In a multicohort study of human immunodeficiency virus/hepatitis C virus-coinfected individuals, the crude incidence of hepatocellular cacinoma increased from 2001 to 2014, while other liver events declined. Age, cirrhosis, and low current CD4 cell count were risk factors.
Keywords: HIV, hepatitis C virus, hepatocellular carcinoma, liver disease, cohort study
Abstract
Background. While liver-related deaths in human immunodeficiency virus (HIV) and hepatitis C virus (HCV)–coinfected individuals have declined over the last decade, hepatocellular carcinoma (HCC) may have increased. We describe the epidemiology of HCC and other liver events in a multicohort collaboration of HIV/HCV–coinfected individuals.
Methods. We studied HCV antibody-positive adults with HIV in the EuroSIDA study, the Southern Alberta Clinic Cohort, the Canadian Co-infection Cohort, and the Swiss HIV Cohort study from 2001 to 2014. We calculated the incidence of HCC and other liver events (defined as liver-related deaths or decompensations, excluding HCC) and used Poisson regression to estimate incidence rate ratios.
Results. Our study comprised 7229 HIV/HCV–coinfected individuals (68% male, 90% white). During follow-up, 72 cases of HCC and 375 other liver events occurred, yielding incidence rates of 1.6 (95% confidence interval [CI], 1.3, 2.0) and 8.6 (95% CI, 7.8, 9.5) cases per 1000 person-years of follow-up, respectively. The rate of HCC increased 11% per calendar year (95% CI, 4%, 19%) and decreased 4% for other liver events (95% CI, 2%, 7%), but only the latter remained statistically significant after adjustment for potential confounders. Older age, cirrhosis, and low current CD4 cell count were associated with a higher incidence of both HCC and other liver events.
Conclusions. In HIV/HCV–coinfected individuals, the crude incidence of HCC increased from 2001 to 2014, while other liver events declined. Individuals with cirrhosis or low current CD4 cell count are at highest risk of developing HCC or other liver events.
As hepatitis C virus (HCV) and human immunodeficiency virus (HIV) have shared modes of transmission, individuals with HIV are more often infected with HCV than the general population [1]. HCV can cause chronic hepatic inflammation, leading to liver fibrosis and cirrhosis that entails a risk of liver decompensation and hepatocellular carcinoma (HCC) [2]. Concomitant HIV infection accelerates this process [3, 4], and liver disease is one of the main causes of death in people with HIV [5]. Some studies indicate that the overall rate of liver-related death is declining in HIV/HCV–coinfected individuals [6], but at the same time, national cohort studies have shown an increasing rate of HCC [7–9].
Our aim was to study HIV/HCV–coinfected individuals in Europe and Canada and describe trends in the incidences of HCC and other liver events from 2001 to 2014 and identify risk factors for the development of HCC and other liver events.
METHODS
Study Population
We included all HIV/HCV–coinfected individuals in 4 prospective cohorts of HIV-positive individuals: the EuroSIDA study [10], which enrolls from 111 clinics across Europe, Israel, and Argentina; the Southern Alberta Clinic Cohort [11], which enrolls from Southern Alberta, Canada; the Canadian Co-infection Cohort study (CTN222) [12], which enrolls HIV/HCV–coinfected individuals from 18 centers across Canada; and the Swiss HIV Cohort Study [13]. All cohorts collect and update data at least every 6 months using standardized collection forms.
We defined HCV coinfection as being HCV antibody-positive. Baseline was defined as the latest of first cohort or clinic visit; first positive HCV antibody test; or 1 January 2001 (since data on non-AIDS-defining cancers, including HCC, were collected prospectively from this date).
Data from all participating cohorts were pooled together by use of the HIV Cohort Data Exchange Protocol [14].
Outcomes
We studied 2 separate outcomes: HCC and other liver events, defined as liver decompensation or liver-related death. Liver decompensation was defined as hepatic encephalopathy (grade 3 or 4), hepatorenal syndrome, ascites, variceal bleeding, or spontaneous bacterial peritonitis. Liver-related death was defined as death caused by chronic viral hepatitis or other liver failure, excluding HCC. The diagnoses were based on pathology reports, hospital discharge summaries, or consultation notes. For HCC, in the absence of the above, the diagnosis could also be based on a strong suspicion supported by evidence from radiological imaging or biochemical assay. All events were subsequently centrally reviewed.
Statistical Analyses
Crude incidence rates of both HCC and other liver events were calculated by calendar time, grouped into 2-year periods, and stratified by latest cirrhosis status and CD4 cell count, also testing for interaction between cirrhosis status and current CD4 cell count. When calculating incidence of other liver events, individuals with a liver decompensation prior to baseline were excluded from follow-up (N = 179).
Poisson generalized estimating equations assuming autoregressive (AR1) correlation were used to investigate the association between various demographic, HIV, and lifestyle-related characteristics and the incidence of HCC and other liver-related events, separately. Variables significant in the univariate regression models (P < .1) were included in multivariate regression models. Sensitivity analyses were performed that excluded HCC cases with HIV/HCV/hepatitis B virus (HBV) coinfection or those with any cancer diagnosis (other than HCC) prior to the end of follow-up.
All statistical tests were 2 sided with a type I error rate of 5%. Statistical analyses were performed using SAS 9.3 (Statistical Analysis Software, Cary, North Carolina).
Predictor Variables
The following variables were analyzed: age*, sex, race (white, other/unknown), region (Europe East/Argentina, Europe West, Canada), HIV risk group (men who have sex with men [MSM], injection drug use [IDU], heterosexual, other/unknown), body mass index (BMI) category*, ever smoked*, ever abused alcohol*, diabetes mellitus*, HBV coinfection*, ever HBV active drugs*, ever HCV active drugs*, ever combination antiretroviral therapy (cART)*, ever AIDS*, detectable HIV RNA*, CD4 cell count current*, CD4 cell count nadir*, cirrhosis*, and calendar year of event. Asterisk indicates variables that were updated at each visit.
BMI was calculated as weight in kilograms/squared height in meters and categorized as either underweight (BMI < 20), normal weight (BMI 20–25), overweight (BMI 25–30), or obese (BMI > 30). Alcohol use was not uniformly collected in all cohorts. Where available, alcohol abuse was defined as consuming more than 25 and 20 units per week for men and women, respectively. Diabetes mellitus was defined as having a diagnosis of insulin-dependent diabetes mellitus or taking diabetic medication or insulin. HBV coinfection was defined as the presence of hepatitis B surface antigen in serum, and cART was defined as receiving at least 3 antiretrovirals from any class. Detectable HIV RNA was defined as having plasma HIV RNA >400 copies/mL.
Cirrhosis was defined using a hierarchical structure with cutoffs from earlier publications [15–17]. Liver biopsy with a METAVIR score of F4 was considered the highest level of evidence, followed by FibroScan elasticity of ≥12.5 kPa, then aspartate aminotransferase-to-platelet ratio index (APRI) of ≥2, and then plasma hyaluronic acid level of ≥200 ng/mL. A patient was assumed cirrhotic from the date of measurement and onward if the measurement fulfilled at least 1 of the criteria mentioned above. If markers were inconsistent, the marker of highest evidence level decided the cirrhosis status. Individuals in whom we could not assess cirrhosis status were classified as “unknown.”
RESULTS
Baseline Characteristics
We included 7229 individuals in our study (4132 from the EuroSIDA study; 2044 from the Swiss HIV Cohort study; 840 from the Canadian Co-infection Cohort; 213 from the Southern Alberta Clinic Cohort). Table 1 shows the baseline characteristics of our study population. The majority were male (68%), white (90%), and primarily from western Europe (58%). Median age was 38 years (interquartile range [IQR]: 36, 43). The main HIV risk group was IDU (59%), and 5% were HIV/HCV/HBV–coinfected.
Table 1.
Baseline Characteristics of 7229 Human Immunodeficiency Virus/Hepatitis C Virus–Coinfected Individuals
| Characteristic | N (%) |
|---|---|
| Age, years, median (IQR) | 38.1 (32.6, 43.4) |
| Female sex, N (%) | 2304 (31.9) |
| White race, N (%) | 6530 (90.3) |
| Region, N (%) | |
| Western Europe | 4200 (58.1) |
| Eastern Europe/Argentina | 1976 (27.3) |
| Canada | 1053 (14.6) |
| HIV risk group, N (%) | |
| Men who have sex with men | 834 (11.5) |
| Injection drug use | 4289 (59.3) |
| Heterosexual | 1188 (16.4) |
| Other/unknown | 918 (12.7) |
| Body mass index category, N (%)a | |
| Underweight | 111 (4.1) |
| Normal weight | 2003 (74.5) |
| Overweight | 496 (18.5) |
| Obese | 78 (2.9) |
| Ever smoked, N (%)b | 2168 (71.7) |
| Ever abused alcohol, N (%)c | 165 (13.3) |
| Diabetes mellitus, N (%) | 200 (2.8) |
| HBV coinfection, N (%)d | 329 (5.1) |
| Ever HBV active drugs, N (%) | 381 (5.3) |
| Ever hepatitis C virus active drugs, N (%) | 735 (10.2) |
| Ever combination antiretroviral therapy, N (%) | 5138 (71.1) |
| Ever AIDS, N (%) | 1822 (25.2) |
| Detectable HIV RNA, N (%)e | 2540 (41.6) |
| CD4 cell count, cells/mm3, median (IQR)f | 388 (240, 571) |
| CD4 cell count nadir, cells/mm3, median (IQR)g | 208 (95, 351) |
| Cirrhosis, N (%)h | 342 (5.9) |
| Year of baselinei, N (%) | |
| 2001–2002 | 3185 (44.1) |
| 2003–2004 | 799 (11.1) |
| 2005–2006 | 892 (12.3) |
| 2007+ | 2353 (32.5) |
Abbreviations: HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range.
a Body mass index was categorized in 2668 individuals.
b Smoking status was determined in 3025 individuals.
c Alcohol abuse was determined in 1241 individuals.
d HBV coinfection was determined in 6485 individuals.
e Detectable HIV RNA was determined in 6099 individuals.
f CD4 cell count was determined in 6802 individuals.
g CD4 cell count nadir was determined in 7098 individuals.
h Cirrhosis was determined in 5799 individuals.
i Baseline was defined as the latest of first visit; first positive hepatitis C virus antibody test; or 1 January 2001.
Incidence Rates of HCC and Other Liver Events
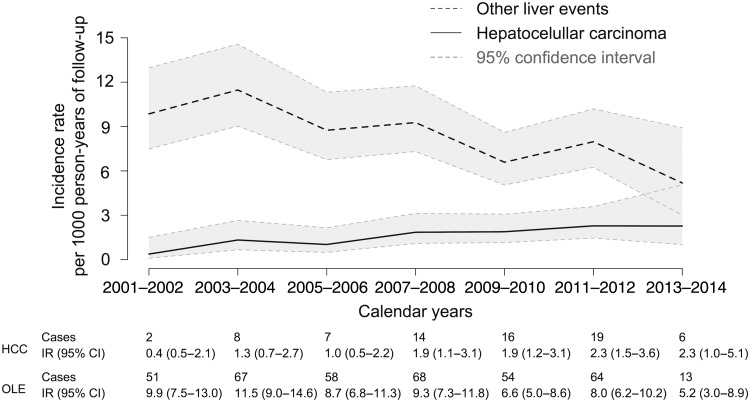
From 2001 to 2014, 72 cases of HCC (with 45 192 person-years of follow-up) and 375 cases of other liver events (with 43 718 person-years of follow-up) occurred, resulting in overall incidence rates of 1.6 (95% confidence interval [CI], 1.3, 2.0) cases of HCC per 1000 person-years of follow-up and 8.6 (95% CI, 7.8, 9.5) cases of other liver events per 1000 person-years of follow-up. Figure 1 shows the incidence rates of HCC and other liver events as a function of calendar years. The incidence of HCC increased by 11% per year (95% CI, 4%, 19%; P = .002), from 0.4 cases per 1000 person-years of follow-up in 2001–2002 to 2.3 cases per 1000 person-years of follow-up in 2013–2014. In contrast, the incidence of other liver events decreased by 4% per year (95% CI, 2%, 7%; P = .002) from 9.9 cases per 1000 person-years of follow-up in 2003–2004 to 5.2 cases per 1000 person-years of follow-up in 2013–2014.
Figure 1.
Trend in incidence rates (with 95% confidence intervals [CI]) of hepatocellular carcinoma (HCC) and other liver events in 7229 human immunodeficiency virus/hepatitis C virus coinfected individuals from 2001 to 2014. Abbreviations: IR, incidence rate; OLE, other liver event.
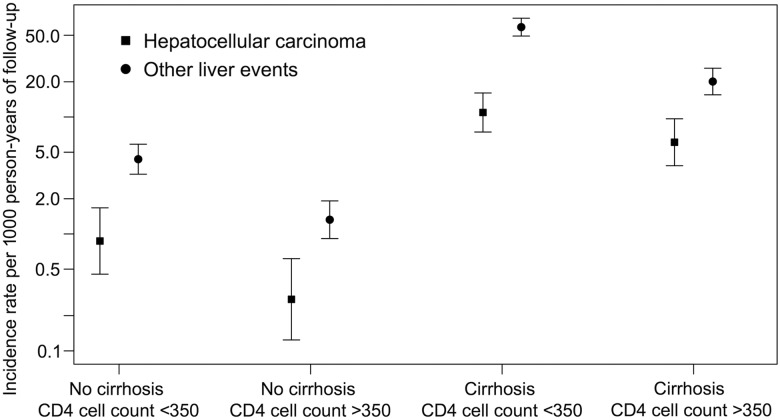
In cirrhotics, the overall incidence rate of HCC was 7.9 (95% CI, 5.9, 10.5) cases per 1000 person-years of follow-up compared with 0.5 (95% CI, .3, .7) cases per 1000 person-years of follow-up in noncirrhotics. For other liver events, the incidence rates were 35.9 (95% CI, 31.1, 41.4) and 2.4 (95% CI, 1.9, 3.0) cases per 1000 person-years of follow-up for cirrhotics and noncirrhotics, respectively.
In both cirrhotics and noncirrhotics, the incidence rates of both outcomes were lower in those with a current CD4 cell count >350 cells/mm3 (Figure 2). In cirrhotics, the incidence rate of HCC decreased from 10.9 (95% CI, 7.4, 16.1) cases per 1000 person-years of follow-up in those with a CD4 cell count <350 cells/mm3 to 6.1 (95% CI, 3.8, 9.7) cases per 1000 person-years of follow-up in those with a CD4 cell count >350 cells/mm3. For other liver events, the effect was more drastic, as the incidence rate went from 58.7 (95% CI, 49.2, 70.0) to 20.1 (95% CI, 15.5, 26.4) cases per 1000 person-years of follow-up, respectively. There was no significant interaction between cirrhosis and current CD4 cell count for any outcome (both P > .2), but the analyses had low power.
Figure 2.
Incidence rates (with 95% confidence intervals) for hepatocellular carcinoma and other liver events in human immunodeficiency virus/hepatitis C virus coinfected individuals stratified by cirrhosis status and current CD4 cell count (cells/mm3). Note: Formal analysis of interaction between cirrhosis and current CD4 cell count was not significant for both outcomes (all P values >.2).
Characteristics at Event
Table 2 shows the characteristics at event in those who developed HCC or other liver events and at the end of follow-up in the remaining cohort. Median age at event was 49.6 years for HCC vs 43.9 years for other liver events. Within 2 years of event, 75% of HCC cases and 70% of other liver events cases had cirrhosis. Only 8% of HCC cases and 6% of other liver events cases were HBV–coinfected. Thirty-two percent of HCC cases had ever received HCV active drugs vs 18% of other liver events cases. Almost all had ever received cART (99% of HCC cases and 91% of other liver events cases), but their most recent (within 6 months) CD4 cell counts were quite low with a median of 286 cells/mm3 (IQR, 201, 438) in HCC cases and 242 cells/mm3 (IQR, 110, 397) in other liver event cases.
Table 2.
Characteristics of Human Immunodeficiency Virus/Hepatitis C Virus–Coinfected Individuals Within 6 Months of Diagnosis of Hepatocellular Carcinoma or Other Liver Event or Within 6 Months of End of Follow-Up in the Remaining Cohort
| Characteristic | No HCC or Other Liver Event (n = 6787) | HCC (n = 72) | Other Liver Event (n = 375) |
|---|---|---|---|
| Age, years, median (IQR) | 44.8 (37.6, 50.8) | 49.6 (46.0, 55.8) | 43.9 (38.6, 49.6) |
| Follow-up time, years, median (IQR) | 5.3 (2.5, 9.7) | 6.0 (2.4, 9.2) | 3.9 (1.6, 6.7) |
| Female sex, N (%) | 2182 (32.1) | 17 (23.6) | 106 (28.3) |
| White race, N (%) | 6124 (90.2) | 68 (94.4) | 343 (91.5) |
| Region, N (%) | |||
| Western Europe | 3882 (57.2) | 55 (76.4) | 266 (70.9) |
| Eastern Europe /Argentina | 1898 (28.0) | 6 (8.3) | 73 (19.5) |
| Canada | 1007 (14.8) | 11 (15.3) | 36 (9.6) |
| HIV risk group, N (%) | |||
| Men who have sex with men | 802 (11.8) | 6 (8.3) | 27 (7.2) |
| Injection drug use | 4003 (59.0) | 40 (55.6) | 249 (66.4) |
| Heterosexual | 1129 (16.6) | 17 (23.6) | 43 (11.5) |
| Other/unknown | 853 (12.6) | 9 (12.5) | 56 (14.9) |
| Body mass index category, N (%)a | |||
| Underweight | 179 (6.8) | 3 (12.0) | 23 (13.9) |
| Normal weight | 1794 (68.6) | 17 (68.0) | 109 (65.7) |
| Overweight | 527 (20.2) | 4 (16.0) | 27 (16.3) |
| Obese | 115 (4.4) | 1 (4.0) | 7 (4.2) |
| Ever smoked, N (%)b | 2987 (80.1) | 25 (75.8) | 180 (86.5) |
| Ever abused alcohol, N (%)c | 610 (18.6) | 4 (20.0) | 22 (30.6) |
| Diabetes mellitus, N (%) | 313 (4.6) | 6 (8.3) | 29 (7.7) |
| HBV coinfection, N (%)d | 292 (4.5) | 6 (8.3) | 23 (6.3) |
| Ever HBV active drugs, N (%) | 2633 (38.8) | 34 (47.2) | 136 (36.3) |
| Ever hepatitis C virus active drugs, N (%) | 1537 (14.6) | 23 (31.9) | 68 (18.1) |
| Ever combination antiretroviral therapy, N (%) | 5964 (87.9) | 71 (98.6) | 340 (90.7) |
| Ever AIDS, N (%) | 2163 (31.9) | 28 (38.9) | 167 (44.5) |
| Detectable HIV RNA, N (%)e | 1189 (25.1) | 9 (15.5) | 123 (38.4) |
| CD4 cell count current, cells/mm3, median (IQR)f | 470 (289, 670) | 286 (201, 438) | 242 (110, 397) |
| Cirrhosis, N (%)g | 1447 (25.0) | 45 (75.4) | 189 (69.7) |
Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; IQR, interquartile range.
a Body mass index was categorized in 2615 individuals without an event, 25 individuals with HCC, and 166 individuals with other liver events.
b Smoking status was determined in 3728 individuals without an event, 33 individuals with HCC, and 208 individuals with other liver events.
c Alcohol abuse was determined in 3278 individuals without an event, 20 individuals with HCC, and 72 individuals with other liver events.
d HBV coinfection was determined in 6489 individuals without an event, 69 individuals with HCC, and 366 individuals with other liver events.
e Detectable HIV RNA was determined in 4746 individuals without an event, 58 individuals with HCC, and 320 individuals with other liver events.
f CD4 cell count current was determined in 6254 individuals without an event, 69 individuals with HCC, and 349 individuals with other liver events.
g Cirrhosis was determined in 5799 individuals without an event, 61 individuals with HCC, and 271 individuals with other liver events.
Of the 11 who developed HCC and had an HCV RNA measurement at least 6 months after completing interferon-based treatment, none had achieved a sustained virologic response (SVR).
Risk Factors for HCC and Other Liver Events
In multivariate analysis, being older, HBV coinfection, lower current CD4 cell count, and cirrhosis were associated with a higher incidence of HCC (Table 3). Notably, we found no impact of alcohol abuse, diabetes mellitus, or detectable HIV RNA on the incidence of HCC in univariate analysis.
Table 3.
Univariate and Multivariate Analyses of Hepatocellular Carcinoma in Human Immunodeficiency Virus/Hepatitis C Virus–Coinfected Individuals
| Variates | Univariate Analysis |
Multivariate Analysisa |
||
|---|---|---|---|---|
| IRR (95% CI) | P Value | IRR (95% CI) | P Value | |
| Age at baseline, per 10-y increase | 2.43 (2.06, 2.88) | <.01 | 2.36 (1.89, 2.94) | <.01 |
| Sex, female vs male | 0.61 (.36, 1.06) | .08 | 1.01 (.58, 1.77) | .96 |
| Race, other/unknown vs white | 0.65 (.24, 1.77) | .40 | ||
| Region | ||||
| Western Europe | Reference | Reference | ||
| Eastern Europe /Argentina | 0.32 (.14, 0.74) | .01 | 0.65 (.28, 1.51) | .31 |
| Canada | 1.27 (.6, 2.42) | .47 | 0.68 (.32, 1.45) | .32 |
| HIV risk group | ||||
| Men who have sex with men | Reference | |||
| Injection drug use | 1.06 (.45, 2.50) | .89 | ||
| Heterosexual | 1.79 (.71, 4.51) | .22 | ||
| Other/unknown | 1.07 (.38, 3.01) | .89 | ||
| Body mass index category | ||||
| Underweight | 2.40 (.70, 8.22) | .16 | ||
| Normal weight | Reference | |||
| Overweight | 0.91 (.31, 2.71) | .87 | ||
| Obese | 1.26 (.17, 9.39) | .82 | ||
| Unknown | 1.14 (.65, 1.98) | .65 | ||
| Ever smoked | ||||
| No | Reference | |||
| Yes | 0.84 (.38, 1.85) | .66 | ||
| Unknown | 1.07 (.50, 2.28) | .87 | ||
| Ever abused alcohol | ||||
| No | Reference | |||
| Yes | 1.34 (.45, 4.01) | .61 | ||
| Unknown | 0.66 (.38, 1.15) | .14 | ||
| Diabetes mellitus, yes vs no | 1.95 (.86, 4.46) | .11 | ||
| HBV coinfection | ||||
| No | Reference | Reference | ||
| Yes | 2.17 (.94, 5.02) | .07 | 2.46 (1.03, 5.87) | .04 |
| Unknown | 0.72 (.23, 2.28) | .58 | 0.96 (.31, 2.96) | .95 |
| Ever HBV active drugs, yes vs no | 1.92 (1.12, 3.04) | <.01 | 1.00 (.59, 1.68) | .99 |
| Ever hepatitis C virus active drugs, yes vs no | 2.06 (1.26, 3.38) | <.01 | 1.27 (.74, 2.16) | .39 |
| Ever combination antiretroviral therapy, yes vs no | 12.03 (1.67, 86.58) | <.01 | 5.79 (.77, 43.69) | .09 |
| Ever AIDS, yes vs no | 1.51 (.94, 2.42) | .09 | 1.20 (.69, 2.06) | .52 |
| Detectable HIV RNA | ||||
| No | Reference | |||
| Yes | 0.56 (.28, 1.14) | .11 | ||
| Unknown | 0.83 (.46, 1.50) | .53 | ||
| CD4 cell count current, per log2 increase in cells/mm3 | 0.74 (.66, .83) | <.01 | 0.78 (.65, .95) | .01 |
| CD4 cell count nadir, per log2 increase in cells/mm3 | 0.88 (.80,.97) | <.01 | 1.07 (.90, 1.27) | .43 |
| Cirrhosis | ||||
| No | Reference | |||
| Yes | 17.21 (9.74, 30.41) | <.01 | 12.92 (6.97, 23.95) | <.01 |
| Unknown | 5.51 (2.56, 11.87) | <.01 | 5.80 (2.52, 13.39) | <.01 |
| Calendar year of event, per year increase | 1.11 (1.04, 1.19) | <.01 | 1.05 (.98, 1.13) | .17 |
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IRR, incidence rate ratio.
a Variables with a P value of <.10 in the univariate analysis were included in the multivariate analysis.
Later calendar years were significantly associated with a higher incidence of HCC in univariate analysis, but not in multivariate analysis, indicating that changes in other variables explained the increase over time. In a supplementary analysis, increases in the proportion with cirrhosis mostly explained the increase in HCC over time, as it was only after adjustment for cirrhosis that there no longer was a significant increase in HCC over time (adjusted incidence rate ratio per calendar year increase 1.05; 95% CI, .98, 1.13).
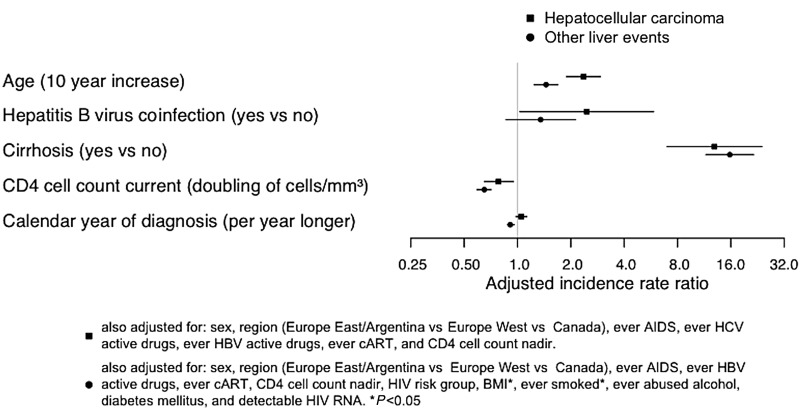
The multivariate analysis of other liver events yielded similar risk factors (Figure 3 and Supplementary 1). Being older, lower CD4 cell count, and cirrhosis (but not HBV coinfection) were associated with a higher incidence of other liver events. Additionally, the HIV risk group “other/unknown” compared with MSM, being underweight, and having ever smoked were also associated with a higher incidence of other liver events. In both uni- and multivariate analysis, later calendar years were associated with a lower incidence of other liver events with statistical significance.
Figure 3.
Adjusted incidence rate ratios (with 95% confidence intervals) for a selection of risk factors for hepatocellular carcinoma and other liver events in multivariate time-updated Poisson regression models. Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Exclusion of those with HBV coinfection or those with any other cancer diagnosis prior to end of follow-up did not change the results (data not shown).
In a supplementary analysis of individuals with cirrhosis (Supplementary 2), we found that having a current APRI >3 (compared with current APRI between 2 and 3) was independently associated with a higher incidence of other liver events. For HCC, the trend was similar but not statistically significant.
DISCUSSION
We observed opposing trends in the crude incidence of HCC and other liver events in HIV/HCV–coinfected individuals from 2001 to 2014. HCC increased from 0.4 to 2.3 cases per 1000 person-years of follow-up, whereas other liver events decreased from 9.9 to 5.2 cases per 1000 person-years of follow-up.
An increase in HCC incidence has previously been shown in retrospective national studies of HIV/HCV–coinfected individuals. In Spain, Merchante et al [8] showed that the incidence increased from 2000 to 2010 (0.2–1.4 cases per 1000 person-years of follow-up). In the Veterans Affairs Cohort in the United States, Ioannou et al [9] found that the age-adjusted prevalence of HCC increased from 0.15% in 1996 to 1.06% in 2009. They linked the rise in incidence with the increased survival in individuals with HIV, as HCV-related development of cirrhosis and subsequent HCC takes several years [18]. Our results support this reasoning, as changes in the proportion of individuals with cirrhosis, which increased by 8% per year (data not shown), mostly explained the increase in HCC per calendar year in multivariate analysis.
However, while the proportion of cirrhotics and crude incidence of HCC was increasing, we found that the incidence of other liver events, that is, liver decompensations and liver-related deaths, decreased, even after adjustment. Other recent studies of HIV/HCV–coinfected individuals, but not all [19], have shown similar trends [5–7, 20]. This could be explained by the increased uptake of cART (which have been found to lower the progression of hepatic fibrosis and disease [21, 22]), improved HCV treatment uptake [23], and possibly the discontinuation of older hepatotoxic antiretrovirals [24]. In our multivariate analysis, having ever received cART or HCV active drugs did not affect the decrease of other liver events per calendar year. However, an increase in current CD4 cell count did protect against other liver events, and the population had higher CD4 cell counts at the end of follow-up. Improvements in HIV and HCV treatment have undoubtedly reduced the risk of liver decompensations and liver-related death in the last decade, but our data suggest that other explanatory factors have yet to be accounted for.
It seems paradoxical that improvements in liver-related morbidity in HIV/HCV–coinfected patients, demonstrated by a lower incidence of other liver events, would simultaneously yield a higher incidence of HCC. Perhaps an improved management of liver cirrhosis and HIV treatment can increase the threshold for liver decompensation in cirrhotic HIV/HCV–coinfected individuals, but thus increasing longevity such that viral hepatocarcinogenesis has enough time to manifest itself as HCC. This hypothesis is somewhat supported in our finding that for cirrhotics, the decrease in incidence rate was much more pronounced for other liver events than for HCC when comparing those with a current CD4 cell count <350 cells/mm3 to those with a count >350 cells/mm3, though formal analysis for interaction between cirrhosis and current CD4 cell count was not statistically significant.
In our multivariate analysis, the major risk factor for HCC was cirrhosis. We found that 74% of individuals with HCC had cirrhosis within 6 months of HCC diagnosis. As “unknown” cirrhosis status also was associated with a higher incidence of HCC, it is likely that the markers used in our definition of cirrhosis have not identified all cirrhotics, rather than an actual situation in which 26% of individuals developed HCC without cirrhosis. However, as liver biopsies are being replaced by the same noninvasive markers, our result warns that HCC can develop in individuals who do not seem to have cirrhosis based on these markers.
We also found that lower current CD4 cell count was associated with HCC in HIV/HCV–coinfected individuals. Previous studies have also shown this in HIV individuals in general [9, 25, 26], but in HCV/HIV–coinfected individuals, the results have been conflicting. Salmon et al [27] did not find an association, arguing that any association is likely confounded by cirrhosis, as concomitant splenic sequestration of lymphocytes artificially lowers the CD4 cell count [28]. However, Kramer et al [29] found that having a CD4 cell count of <200 cells/mm3 (compared with >350 cells/mm3) was associated with a 1.7 times greater hazard of HCC and that the association remained when the subgroup with cirrhosis was analyzed. In our study, we found a significant protective effect of a doubling in current CD4 cell count after adjustment for cirrhosis, corroborating the independent effect of current immunosuppression as a risk factor for HCC.
In addition to cirrhosis and CD4 cell count, HBV coinfection and being older were independent risk factors for HCC. However, these factors (except HBV coinfection) were also risk factors for other liver events. Even though the effect sizes were different, the clinician should perceive the aforementioned risk factors as general predictors of liver disease and death, and not HCC exclusively, especially as the incidence of HCC, though increasing, remains low.
Alcohol abuse and diabetes mellitus have been associated with an increased risk of HCC among HCV-monoinfected individuals. We found no influence of a history of alcohol abuse on the risk of HCC (or other liver events after adjustment), but since our data on alcohol abuse were heterogeneous and scarce, we cannot rule out that (continued) alcohol abuse impacts the risk of developing HCC or other liver events. We found no association between diabetes mellitus and HCC or other liver events, consistent with other studies [9, 27], although the latter study found an association between insulin resistance and the risk of HCC.
Our study has several limitations. First, our diagnosis of cirrhosis was based primarily on noninvasive techniques. However, the measurements and their respective cutoffs have been validated [30], and possible misclassifications would underestimate any real effect of cirrhosis in our analyses, where it in fact was the strongest predictor. Second, we defined (chronic) HCV coinfection by the presence of HCV antibodies. Since around 20% can clear the infection spontaneously, we might have included some individuals with resolved HCV infection, which may have diluted the incidence rates reported. Third, data on alcohol abuse were only recently added to some of our cohorts and were thus very limited. Fourth, we only had data on HCV treatment, but not treatment outcome (SVR rates), which likely would have been a more precise covariate to include in our regression models. The major strength of our study is that our population was taken from prospective cohorts, representing a large portion of Europe and Canada and with a relatively large proportion of females.
In conclusion, we observed that from 2001 to 2014, the incidence of HCC in HIV/HCV–coinfected individuals increased, largely explained by an increase in the number of individuals with cirrhosis, whereas the rate of other liver events (liver decompensations and liver-related deaths) decreased. Older age, cirrhosis, and lower current CD4 cell count were independent risk factors for both HCC and other liver events. New HCV treatment with direct-acting antivirals and earlier HIV treatment will likely reduce the rates of HCC and other liver events. However, as HCC can develop after SVR is achieved [31], or as a consequence of long-tern alcohol abuse, nonalcoholic steatohepatitis, or other hepatotoxic exposures, continuous surveillance of incidence trends is needed.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The Canadian Co-Infection Cohort acknowledges the participants of the Canadian Co-Infection Cohort (CTN 222) and thank O. Adebayo, C. Allen, B. Beckthold, C. Casavant, I. Chabot, W. Chan, V. Chiang, S. De Coutere, B. Ganase, L. Gokool, S. Kassir, J. Kocilowicz, J. Latendre-Paquette, N. McFarland, A. Mcneil, J. Milloy, C. Morrisseau, M. Perez, M. –C. Plaisance, L. Puri, and Annie Qiu for their assistance with study coordination, participant recruitment, and care. Coinvestigators of the Canadian Co-Infection Cohort are L. Barrett, J. Cohen, B. Conway, C. Cooper, P. Côté, J. Cox, J. Gill, S. Haider, M. Hull, V. Martel-Laferriere, J. Montaner, E. Moodie, N. Pick, A. Rachlis, D. Rouleau, A. Sadr, S. Sanche, R. Sandre, M. Tyndall, M.-L. Vachon, S. Walmsley, A. Wong, and D. Wong.
Financial support. This work was supported within the framework of the EuroSIDA study programs (supported by the European Commission BIOMED 1 [CT94–1637], BIOMED 2 [CT97–2713], the 5th Framework [QLK2-2000-00773], the 6th Framework [LSHP-CT-2006-018632], the 7th Framework [FP7/ 2007-2013, and EuroCoord grant agreement 260694]), the Swiss National Science Foundation (grant 108787), and unrestricted grants from Janssen R&D, Merck and Co. Inc., Pfizer Inc., and GlaxoSmithKline LLC; the Swiss HIV Cohort study (supported by the Swiss National Science Foundation [grant 148522] and the Swiss HIV Cohort Study Research Foundation); the Canadian Co-Infection Cohort (supported by the Canadian Institutes of Health Research [CIHR MOP-79529], Fonds de la recherche du Québec-Santé [FRQ-S]: Réseau SIDA/maladies infectieuses, and the Canadian HIV Trials Network [CTN-222]). M. B. K. is supported by the Chercheurs Nationaux Career Award from the FRQ-S and by a grant from the Danish National Research Foundation (DNRF126).
Potential conflicts of interest. E. J. has held lectures sponsored by AbbVie, Merck Sharp & Dohme (MSD), Gilead Sciences, Janssen, and Bristol-Myers Squibb (BMS) and has received honoraria for serving on advisory boards from Gilead Sciences, Janssen-Cilag, Glaxo, and BMS. A. L. received honoraria, travel support, and/or consultancy fees from BMS, ViiV, AbbVie, Gilead Sciences, and MSD. A. R. received honoraria for serving on advisory boards and/or travel grants from Janssen-Cilag, MSD, Gilead Sciences, AbbVie, and BMS and received an unrestricted research grant from Gilead Sciences; all remuneration went to his home institution and not to A. R. personally. A. M. received honoraria, travel support, and speaker and/or consultancy fees from BMS, Boehringer Ingelheim, Gilead Sciences, Pfizer, Merck, and Wragge LLC. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
The Swiss HIV Cohort study
V. Aubert, M. Battegay, E. Bernasconi, J. Böni, D. L. Braun, H. C. Bucher, C. Burton-Jeangros, A. Calmy, M. Cavassini, G. Dollenmaier, M. Egger, L. Elzi, J. Fehr, J. Fellay, H. Furrer (chair of the Clinical and Laboratory Committee), C. A. Fux, M. Gorgievski, H. Günthard (president of the study), D. Haerry (deputy of the “Positive Council”), B. Hasse, H. H. Hirsch, M. Hoffmann, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, T. Klimkait, R. Kouyos, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, C. Marzolini, K. Metzner, N. Müller, D. Nadal, D. Nicca, G. Pantaleo, A. Rauch (chair of the Scientific Board), S. Regenass, C. Rudin (chair of the Mother & Child substudy), F. Schöni-Affolter (head of the Data Centre), P. Schmid, R. Speck, M. Stöckle, P. Tarr, A. Trkola, P. Vernazza, R. Weber, and S Yerly. Data from the Swiss HIV Cohort study are gathered by the 5 Swiss university hospitals, 2 Cantonal hospitals, 15 affiliated hospitals, and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers).
EuroSIDA study group (a multicenter study group; national coordinators are listed in parenthesis). Argentina: (M. Losso), M. Kundro, Hospital JM Ramos Mejia, Buenos Aires. Austria: (N. Vetter), Pulmologisches Zentrum der Stadt Wien, Vienna; R. Zangerle, Medical University Innsbruck. Belarus: (I. Karpov), A. Vassilenko, Belarus State Medical University, Minsk; V. M. Mitsura, Gomel State Medical University; D. Paduto, Regional AIDS Centre, Svetlogorsk. Belgium: (N. Clumeck), S. De Wit, M. Delforge, Saint-Pierre Hospital, Brussels; E. Florence, Institute of Tropical Medicine, Antwerp; L. Vandekerckhove, University Ziekenhuis Gent. Bosnia-Herzegovina: (V. Hadziosmanovic), Klinicki Centar Univerziteta Sarajevo. Bulgaria: (K. Kostov), Infectious Diseases Hospital, Sofia. Croatia: (J. Begovac), University Hospital of Infectious Diseases, Zagreb. Czech Republic: (L. Machala), D. Jilich, Faculty Hospital Bulovka, Prague; D. Sedlacek, Charles University Hospital, Plzen. Denmark: G. Kronborg, T. Benfield, Hvidovre Hospital, Copenhagen; J. Gerstoft, T. Katzenstein, Rigshospitalet, Copenhagen; N. F. Møller, C. Pedersen, Odense University Hospital; L. Ostergaard, Skejby Hospital, Aarhus, U. B. Dragsted, Roskilde Hospital; L. N. Nielsen, Hillerod Hospital. Estonia: (K. Zilmer), West-Tallinn Central Hospital; J. Smidt, Nakkusosakond Siseklinik, Kohtla-Järve. Finland: (M. Ristola), I. Aho, Helsinki University Central Hospital. France: (J.-P. Viard), Hôtel-Dieu, Paris; P.-M. Girard, Hospital Saint-Antoine, Paris; L. Cotte, Hôpital de la Croix Rousse, Lyon; C. Pradier, E. Fontas, Hôpital de l'Archet, Nice; F. Dabis, D. Neau, Unité INSERM, Bordeaux; C. Duvivier, Hôpital Necker-Enfants Malades, Paris. Germany: (J. Rockstroh), Universitäts Klinik Bonn; R. Schmidt, Medizinische Hochschule Hannover; O. Degen, University Medical Center Hamburg-Eppendorf, Infectious Diseases Unit; H. J. Stellbrink, IPM Study Center, Hamburg; C. Stefan, JW Goethe University Hospital, Frankfurt; J. Bogner, Medizinische Poliklinik, Munich; G. Fätkenheuer, Universität Köln, Cologne. Georgia: (N. Chkhartishvili) Infectious Diseases, AIDS & Clinical Immunology Research Center, Tbilisi. Greece: (J. Kosmidis), P. Gargalianos, G. Xylomenos, P. Lourida, Athens General Hospital; H. Sambatakou, Ippokration General Hospital, Athens. Hungary: (J. Szlávik), Szent Lásló Hospital, Budapest. Iceland: (M. Gottfredsson), Landspitali University Hospital, Reykjavik. Ireland: (F. Mulcahy), St. James's Hospital, Dublin. Israel: (I. Yust), D. Turner, M. Burke, Ichilov Hospital, Tel Aviv; E. Shahar, G. Hassoun, Rambam Medical Center, Haifa; H. Elinav, M. Haouzi, Hadassah University Hospital, Jerusalem; D. Elbirt, Z. M. Sthoeger, AIDS Center (Neve Or), Jerusalem. Italy: (A. D′Arminio Monforte), Istituto Di Clinica Malattie Infettive e Tropicale, Milan; R. Esposito, I. Mazeu, C. Mussini, Università Modena; F. Mazzotta, A. Gabbuti, Ospedale S Maria Annunziata, Firenze; V. Vullo, M. Lichtner, University di Roma la Sapienza, Rome; M. Zaccarelli, A. Antinori, R. Acinapura, M. Plazzi, Istituto Nazionale Malattie Infettive Lazzaro Spallanzani, Rome; A. Lazzarin, A. Castagna, N. Gianotti, Ospedale San Raffaele, Milan; M. Galli, A. Ridolfo, Osp. L. Sacco, Milan. Latvia: (B. Rozentale), Infectology Centre of Latvia, Riga. Lithuania: (V. Uzdaviniene), Vilnius University Hospital Santariskiu Klinikos; R. Matulionyte, Center of Infectious Diseases, Vilnius University Hospital Santariskiu Klinikos. Luxembourg: (T. Staub), R. Hemmer, Centre Hospitalier, Luxembourg. The Netherlands: (P. Reiss), Academisch Medisch Centrum bij de Universiteit van Amsterdam. Norway: (V. Ormaasen), A. Maeland, J. Bruun, Ullevål Hospital, Oslo. Poland: (B. Knysz), J. Gasiorowski, M. Inglot, Medical University, Wroclaw; A. Horban, E. Bakowska, Centrum Diagnostyki i Terapii AIDS, Warsaw; R. Flisiak, A. Grzeszczuk, Medical University, Bialystok; M. Parczewski, M. Pynka, K. Maciejewska, Medical University, Szczecin; M. Beniowski, E. Mularska, Osrodek Diagnostyki i Terapii AIDS, Chorzow; T. Smiatacz, M. Gensing, Medical University, Gdansk; E. Jablonowska, E. Malolepsza, K. Wojcik, Wojewodzki Szpital Specjalistyczny, Lodz; I. Mozer-Lisewska, Poznan University of Medical Sciences. Portugal: (M. Doroana), L. Caldeira, Hospital Santa Maria, Lisbon; K. Mansinho, Hospital de Egas Moniz, Lisbon; F. Maltez, Hospital Curry Cabral, Lisbon. Romania: (R. Radoi), C. Oprea, Spitalul de Boli Infectioase si Tropicale; V. Babes, Bucarest. Russia: (A. Rakhmanova) (deceased), Medical Academy Botkin Hospital, St Petersburg; A. Rakhmanova, St Petersburg AIDS Centre; T. Trofimora, Novgorod Centre for AIDS; I. Khromova, Centre for HIV/AIDS & and Infectious Diseases, Kaliningrad; E. Kuzovatova, Nizhny Novgorod Scientific and Research Institute. Serbia: (D. Jevtovic), Institute for Infectious and Tropical Diseases, Belgrade. Slovakia: A. Shunnar, D. Staneková, Dérer Hospital, Bratislava. Slovenia: (J. Tomazic), University Clinical Centre Ljubljana. Spain: (J. M. Gatell), J. M. Miró, Hospital Clinic Universitari de Barcelona; S. Moreno, J. M. Rodriguez, Hospital Ramon y Cajal, Madrid; B. Clotet, A. Jou, R. Paredes, C. Tural, J. Puig, I. Bravo, Hospital Germans Trias i Pujol, Badalona; P. Domingo, M. Gutierrez, G. Mateo, M. A. Sambeat, Hospital Sant Pau, Barcelona; J. M. Laporte, Hospital Universitario de Alava, Vitoria-Gasteiz. Sweden: (K. Falconer), A. Thalme, A. Sonnerborg, Karolinska University Hospital, Stockholm; A. Blaxhult, Venhälsan-Sodersjukhuset, Stockholm; L. Flamholc, Malmö University Hospital. Switzerland: (B. Ledergerber), R. Weber, University Hospital Zurich; M. Cavassini, University Hospital Lausanne; A. Calmy, University Hospital Geneva; H. Furrer, University Hospital Bern; M. Battegay, University Hospital Basel; P. Schmid, Cantonal Hospital St. Gallen. Ukraine: (E. Kravchenko), Kiev Centre for AIDS; V. Frolov, G. Kutsyna, I. Baskakov, Luhansk State Medical University; A. Kuznetsova, Kharkov State Medical University; G. Kyselyova, Crimean Republican AIDS Centre, Simferopol; M. Sluzhynska, Lviv Regional HIV/AIDS Prevention and Control Center. United Kingdom: (B. Gazzard), St. Stephen's Clinic, Chelsea and Westminster Hospital, London; A. M. Johnson, E. Simons, S. Edwards, Mortimer Market Centre, London; A. Phillips, M. A. Johnson, A. Mocroft, Royal Free and University College Medical School, London (Royal Free Campus); C. Orkin, Royal London Hospital; J. Weber, G. Scullard, Imperial College School of Medicine at St. Mary's, London; A. Clarke, Royal Sussex County Hospital, Brighton; C. Leen, Western General Hospital, Edinburgh.
The following centers have contributed data to EuroSIDA: Hôpital de la Pitié-Salpétière, Paris, France. Bernhard Nocht Institut für Tropenmedizin, Hamburg, Germany. 1st I.K.A Hospital of Athens, Greece. Ospedale Riuniti, Divisione Malattie Infettive, Bergamo, Italy. Ospedale di Bolzano, Divisione Malattie Infettive, Bolzano, Italy. Ospedale Cotugno, III Divisione Malattie Infettive, Napoli, Italy. Hospital Carlos III, Departamento de Enfermedades Infecciosas, Madrid, Spain. Odessa Region AIDS Center, Ukraine.
EuroSIDA Steering Committee: J. Gatell, B. Gazzard, A. Horban, I. Karpov, B. Ledergerber, M. Losso, A. d′Arminio Monforte, C. Pedersen, A. Rakhmanova, M. Ristola, A. Phillips, P. Reiss, J. Lundgren, J. Rockstroh, and S. De Wit.
EuroSIDA Coordinating Centre Staff: O. Kirk, L. Peters, C. Matthews, A. H. Fischer, A. Bojesen, D. Raben, D. Kristensen, K. Grønborg Laut, J. F. Larsen, and D. Podlekareva.
Statistical Centre Staff: A. Mocroft, A. Phillips, A. Cozzi- Lepri, L. Shepherd, and A. Schultze.
References
- 1.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(1 suppl):S6–9. [DOI] [PubMed] [Google Scholar]
- 2.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015; 385:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo Re V III, Kallan MJ, Tate JP et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med 2014; 160:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Sierra C, Arizcorreta A, Diaz F et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis 2003; 36:491–8. [DOI] [PubMed] [Google Scholar]
- 5.Smith CJ, Ryom L, Weber R et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 6.Grint D, Peters L, Rockstroh JK et al. Liver-related death among HIV/hepatitis C virus-co-infected individuals: implications for the era of directly acting antivirals. AIDS 2015; 29:1205–15. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal E, Roussillon C, Salmon-Ceron D et al. Liver-related deaths in HIV-infected patients between 1995 and 2010 in France: the Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalite 2010 survey. HIV Med 2015; 16:230–9. [DOI] [PubMed] [Google Scholar]
- 8.Merchante N, Merino E, Lopez-Aldeguer J et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis 2013; 56:143–50. [DOI] [PubMed] [Google Scholar]
- 9.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013; 57:249–57. [DOI] [PubMed] [Google Scholar]
- 10.Mocroft A, Ledergerber B, Katlama C et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 2003; 362:22–9. [DOI] [PubMed] [Google Scholar]
- 11.Krentz HB, Gill MJ. The effect of churn on “community viral load” in a well-defined regional population. J Acquir Immune Defic Syndr 2013; 64:190–6. [DOI] [PubMed] [Google Scholar]
- 12.Klein MB, Saeed S, Yang H et al. Cohort profile: the Canadian HIV-hepatitis C Co-infection Cohort study. Intern J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 13.Swiss HIVCS, Schoeni-Affolter F, Ledergerber B et al. Cohort profile: the Swiss HIV Cohort study. Intern J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 14.Kjaer J, Ledergerber B. HIV cohort collaborations: proposal for harmonization of data exchange. Antiviral Therapy 2004; 9:631–3. [PubMed] [Google Scholar]
- 15.Wai CT, Greenson JK, Fontana RJ et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Winnock M, Pambrun E et al. Comparison of transient elastography (FibroScan), FibroTest, APRI and two algorithms combining these non-invasive tests for liver fibrosis staging in HIV/HCV coinfected patients: ANRS CO13 HEPAVIH and FIBROSTIC collaboration. HIV Med 2014; 15:30–9. [DOI] [PubMed] [Google Scholar]
- 17.Halfon P, Bourliere M, Penaranda G et al. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol 2005; 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castells L, Vargas V, Gonzalez A, Esteban J, Esteban R, Guardia J. Long interval between HCV infection and development of hepatocellular carcinoma. Liver 1995; 15:159–63. [DOI] [PubMed] [Google Scholar]
- 19.Klein MB, Althoff KN, Jing Y et al. Has modern ART reduced endstage liver disease risk in HIV-hepatitis coinfection? In: 22nd Conference on Retroviruses and Opportunistic Infections Seattle, WA, USA, 2015. Abstract 638. [Google Scholar]
- 20.van der Helm J, Geskus R, Sabin C et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology 2013; 144:751–60 e2. [DOI] [PubMed] [Google Scholar]
- 21.Brau N, Salvatore M, Rios-Bedoya CF et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol 2006; 44:47–55. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JP, Horsburgh CR Jr, Williams PL et al. CD4 recovery on antiretroviral therapy is associated with decreased progression to liver disease among hepatitis C virus-infected injecting drug users. Open Forum Infect Dis 2015; 2:ofv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grint D, Peters L, Schwarze-Zander C et al. Temporal changes and regional differences in treatment uptake of hepatitis C therapy in EuroSIDA. HIV Med 2013; 14:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovari H, Weber R. Influence of antiretroviral therapy on liver disease. Curr Opin HIV AIDS 2011; 6:272–7. [DOI] [PubMed] [Google Scholar]
- 25.Clifford GM, Rickenbach M, Polesel J et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS 2008; 22:2135–41. [DOI] [PubMed] [Google Scholar]
- 26.Bruyand M, Dabis F, Vandenhende MA et al. HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998–2008. J Hepatol 2011; 55:1058–62. [DOI] [PubMed] [Google Scholar]
- 27.Salmon D, Bani-Sadr F, Loko MA et al. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: results from ANRS CO13 HEPAVIH. J Hepatol 2012; 56:862–8. [DOI] [PubMed] [Google Scholar]
- 28.McGovern BH, Golan Y, Lopez M et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis 2007; 44:431–7. [DOI] [PubMed] [Google Scholar]
- 29.Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. J Acquir Immune Defic Syndr 2015; 68:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resino S, Sanchez-Conde M, Berenguer J. Coinfection by human immunodeficiency virus and hepatitis C virus: noninvasive assessment and staging of fibrosis. Curr Opin Infect Dis 2012; 25:564–9. [DOI] [PubMed] [Google Scholar]
- 31.Merchante N, Merino E, Rodriguez-Arrondo F et al. HIV/hepatitis C virus-coinfected patients who achieved sustained virological response are still at risk of developing hepatocellular carcinoma. AIDS 2014; 28:41–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.