E. cloacae) with the same MLST in ceftazidime-avibactam patients, the observed MIC increases were exactly 4-fold and could be considered within the error of the susceptibility test. Whole-genome sequencing was used to investigate differences between the baseline and TOC isolates in these cases. Interestingly, in one K. pneumoniae, there was acquisition of a single plasmid containing the genes for SCO-1 carbapenemase and CTX-M-15 and OXA-9 β-lactamases, as well as other antibiotic resistance genes. The ceftazidime-avibactam MIC was 0.25 mg/L at baseline for this strain and 1 mg/L at TOC. In the 2 E. coli isolates, there were no obvious differences in penicillin binding proteins or β-lactamase resistance–related genes in the sequences to suggest resistance emergence. In the E. cloacae, variation in ampG was observed; however, the effect of this variation on the regulation of AmpC in this strain is unknown.
Table 3.
Per-Pathogen Favorable Microbiological Response Rates at Test of Cure (Microbiological Modified Intent-to-Treat Population)
| Pathogen | Favorable Response Rate, no./No. (%) |
Difference, % (95% CI) | |
|---|---|---|---|
| Ceftazidime-Avibactam (n = 393) | Doripenem (n = 417) | ||
| All baseline pathogens | |||
| Overall | 311/400 (77.8) | 297/419 (70.9) | 6.9 (.88 to 12.81) |
| Enterobacteriaceae | 299/382 (78.3) | 281/398 (70.6) | 7.7 (1.54 to 13.75) |
| Citrobacter freundii complex | 4/4 (100.0) | 1/2 (50.0) | 50.0 (23.13 to 91.76) |
| Citrobacter koseri | 0/1 (0.0) | 1/1 (100.0) | −100.0 (−100.00 to 58.69) |
| Enterobacter aerogenes | 1/1 (100.0) | 1/1 (100.0) | 0.0 (−88.48 to 88.48) |
| Enterobacter cloacae | 6/11 (54.5) | 9/13 (69.2) | −14.7 (−50.01 to 23.88) |
| Escherichia coli | 229/292 (78.4) | 220/306 (71.9) | 6.5 (−.41 to 13.41) |
| Klebsiella oxytoca | 5/6 (83.3) | 1/1 (100.0) | −16.7 (−59.15 to 71.03) |
| Klebsiella pneumoniae | 33/44 (75.0) | 35/56 (62.5) | 12.5 (−6.15 to 29.84) |
| Morganella morganii | 4/4 (100.0) | 0/0 | … |
| Proteus mirabilis | 16/17 (94.1) | 9/13 (69.2) | 24.9 (−2.79 to 53.59) |
| Proteus vulgaris group | 0/0 | 2/2 (100.0) | … |
| Providencia rettgeri | 0/1 (0.0) | 0/1 (0.0) | 0.0 (−88.48 to 88.48) |
| Serratia marcescens | 1/1 (100.0) | 2/2 (100.0) | 0.0 (−85.21 to 74.23) |
| Other gram-negative pathogens | 12/18 (66.7) | 16/21 (76.2) | −9.5 (−37.59 to 18.91) |
| Burkholderia cepacia complex | 0/0 | 1/1 (100.0) | … |
| Pseudomonas aeruginosa | 12/18 (66.7) | 15/20 (75.0) | −8.3 (−36.77 to 20.66) |
| Ceftazidime-nonsusceptible pathogensa | |||
| Overall | 48/75 (64.0) | 51/85 (60.0)b | 4.0 (−11.11 to 18.81) |
| Enterobacteriaceae | 43/68 (63.2) | 46/79 (58.2) | 5.0 (−10.87 to 20.50) |
| Citrobacter freundii complex | 3/3 (100.0) | 0/0 | … |
| Enterobacter cloacae | 3/7 (42.9) | 5/6 (83.3) | −40.5 (−76.04 to 14.76) |
| Escherichia coli | 22/36 (61.1) | 20/37 (54.1) | 7.1 (−15.54 to 28.93) |
| Klebsiella pneumoniae | 13/18 (72.2) | 17/30 (56.7) | 15.6 (−13.30 to 40.34) |
| Morganella morganii | 1/1 (100.0) | 0/0 | … |
| Proteus mirabilis | 1/2 (50.0) | 4/5 (80.0) | −30.0 (−82.00 to 39.20) |
| Providencia rettgeri | 0/1 (0.0) | 0/1 (0.0) | 0.0 (−88.48 to 88.48) |
| Other gram-negative pathogens | 5/7 (71.4) | 5/6 (83.3) | −11.9 (−54.78 to 37.60) |
| Pseudomonas aeruginosa | 5/7 (71.4) | 5/6 (83.3) | −11.9 (−54.78 to 37.60) |
| Ceftazidime-susceptible pathogensa | |||
| Overall | 254/311 (81.7) | 228/312 (73.1) | 8.6 (2.03 to 15.14) |
| Enterobacteriaceae | 247/301 (82.1) | 217/297 (73.1) | 9.0 (2.32 to 15.66) |
| Citrobacter freundii complex | 1/1 (100.0) | 1/2 (50.0) | 50.0 (−64.16 to 93.08) |
| Citrobacter koseri | 0/1 (0.0) | 1/1 (100.0) | −100.0 (−100.00 to 58.69) |
| Enterobacter aerogenes | 1/1 (100.0) | 1/1 (100.0) | 0.0 (−88.48 to 88.48) |
| Enterobacter cloacae | 3/4 (75.0) | 4/7 (57.1) | 17.9 (−41.27 to 62.79) |
| Escherichia coli | 206/254 (81.1) | 193/262 (73.7) | 7.4 (.21 to 14.63) |
| Klebsiella oxytoca | 5/6 (83.3) | 1/1 (100.0) | −16.7 (−59.15 to 71.03) |
| Klebsiella pneumoniae | 20/26 (76.9) | 18/26 (69.2) | 7.7 (−16.81 to 31.50) |
| Morganella morganii | 2/2 (100.0) | 0/0 | … |
| Proteus mirabilis | 15/15 (100.0) | 5/8 (62.5) | 37.5 (11.44 to 69.98) |
| Proteus vulgaris group | 0/0 | 2/2 (100.0) | … |
| Serratia marcescens | 1/1 (100.0) | 2/2 (100.0) | 0.0 (−85.21 to 74.23) |
| Other gram-negative pathogens | 7/10 (70.0) | 11/15 (73.3) | −3.3 (−40.10 to 30.94) |
| Burkholderia cepacia complex | 0/0 | 1/1 (100.0) | … |
| Pseudomonas aeruginosa | 7/10 (70.0) | 10/14 (71.4) | −1.4 (−38.84 to 33.69) |
Patients could have >1 pathogen. Multiple isolates of the same species in the same patient are counted only once. Test of cure occurred 21–25 days after randomization.
Abbreviation: CI, confidence interval.
a Ceftazidime-nonsusceptible was defined as a central microbiology reference laboratory minimum inhibitory concentration ≥8 µg/mL for Enterobacteriaceae or ≥16 µg/mL for P. aeruginosa, or local laboratory disk diffusion diameter (from a 30-μg ceftazidime disk) of ≤20 mm for Enterobacteriaceae and ≤17 mm for P. aeruginosa. Nine patients were not included in either subset (ceftazidime-nonsusceptible or ceftazidime-susceptible) because no susceptibility tests were performed (6 patients) or baseline blood or urine susceptibility results were missing (3 bacteremic patients).
b One patient in the doripenem group had 2 ceftazidime-nonsusceptible pathogens isolated at baseline.
Investigator-determined clinical cure rates were high and similar across treatment groups (Table 2). Clinical cure rates for patients with ceftazidime-nonsusceptible E. coli and K. pneumoniae were numerically higher for ceftazidime-avibactam; those for ceftazidime-nonsusceptible E. cloacae and P. aeruginosa were numerically higher for doripenem (data not shown).
Microbiological eradication at TOC using the exploratory <103 CFU/mL cutoff (mMITT population) occurred in 299 of 393 (76.1%) ceftazidime-avibactam and 291 of 417 (69.8%) doripenem patients (difference, 6.3% [95% CI, .17% to 12.38%]; Supplementary Table 2). Eradication at TOC using the more stringent criteria occurred for 10 fewer pathogens in 10 patients: 5 additional cases of persistence for ceftazidime-avibactam and 2 for doripenem (none with increasing MIC) and 3 indeterminate responses for doripenem.
Safety
At least 1 adverse event (AE) occurred in 185 of 511 (36.2%) and 158 of 509 (31.0%) ceftazidime-avibactam and doripenem recipients, respectively (Table 4). AEs were predominantly mild or moderate in intensity, and generally balanced across groups. Twenty-one (4.1%) and 12 (2.4%) patients treated with ceftazidime-avibactam and doripenem, respectively, had ≥1 serious AE, of which most occurred after the last dose of IV treatment. Few AEs led to study drug discontinuation and no deaths occurred (Table 4). AEs reported in ≥2% of patients comprised headache, nausea, diarrhea, and constipation (Table 4); no new safety concerns were identified. Three AEs of Clostridium difficile colitis occurred in 2 (0.4%) ceftazidime-avibactam patients (none were reported for doripenem). No clinically meaningful trends in laboratory values, electrocardiographic parameters, physical examination, or vital signs were identified. Clinically significant changes in these parameters were infrequent and balanced across groups.
Table 4.
Safety Evaluation up to Late Follow-up (Safety Population)
| AE Category | Ceftazidime-Avibactam (n = 511) | Doripenem (n = 509) |
|---|---|---|
| Any AE | 185 (36.2) | 158 (31.0) |
| Any AE with an outcome of death | 0 | 0 |
| Any serious AE | 21 (4.1) | 12 (2.4) |
| Any AE leading to discontinuation of study drug | 7 (1.4) | 6 (1.2) |
| Any AE of severe intensity | 10 (2.0) | 7 (1.4) |
| AEs reported in ≥2% of patients in either treatment group by system organ class and preferred terma | ||
| Nervous system disorders | ||
| Headache | 38 (7.4) | 40 (7.9) |
| Gastrointestinal disorders | ||
| Nausea | 15 (2.9) | 10 (2.0) |
| Diarrhea | 14 (2.7) | 6 (1.2) |
| Constipation | 11 (2.2) | 7 (1.4) |
Data are presented as No. (%).
Abbreviation: AE, adverse event.
a MedDRA (version 16.1) classification. Patients with multiple AEs are counted once for each category or system organ class and/or preferred term. Patients with AEs in >1 category are counted once in each of those categories.
DISCUSSION
These findings demonstrate the noninferiority of ceftazidime-avibactam vs doripenem for the treatment of hospitalized patients with cUTI or acute pyelonephritis based on FDA- and EMA-defined endpoints. The CI around the treatment difference for microbiological eradication at TOC (EMA primary endpoint) also demonstrated superiority of ceftazidime-avibactam over doripenem at the 5% significance level. Moreover, an exploratory analysis using a more stringent cutoff (<103 CFU/mL) was consistent with the primary analysis, also showing superiority of ceftazidime-avibactam. The safety profile of ceftazidime-avibactam appeared consistent with that of ceftazidime alone; no new safety concerns were identified.
Baseline pathogens were typical of cUTI and similar between groups. Enterobacteriaceae were isolated from >95% of patients; almost 75% were E. coli. The most frequently isolated non-Enterobacteriaceae was P. aeruginosa (<5% of patients). Per-pathogen eradication rates at TOC numerically favored ceftazidime-avibactam over doripenem for all Enterobacteriaceae, including those most commonly isolated (E. coli, K. pneumoniae, and P. mirabilis). Eradication rates in both groups were lower among patients with ceftazidime-nonsusceptible pathogens compared with the overall population. Investigator-determined clinical cure rates at TOC were approximately 90% across groups, and similar for patients with ceftazidime-nonsusceptible and ceftazidime-susceptible pathogens.
The efficacy of ceftazidime-avibactam for infections caused by ceftazidime-nonsusceptible pathogens in RECAPTURE is consistent with in vitro and preclinical studies [21, 29–31] and available clinical data [32, 33]. The phase 3 REPRISE trial (NCT01644643) randomized patients with cUTI (n = 281 [93%]) or complicated intra-abdominal infection (n = 21 [7%]) caused by ceftazidime-nonsusceptible gram-negative pathogens to ceftazidime-avibactam or best available therapy (approximately 97% received a carbapenem) [33]. Differences in trial design limit direct comparisons. Nonetheless, in common with RECAPTURE, microbiological response rates in REPRISE were consistently higher for ceftazidime-avibactam vs best available therapy at all study visits [33].
RECAPTURE endpoints and noninferiority margins were selected with FDA and EMA guidance; consideration was also given to the need to switch patients from IV to oral antibiotic therapy as early as possible to expedite hospital discharge. Strict criteria were applied for oral switch, ensuring substantial improvement or resolution of infection; the TOC assessments were therefore considered appropriate endpoints.
A possible limitation is that no validated questionnaire was available to assess symptoms in cUTI patients. The PSAQ, which was based on a validated questionnaire for uncomplicated UTI [34], was able to detect symptomatic improvement over time; it was therefore considered a suitable endpoint, and could serve as a useful tool in monitoring patient response in future studies.
The choice of doripenem as comparator was based on its efficacy in cUTI [35, 36], expected activity against ceftazidime-resistant pathogens, dosing schedule, and recommended use in severe UTI [37], as well as its availability; other carbapenems were not approved for cUTI in all study regions. Of note, doripenem was withdrawn in Europe in July 2014 for reasons related to its efficacy and safety in nosocomial pneumonia, but it remains available for treatment of cUTI in the United States [38]. In addition, although reduced efficacy in patients with renal impairment was not evident in RECAPTURE, it is important to note that the ceftazidime-avibactam renal dose adjustment protocol differed from the current FDA label [22].
In summary, the microbiological profile of pathogens isolated in RECAPTURE illustrates the need for effective alternative antimicrobials: nearly 20% were ceftazidime-nonsusceptible. The effectiveness of ceftazidime-avibactam in RECAPTURE and REPRISE, including against ceftazidime-nonsusceptible pathogens, highlights its potential clinical value as a carbapenem-sparing treatment in this setting. In vitro and in vivo data suggest that ceftazidime-avibactam is also likely to be effective in infections caused by carbapenemase-producers [29, 31, 39, 40].
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors thank all investigators and patients involved in this clinical trial program. We gratefully acknowledge the contributions of Bob McLaughlin for his expertise in providing Multilocus sequence typing and whole-genome sequencing analyses that contributed to the understanding of the patients with persistence and persistence with increasing minimum inhibitory concentration. Medical writing support was provided by Faye Gould and Mark Waterlow of Prime Medica Ltd, Knutsford, Cheshire, United Kingdom, funded by AstraZeneca.
Author contributions. Study concept and design: F. M. W., P. N., J. A., X. H., G. G. S., and L. B. G. Acquisition, analysis, or interpretation of data: F. M. W., J. D. S., P. N., J. A., X. H., K. Y., and L. B. G. Drafting of the manuscript: P. N., J. A., X. H., G. G. S., and L. B. G. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: J. A., X. H., and L. B. G. Administrative, technical, or material support: P. N., J. A., X. H., K. Y., and L. B. G. Study supervision: F. M. W., J. D. S., and L. B. G. All authors had full access to all trial data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. This work was supported by AstraZeneca and Actavis plc. Ceftazidime-avibactam is being developed by AstraZeneca and Actavis plc, a subsidiary of Allergan Inc.
Potential conflicts of interest. P. N., J. A., G. G. S., and X. H. are employees of AstraZeneca. L. B. G. is a former employee of AstraZeneca. K. Y. is a former contractor for AstraZeneca. F. M. W. is an employee of Justus-Liebig-University, Giessen, Germany, which received institutional funding for the conduct of the study. He is a member of the German Centre for Infection Research (Giessen - Marburg - Langen). J. D. S. is an employee of Detroit Medical Center, Detroit, Michigan, which received institutional funding for the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zimlichman E, Henderson D, Tamir O et al. . Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173:2039–46. [DOI] [PubMed] [Google Scholar]
- 2.Hsueh PR, Hoban DJ, Carmeli Y et al. . Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect 2011; 63:114–23. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol 2010; 7:653–60. [DOI] [PubMed] [Google Scholar]
- 4.Johansen TE, Botto H, Cek M et al. . Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents 2011; 38(suppl):64–70. [DOI] [PubMed] [Google Scholar]
- 5.Levison ME, Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep 2013; 15:109–15. [DOI] [PubMed] [Google Scholar]
- 6.Cek M, Tandogdu Z, Wagenlehner F, Tenke P, Naber K, Bjerklund-Johansen TE. Healthcare-associated urinary tract infections in hospitalized urological patients—a global perspective: results from the GPIU studies 2003–2010. World J Urol 2014; 32:1587–94. [DOI] [PubMed] [Google Scholar]
- 7.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YH, Ko WC, Hsueh PR. Emerging resistance problems and future perspectives in pharmacotherapy for complicated urinary tract infections. Expert Opin Pharmacother 2013; 14:587–96. [DOI] [PubMed] [Google Scholar]
- 9.Zowawi HM, Harris PN, Roberts MJ et al. . The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol 2015; 12:570–84. [DOI] [PubMed] [Google Scholar]
- 10.Tandogdu Z, Cek M, Wagenlehner F et al. . Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J Urol 2014; 32:791–801. [DOI] [PubMed] [Google Scholar]
- 11.Raman G, Avendano E, Berger S, Menon V. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect Dis 2015; 15:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elhanan G, Sarhat M, Raz R. Empiric antibiotic treatment and the misuse of culture results and antibiotic sensitivities in patients with community-acquired bacteraemia due to urinary tract infection. J Infect 1997; 35:283–8. [DOI] [PubMed] [Google Scholar]
- 14.Canton R, Akova M, Carmeli Y et al. . Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 2012; 18:413–31. [DOI] [PubMed] [Google Scholar]
- 15.Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med 2013; 80:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadkarni AS, Schliep T, Khan L, Zeana CB. Cluster of bloodstream infections caused by KPC-2 carbapenemase-producing Klebsiella pneumoniae in Manhattan. Am J Infect Control 2009; 37:121–6. [DOI] [PubMed] [Google Scholar]
- 17.Samuelsen O, Naseer U, Tofteland S et al. . Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J Antimicrob Chemother 2009; 63:654–8. [DOI] [PubMed] [Google Scholar]
- 18.Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc 2011; 86:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golan Y. Empiric therapy for hospital-acquired, gram-negative complicated intra-abdominal infection and complicated urinary tract infections: a systematic literature review of current and emerging treatment options. BMC Infect Dis 2015; 15:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahiri SD, Mangani S, Durand-Reville T et al. . Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC beta-lactamases. Antimicrob Agents Chemother 2013; 57:2496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagace-Wiens P, Walkty A, Karlowsky JA. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of gram-negative bacterial infections. Core Evid 2014; 9:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Actavis Inc. AVYCAZ (ceftazidime-avibactam) for injection, for intravenous use: prescribing information. Available at: http://pi.actavis.com/data_stream.asp?product_group=1957&p=pi&language=E Accessed 18 December 2015.
- 23.Vazquez JA, Gonzalez Patzan LD, Stricklin D et al. . Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 2012; 28:1921–31. [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency. Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. London: EMA, 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/11/WC500153953.pdf Accessed 18 December 2015. [Google Scholar]
- 25.US Food and Drug Administration. Guidance for industry on complicated urinary tract infections: developing drugs for treatment. Draft guidance Available at: http://www.regulations.gov/contentStreamer?documentId=FDA-2012-D-0148-0002&attachmentNumber=1&disposition=attachment&contentType=pdf Accessed 18 December 2015.
- 26.Clinical Laboratory and Standards Institute. Performance standards for antimicrobial susceptibility testing. 22nd informational supplement: Wayne, PA: CLSI, 2012. [Google Scholar]
- 27.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998; 17:873–90. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213–26. [DOI] [PubMed] [Google Scholar]
- 29.Keepers TR, Gomez M, Celeri C, Nichols WW, Krause KM. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against beta-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2014; 58:5297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrob Agents Chemother 2014; 58:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields RK, Clancy CJ, Hao B et al. . Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum beta-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother 2015; 59:5793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazuski JE, Gasink LB, Armstrong J et al. . Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection—results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62:1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmeli Y, Armstrong J, Laud PJ et al. . Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16:661–73. [DOI] [PubMed] [Google Scholar]
- 34.Clayson D, Wild D, Doll H, Keating K, Gondek K. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): the UTI Symptom Assessment questionnaire. BJU Int 2005; 96:350–9. [DOI] [PubMed] [Google Scholar]
- 35.Naber KG, Llorens L, Kaniga K, Kotey P, Hedrich D, Redman R. Intravenous doripenem at 500 milligrams versus levofloxacin at 250 milligrams, with an option to switch to oral therapy, for treatment of complicated lower urinary tract infection and pyelonephritis. Antimicrob Agents Chemother 2009; 53:3782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagenlehner FM, Wagenlehner C, Redman R, Weidner W, Naber KG. Urinary bactericidal activity of doripenem versus that of levofloxacin in patients with complicated urinary tract infections or pyelonephritis. Antimicrob Agents Chemother 2009; 53:1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabe M, Bartoletti R, Bjerklund Johansen TE et al. . Guidelines on urological infections. Available at: http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf Accessed 9 November 2015.
- 38.Shionogi Inc. DORIBAX (doripenem for injection), for intravenous use: prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022106s015lbl.pdf Accessed 18 December 2015.
- 39.Levasseur P, Girard AM, Lavallade L, Miossec C, Pace J, Coleman K. Efficacy of a ceftazidime-avibactam combination in a murine model of septicemia caused by Enterobacteriaceae species producing AmpC or extended-spectrum beta-lactamases. Antimicrob Agents Chemother 2014; 58:6490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. In vivo efficacy of humanized exposures of ceftazidime-avibactam in comparison with ceftazidime against contemporary Enterobacteriaceae isolates. Antimicrob Agents Chemother 2014; 58:6913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.