In a large real-world observational study, treatment outcomes with sofosbuvir (SOF) and ribavirin (RBV) for genotype 3 hepatitis C virus infection were disappointing, particularly in patients with cirrhosis. Treatment with SOF, peginterferon, and RBV was more successful but was rarely used.
Keywords: genotype 3, sofosbuvir, ribavirin, cirrhosis, interferon
Abstract
Background. Sofosbuvir (SOF) is active against all hepatitis C virus (HCV) genotypes, and SOF-based therapies lead to high rates of sustained virologic response (SVR). However, genotype 3 (GT3) HCV remains a challenge with lower SVR rates reported, particularly in patients with cirrhosis. This study reports the effectiveness and safety of SOF-based therapy in patients with GT3 HCV treated in clinical practice.
Methods. Hepatitis C Virus Therapeutic Registry and Research Network is an international, prospective observational study evaluating patients treated in usual clinical practice. Patients with GT3 HCV were analyzed to assess predictors of treatment response and adverse events using descriptive statistics and multivariable logistic regression.
Results. Treatment outcomes were available for 197 patients treated with SOF and ribavirin (RBV), with or without peginterferon, including 54% with cirrhosis and 49% who failed prior therapy. Of 178 patients treated with SOF/RBV, 60% achieved SVR at 12 weeks (SVR12), compared with 84% of 19 patients treated with SOF/peginterferon/RBV. For patients treated with SOF/RBV, the SVR12 rate was 58% in treatment-naive patients with cirrhosis, and 42% in those with cirrhosis who failed prior therapy. In noncirrhotic patients, SVR12 rates were 89% in treatment-naive and 88% in treatment-experienced patients. After controlling for age and sex, absence of cirrhosis (odds ratio [OR], 6.4; 95% confidence interval [CI], 2.78–14.74), albumin levels ≥3.2 g/dL (OR, 12.48; 95% CI, 3.86–40.33), and platelet count >105 cells/µL (OR, 7.44; 95% CI, 3.51–15.78) were associated with greater odds of SVR12.
Conclusions. SVR rates were acceptable in patients with GT3 HCV without cirrhosis; however, in those with cirrhosis, treatment with SOF/RBV was suboptimal, highlighting the need for new therapies for this population.
Chronic hepatitis C virus (HCV) infection continues to be a major global public health problem, with recent estimates suggesting that >103 million people are infected worldwide [1]. There is wide geographical variation in HCV genotype distribution; genotype 1 is most common (46%), followed by genotype 3 (GT3) (22%) [1]. Chronic infection leads to progressive liver fibrosis that may eventually lead to cirrhosis, putting patients at risk of liver failure and/or hepatocellular carcinoma (HCC) [2–4]. Several studies have shown that both the risk for fibrosis progression and the risk of developing HCC are increased among patients with HCV GT3 [5, 6].
Therapy for chronic HCV was originally based on interferon, and resulted in different response rates by genotype [7]. Genotypes 2 (GT2) and 3 are relatively interferon sensitive and required shorter duration of therapy with lower doses of ribavirin (RBV) to achieve high rates of sustained virologic response (SVR). Even in the interferon era, data suggested that GT3 was more difficult to cure than GT2, particularly in patients with established cirrhosis [8]. The discovery of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV infection. Sofosbuvir (SOF) is a well-tolerated nucleotide polymerase inhibitor with activity against all HCV genotypes. However, despite initially promising results, large clinical trials reported lower rates of SVR in patients with GT3 infection receiving SOF-based therapy than with other HCV genotypes [9].
The FUSION, FISSION, and POSITRON trials showed that the combination of SOF and RBV was more effective and much better tolerated than pegylated interferon (peg-IFN)/RBV in patients with GT2 and GT3 HCV [10, 11]. However, a breakdown of the results by genotype and cirrhosis status revealed that patients with GT3, particularly those with cirrhosis, had high rates of relapse. With extension of therapy to 24 weeks, the SVR rates rose to 93% in treatment-naive patients; however, in patients who had failed prior peg-IFN/RBV, the SVR rates were 85% without cirrhosis, but only 60% in those with cirrhosis [12].
Studies then evaluated adding peg-IFN to SOF/RBV for 12 weeks and showed that SVR rates increased to 85%, even in treatment-experienced patients with cirrhosis [13, 14]. Based on these results, guidelines recommend either a 12-week course of SOF/peg-IFN/RBV or a 24-week course of SOF/RBV for patients with GT3 infection [15, 16]. Daclatasvir (DCV), a relatively pan-genotypic NS5A inhibitor, has also been combined with SOF, and the combination has been recommended as an alternative therapy for GT3 infection [15, 16].
The efficacy and safety of these regimens are based on treatments administered to patients who fulfilled the eligibility criteria for clinical trials. Whether these results apply in daily clinical practice is unknown. This study reports the safety and effectiveness of 2 SOF-based regimens for the treatment of chronic GT3 HCV in an international, prospective observational study.
METHODS
Study Population and Design
Hepatitis C Virus Therapeutic Registry and Research Network (HCV-TARGET) is an international, prospective observational study enrolling patients from academic (n = 44) and community (n = 17) centers in the United States, Canada, Germany, and Israel who receive antiviral treatment for chronic HCV infection. Sequentially enrolled patients with no history of liver transplant or prior DAA treatment, ≥18 years old with GT3 HCV who received treatment with SOF and RBV, with or without peg-IFN, were included. The treatment regimen was chosen by the treating physician. Redacted medical records were collected by a central team of trained coders and reviewed, and baseline and on-treatment demographic, clinical, and virologic data were entered into a common, standardized database. Data were managed using REDCap electronic data capture tools hosted at the University of North Carolina at Chapel Hill and reviewed for completeness and accuracy by study monitors. Extreme or unlikely values were verified or resolved with additional queries.
The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by each local institutional review board (IRB). All patients provided written informed consent or an informed consent waiver was granted by the IRB overseeing the site.
Definitions
The following clinical definitions were applied to the study population.
Cirrhosis
Cirrhosis was defined by an algorithm implemented for HCV-TARGET analyses [17]. The primary indicator of cirrhosis was a liver biopsy with Metavir score F4. Patients with a liver biopsy reported as Metavir score F3 with at least 1 secondary indicator were also defined as having cirrhosis. Secondary indicators included serum fibrosis scores above thresholds for cirrhosis (FibroSure/FibroTest, FibroSpect, Hepascore); transient elastography (Fibroscan) ≥12.5 kPa; or signs of portal hypertension (esophageal/gastric varices, portal gastropathy, or platelet count <140 000/µL). In the absence of a biopsy, patients with ≥2 secondary indicators were defined as having cirrhosis [18].
Decompensated cirrhosis was defined as documented presence of current or past ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatic hydrothorax or variceal hemorrhage, or use of medications specifically prescribed for one of these indications.
Adverse Event
An adverse event (AE) was defined as any reported AE regardless of the need for dose reduction or discontinuation of HCV therapy.
Serious Adverse Event
A serious adverse event (SAE) was defined as an AE that either required hospitalization or met criteria for expedited reporting per US Food and Drug Administration form MEDWATCH 3500.
Anemia
Anemia was reported as an AE, or documented RBV dose reduction, treatment with erythropoietin, or blood transfusion.
Statistical Analyses
Demographics, baseline laboratory values, and frequencies of AEs were collected and analyzed by treatment regimen for the evaluable population (N = 197), which comprised patients who ended treatment with a known virologic outcome or were confirmed to be lost to posttreatment follow-up (counted as non–virologic failures). Those lost to follow-up during treatment or who withdrew consent were excluded.
The per-protocol population (n = 174) comprised patients who completed treatment or ended treatment early due to virologic failure only, and have a known virologic outcome.
The unadjusted rates of SVR were calculated for the evaluable and per-protocol populations, and multivariable analyses of factors associated with response and results in subgroups of interest are reported for the per-protocol population, including treatment history, presence of cirrhosis, and features of cirrhosis. Confidence intervals (CIs) of unadjusted rates were calculated using exact binomial methods.
The association between baseline covariates and SVR was estimated by logistic regression adjusted for age and sex for the per-protocol population. Covariates significant after adjustment for age and sex without significant collinearity were combined into multivariable models. Predictor variables were selected a priori based on consensus of clinical expertise and included well-established covariates associated with SVR: treatment regimen, age, sex, race, albumin level (<3.2 g/dL, ≥3.2 g/dL), platelet count (<105 cells/µL, ≥105 cells/µL), creatinine clearance, total bilirubin (mg/dL), hemoglobin (g/dL), cirrhosis status, and history of prior antiviral treatment as well as Model for End-Stage Liver Disease score and history of decompensating event in patients with cirrhosis. Results are presented as an odds ratio (OR) with 95% CIs. Analyses were performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
Patient Disposition
Between December 2013 and January 2015, 210 patients with GT3 HCV infection with no history of liver transplant or prior DAA therapy started treatment with SOF/RBV or SOF/peg-IFN/RBV. A total of 185 patients completed the prescribed regimen. Eighteen patients discontinued treatment early due to AEs [4], lack of efficacy [4], noncompliance [3], or other reasons [3] (eg, loss of insurance or planned surgery). Twelve patients were excluded due to loss to follow-up during treatment [4], withdrawal of consent [1], or ongoing posttreatment follow-up at the time of reporting [5].
The majority of patients (178/197 [90%]) were treated with SOF/RBV, with 87% receiving 24±2 weeks of therapy, while 19 (10%) patients received 12 (n = 12), 16 (n = 1), or 24 (n = 5) weeks of SOF/peg-IFN/RBV, including 1 patient who discontinued treatment at week 4. In total, 112 (57%) were male, 81% were white, and the mean age was 56 years (range, 27–77 years). Just under half (49%) were treatment experienced and 3 patients (1.5%) were coinfected with human immunodeficiency virus (HIV). Of 107 (54%) patients with cirrhosis, 49 (46%) had a history of prior hepatic decompensation. Of cirrhotic patients with complete MELD data (70%), the median baseline MELD score was 10 (range, 6–24). MELD score was <10 in 49% (n = 37), 10–15 in 44% (n = 33), and >15 in 7% (n = 5) of patients with cirrhosis (Table 1; Supplementary Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients With Genotype 3 Hepatitis C Virus Who Completed Therapy With Sofosbuvir and Ribavirin With or Without Peginterferon
| Characteristic | SOF/Peg-IFN/RBV (n = 19) | SOF/RBV (n = 178) | Total (N = 197) |
|---|---|---|---|
| Age, y | |||
| 18–39 | 3 (15.8) | 19 (10.7) | 22 (11.2) |
| 40–64 | 15 (78.9) | 140 (78.7) | 155 (78.7) |
| ≥65 | 1 (5.3) | 19 (10.7) | 20 (10.2) |
| Median (range) | 56.0 (33–67) | 56.0 (27–77) | 56.0 (27–77) |
| Male sex | 13 (68.4) | 99 (55.6) | 112 (56.9) |
| Race | |||
| White | 14 (73.7) | 146 (82.0) | 160 (81.2) |
| Black | 0 (0.0) | 4 (2.2) | 4 (2.0) |
| Other/missing | 5 (26.3) | 28 (15.7) | 33 (16.8) |
| Hispanic ethnicity | 2 (10.5) | 15 (8.4) | 17 (8.6) |
| Prior HCV treatment | |||
| Naive | 7 (36.8) | 94 (52.8) | 101 (51.3) |
| Experienced | 12 (63.2) | 84 (47.2) | 96 (48.7) |
| Cirrhosis | 11 (57.9) | 96 (53.9) | 107 (54.3) |
| History of hepatic decompensation | 3 (15.8) | 46 (25.8) | 49 (24.9) |
| Baseline MELD score ≥10 | 2 (25.0) | 36 (53.7) | 38 (50.7) |
| HCV RNA, log10 IU/mL, mean (range) | 6.3 (4–7) | 5.9 (0–8) | 5.9 (0–8) |
| Total bilirubin, mg/dL, median (range) | 0.9 (0.2–2.8) | 0.7 (0.1–6.6) | 0.8 (0.1–6.6) |
| Albumin, g/dL, median (range) | 4.0 (2.4–5.0) | 3.9 (2.3–5.0) | 3.9 (2.3–5.0) |
| ALT, mean IU/L, median (range) | 74.0 (28.0–375.0) | 73.0 (12.0–362.0) | 73.0 (12.0–375.0) |
| Platelet count, ×103 cells/µL, median (range) | 132 (59.0–302.0) | 134 (22.0–418.0) | 133 (22.0–418.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; peg-IFN, pegylated interferon; RBV, ribavirin; SOF sofosbuvir.
Treatment Response
SVR12 was achieved by 107 of 178 (60.1%) patients treated with SOF/RBV. The reasons for virologic failure included relapse in 50 (28%) patients, viral breakthrough in 6 patients (3.4%), and lack of efficacy in 1 patient (<0.5%). Fourteen patients (8%) were lost to posttreatment follow-up and were counted as failing treatment. Of those who received SOF/peg-IFN/RBV, 16 of 19 (84%) achieved SVR12; 1 was a nonresponder and 2 relapsed (Table 2).
Table 2.
Virologic Response in Patients With Genotype 3 Hepatitis C Virus Who Completed Therapy With Sofosbuvir and Ribavirin With or Without Peginterferon
| Treatment Regimen | SOF/Peg-IFN/RBV (n = 19) |
SOF/RBV (n = 178) |
Total (N = 197) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall SVR12 (EP) | 16/19 (84.2%) |
107/178 (60.1%) |
123/197 (62.4%) | ||||||
| PP SVR12 | 16/18 (88.9%) |
104/156 (66.7%) |
120/174 (69.0%) | ||||||
| Noncirrhotic |
Cirrhotic |
Noncirrhotic |
Cirrhotic |
||||||
| Overall SVR12 (EP) | 87.5% (7/8) |
81.8% (9/11) |
78.1% (64/82) |
44.8% (43/96) |
|||||
| PP SVR12 | 87.5% (7/8) |
90% (9/10) |
88.7% (63/71) |
48.2% (41/85) |
|||||
| TN | TE | TN | TE | TN | TE | TN | TE | ||
| SVR12 (EP) | 100% (4/4) | 75% (3/4) | 66.7% (2/3) | 87.5% (7/8) | 75.0% (42/56) | 84.6% (22/26) | 55.3% (21/38) | 37.9% (22/58) | |
| SVR12 (PP) | 100% (4/4) | 75% (3/4) | 100% (2/2) | 87.5% (7/8) | 89.1% (41/46) | 88.0% (22/25) | 57.6% (19/33) | 42.3% (22/52) | |
| Virologic failure (EP) | |||||||||
| Breakthrough | … | … | … | … | 1 | 1 | 2 | 2 | 6 |
| Relapse | … | 1 | … | 1 | 7 | 3 | 12 | 28 | 52 |
| Nonresponse | … | … | 1 | … | … | … | … | 1 | 2 |
| Non–virologic failure | … | … | … | … | 6 | … | 3 | 5 | 14 |
Abbreviations: EP, evaluable population; peg-IFN, pegylated interferon; PP, per protocol; RBV, ribavirin; SOF sofosbuvir; SVR12, sustained virologic response at 12 weeks; TE, treatment experienced; TN, treatment naive.
In the per-protocol population, the SVR12 rate was similar in patients without cirrhosis who received SOF/RBV, whether treatment naive (41/46 [89%]) or experienced (22/25 [88%]). In patients with cirrhosis treated with SOF/RBV, the SVR12 rate was 58% (19 of 33) in treatment-naive patients and fell to 42% (22 of 52) in those who had failed prior therapy (Table 2). In contrast, of 8 treatment-experienced patients with cirrhosis who received SOF/peg-IFN/RBV, 7 (88%) achieved SVR12 (Table 2). Overall, treatment with SOF/RBV was associated with a lower rate of SVR than treatment with SOF/peg-IFN/RBV (OR, 0.25; 95% CI, .06–1.13), but the difference did not reach statistical significance given the few patients treated with triple therapy. After controlling for the presence of cirrhosis, neither treatment history nor treatment duration was associated with SVR.
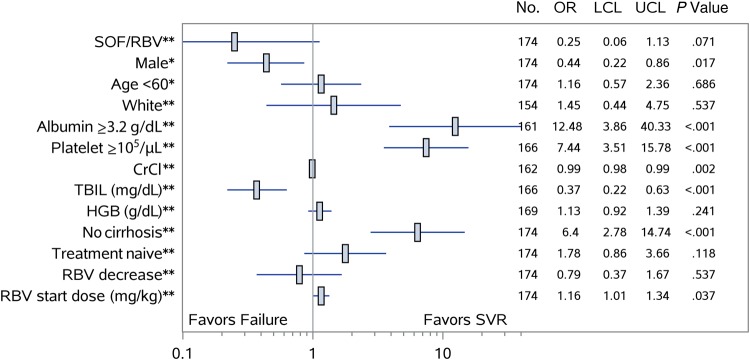
After controlling for age and sex, the absence of cirrhosis (OR, 6.4; 95% CI, 2.78–14.74), albumin levels of ≥3.2 g/dL (OR, 12.48; 95% CI, 3.86–40.33), and platelet count >105 cells/µL (OR, 7.44; 95% CI, 3.51–15.78) were associated with greater odds of achieving SVR12, whereas male sex (OR, 0.44; 95% CI, .22–.86) and increasing baseline bilirubin levels (OR, 0.37; 95% CI, .22–.63) were negative predictors of response (Figure 1).
Figure 1.
Baseline predictors of sustained virologic response (SVR) among patients with genotype 3 hepatitis C virus treated with sofosbuvir (SOF) and ribavirin (RBV) with or without peginterferon with available virologic outcomes. Factors associated with SVR are shown with an estimate of the odds ratios (ORs) with 95% confidence interval determined by multivariable logistic regression for each variable controlled for either age and sex**, or age or sex*. Abbreviations: CrCl, creatinine clearance; HGB, hemoglobin; LCL, lower confidence limit; TBIL, total bilirubin; UCL, upper confidence limit.
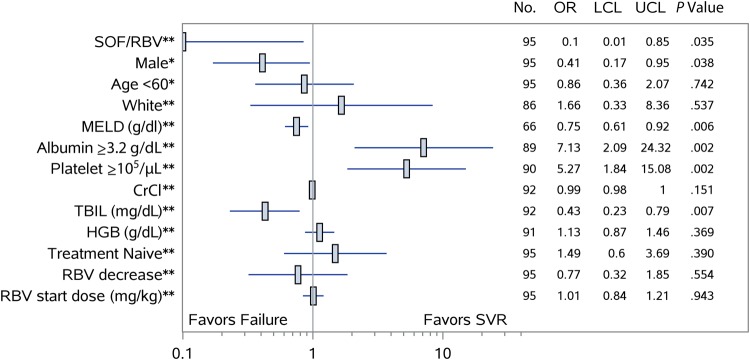
Among patients with cirrhosis, after controlling for age and sex, patients with baseline albumin ≥3.2 g/dL (OR, 7.13; 95% CI, 2.09–24.32) and those with platelet count >105 cells/µL (OR, 5.27; 95% CI, 1.84–15.08) were more likely to achieve SVR. Increasing baseline total bilirubin (OR, 0.43; 95% CI, .23–.79), baseline MELD score (OR, 0.75; 95% CI, .61–.92), and history of decompensating events (OR, 0.29; 95% CI, .12–.70) were each associated with a decreased likelihood of SVR (Figure 2). The SVR rate in patients treated with SOF/RBV with compensated cirrhosis was 64.8% (35/54) compared with 36% (4/11) in those with a history of decompensated disease (P = .08). Baseline HCV RNA was not predictive of SVR. Week 4 HCV RNA values were available in 153 patients (81%) and although patients with undetectable HCV RNA by week 4 were more likely to achieve SVR, this did not reach statistical significance (P = .09).
Figure 2.
Baseline predictors of sustained virologic response (SVR) among patients with genotype 3 hepatitis C virus and cirrhosis treated with sofosbuvir (SOF) and ribavirin (RBV) with or without peginterferon with available virologic outcomes. Factors associated with SVR among patients with cirrhosis are shown with an estimate of the odds ratios (ORs) with 95% confidence interval determined by multivariable logistic regression for each variable controlled for either age and sex**, or age or sex*. Abbreviations: CrCl, creatinine clearance; HGB, hemoglobin; LCL, lower confidence limit; MELD, Model for End-Stage Liver Disease; TBIL, total bilirubin; UCL, upper confidence limit.
Adverse Events
At least 1 AE was reported by 86% (169/197), whether treated with SOF/RBV or SOF/peg-IFN/RBV (Table 3). The most commonly reported AEs were fatigue (40%), headache (21%), and anemia (20%) (Table 3). Rash was reported in 5 (26%) patients treated with SOF/peg-IFN/RBV and 23 (13%) of those treated with SOF/RBV. Depression was documented in 25 (14%) patients on SOF/RBV compared to none on SOF/peg-IFN/RBV. In total, 11 patients experienced hepatic decompensation during therapy, all of whom had a prior history of decompensation (Table 4). Hepatic encephalopathy (n = 10) was the most frequently reported decompensating event, followed by ascites (n = 2) and variceal hemorrhage (n = 2).
Table 3.
Adverse Events and Management of Anemia
| Event | SOF/Peg-IFN/RBV (n = 19) | SOF/RBV (n = 178) | Total (N = 197) |
|---|---|---|---|
| Any adverse event | 15 (79.0) | 154 (86.5) | 169 (85.8) |
| Fatigue | 8 (42.1) | 70 (39.3) | 78 (39.6) |
| Headache | 4 (21.1) | 38 (21.4) | 42 (21.3) |
| Anemia | 3 (15.8) | 37 (20.8) | 40 (20.3) |
| Insomnia | 2 (10.5) | 35 (19.7) | 37 (18.8) |
| Nausea | 1 (5.3) | 33 (18.5) | 34 (17.3) |
| Rash | 5 (26.3) | 23 (12.9) | 28 (14.2) |
| Dyspnea | 2 (10.5) | 25 (14.0) | 27 (13.7) |
| Influenza-like illness | 5 (26.3) | 21 (11.8) | 26 (13.2) |
| Depression | 0 (0.0) | 25 (14.0) | 25 (12.7) |
| Dizziness | 2 (10.5) | 21 (11.8) | 23 (11.7) |
| Irritability | 3 (15.8) | 19 (10.7) | 22 (11.2) |
| Pruritus | 2 (10.5) | 20 (11.2) | 22 (11.2) |
| Decreased appetite | 3 (15.8) | 15 (8.4) | 18 (9.1) |
| Abdominal pain | 3 (15.8) | 13 (7.3) | 16 (8.1) |
| Asthenia | 2 (10.5) | 13 (7.3) | 15 (7.6) |
| Diarrhea | 2 (10.5) | 11 (6.2) | 13 (6.6) |
| Muscle spasms | 2 (10.5) | 11 (6.2) | 13 (6.6) |
| Cough | 2 (10.5) | 11 (6.2) | 13 (6.6) |
| Serious adverse events (18 events)a | 0 (0.0) | 13 (7.3) | 13 (6.6) |
| Anemia management | |||
| RBV dose reduction | 2 (10.5) | 33 (18.5) | 35 (17.8) |
| Erythropoietin use | 0 (0.0) | 7 (3.9) | 7 (3.6) |
| Blood transfusion | 0 (0.0) | 4 (2.2) | 4 (2.0) |
| RBV discontinuation | 0 (0.0) | 1 (0.6) | 1 (0.5) |
Data are presented as No. (%) of patients.
Abbreviations: peg-IFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir.
a Anemia, colitis, gastrointestinal hemorrhage, esophageal varices hemorrhage, chest pain, hepatocellular carcinoma (2), hepatic encephalopathy (4), dizziness (2), depression, psychotic disorder, renal failure acute (2), epistaxis.
Table 4.
Details on 14 Decompensation Events in 11 Patients During Antiviral Therapy
| Patient ID | Decompensation Event | Regimen | Releated to Treatment | Timing of Event, wk | Past History of Decompensation | Treatment Outcome |
|---|---|---|---|---|---|---|
| 1 | Hepatic encephalopathy | SOF/RBV | N | 8.3 | Y | SVR |
| 2 | Hepatic encephalopathy | SOF/RBV | Y | 16.1 | Y | Relapse |
| 3 | Variceal hemorrhage | SOF/RBV | N | 11.7 | Y | Relapse |
| 4 | Hepatic encephalopathy | SOF/RBV | Y | 4.9 | Y | Relapse |
| 4 | Hepatic encephalopathy | SOF/RBV | Y | 24.4 | Y | Relapse |
| 5 | Variceal hemorrhage | SOF/RBV | Y | 0.4 | Y | SVR |
| 6 | Hepatic encephalopathy | SOF/RBV | Y | 0.1 | Y | Relapse |
| 7 | Hepatic encephalopathy | SOF/RBV | Y | 19.4 | Y | SVR |
| 7 | Hepatic encephalopathy | SOF/RBV | Y | 16.6 | Y | SVR |
| 8 | Hepatic encephalopathy | SOF/RBV | Y | 0.1 | Y | LTFU |
| 8 | Ascites | SOF/RBV | Y | 9 | Y | LTFU |
| 9 | Hepatic encephalopathy | SOF/RBV | N | 4.3 | Y | BT |
| 10 | Hepatic encephalopathy | SOF/RBV | Y | 4.9 | Y | BT |
| 11 | Ascites | SOF/RBV | Y | 13.3 | Y | SVR |
Abbreviations: BT, breakthrough (on treatment); LTFU, lost to posttreatment follow-up; SOF/RBV, sofosbuvir/ribavirin; SVR, sustained virologic response.
Anemia was reported in 37 (21%) patients on SOF/RBV and 3 (16%) patients on SOF/peg-IFN/RBV. The median hemoglobin decline was 2.1 g/dL in SOF/RBV-treated patients, and 2.7 g/dL in patients treated with SOF/peg-IFN/RBV. Anemia led to RBV dose reduction in 33 (18.5%) and discontinuation in 2 (10.5%) patients. The initial RBV dose ranged from 4.6 mg/kg to 19.2 mg/kg (mean, 13.1 mg/kg). As shown in the forest plots (Figures 1 and 2), initial RBV dose was not associated with SVR in those with cirrhosis but was associated with SVR in the overall population, suggesting the effect was greatest in those without cirrhosis. The correlation between RBV dose and SVR is shown in Figure 3. RBV dose reduction was not associated with SVR (Figure 1 and 2). Addition of erythropoietin was needed by 7 patients (4%) and a blood transfusion by 4 (2%) individuals, all in patients treated with SOF/RBV. A total of 18 SAEs were reported in 13 patients (all treated with SOF/RBV), of which 8 SAEs in 7 patients were likely related to HCV therapy per the treating physician.
Figure 3.
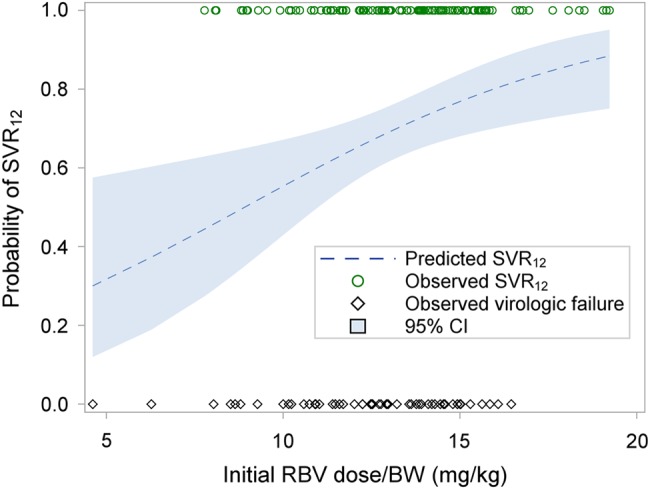
Correlation of probability of sustained virologic response at 12 weeks (SVR12) and initial ribavirin (RBV) dose (by body weight [BW]; mg/kg). The impact of the initial dose of RBV is shown by estimating the probability of SVR according to initial RBV dose using observed SVR (circle) and observed virologic failure (diamond), The shaded area shows the 95% confidence interval (CI) for the estimated correlation.
DISCUSSION
This large observational study evaluated the effectiveness and safety of 2 SOF-based therapies in patients with GT3 HCV infection. Although treatment was generally safe and fairly well tolerated, the SVR12 results were suboptimal (69%), particularly in patients with cirrhosis treated with SOF/RBV alone, highlighting the need for improved therapies for this population.
As reported in clinical trials, the main determinant of outcome in GT3 infection was the presence of cirrhosis [10–12]. Less than half (48%) of those with cirrhosis treated with SOF/RBV achieved SVR12. Notably, cirrhosis was a negative predictor of response even in patients who were previously untreated. Although the VALENCE trial reported a 92% SVR rate in treatment-naive patients with cirrhosis, only 13 such patients were included in the trial [12]. In this real-world study, only 19 of the 33 (58%) treatment-naive patients with cirrhosis achieved SVR12. Whether this reflects differences between clinical trials and clinical practice, or inclusion of patients with more advanced disease, or highlights the uncertainty of drawing conclusions from the small numbers included in the trial is difficult to determine.
How cirrhosis affects response to therapy is not clear but is likely multifactorial [20]. Data from patients with genotype 1 infection suggest that even in a cirrhotic liver, SOF is effectively taken up by hepatocytes and phosphorylated to the active compound [20]. Shunting due to portal hypertension may affect local drug concentrations and cirrhosis has important effects on innate and adaptive immune function, which are likely still important for viral clearance even with potent DAA therapy [20]. Younossi and colleagues recently reported that patients with GT3 infection had lower levels of lipids and, specifically, lower levels of metabolites from late in the cholesterol biosynthesis pathway [21]. Although the specific mechanisms remain unclear, these findings support the hypothesis that the unique effects of GT3 HCV on lipid metabolism may affect responses to antiviral therapy, an effect that appears to be more pronounced in the presence of cirrhosis. Unfortunately, cirrhosis is important even when SOF is combined with other approved DAAs for GT3 infection. Only 63% of patients with cirrhosis achieved SVR when SOF was combined with DCV for 12 weeks [22]. In the small ALLY 3+ study, patients with GT3 HCV and cirrhosis who received SOF/DCV plus RBV for 12 or 16 weeks achieved SVR rates of 83%–89%; however, the small sample size makes it difficult to draw strong conclusions or to distinguish the preferred duration [23]. Additional data from the French compassionate use program showed that 24 weeks of SOF/DCV with or without RBV led to SVR rates >80% despite inclusion of some patients with decompensated disease [24]. SOF/RBV plus LDV has also been evaluated in GT3 HCV. With the very limited activity of LDV against GT3 in vitro, it was perhaps surprising to see SVR rates as high as 73% in patients with cirrhosis [25]. These results clearly leave room for improvement for patients with GT3 infection and cirrhosis.
The disappointing outcomes with SOF/RBV therapy led to evaluation of the reintroduction of peg-IFN. Similar to the initial small studies evaluating this approach, the SOF/peg-IFN/RBV regimen proved quite effective in this cohort, most notably in the 8 patients with cirrhosis who had failed prior therapy, 7 of whom achieved SVR. A randomized controlled trial comparing SOF/RBV for 16 or 24 weeks to SOF/peg-IFN/RBV for 12 weeks in patients with GT3 HCV [14] showed that the inclusion of peg-IFN increased response rates in all patient subgroups but most notably in those with cirrhosis, whether treatment naive or experienced. Peg-IFN was well tolerated, with only 1 patient discontinuing treatment prematurely. Similarly, in this real-world experience, peg-IFN was well tolerated, with similar rates of AEs and higher rates of SVR reported compared with SOF/RBV alone. However, despite these encouraging results, SOF/peg-IFN/RBV was prescribed to only 19 of the 197 (9.6%) patients with GT3 who have started therapy in HCV-TARGET to date. Clearly there is a great reluctance by both clinicians and patients to accept interferon-based therapies. Fortunately, recent studies have shown that SOF combined with velpatasvir, a pan-genotypic NS5A inhibitor, is highly effective for GT3 HCV infection, leading to SVR12 rates of 90% even in patients with cirrhosis, without the need for RBV [26]. Other promising DAA combinations hold promise for GT3 HCV as well [27]. With this in mind, although SOF/peg-IFN/RBV may be considered for patients, particularly those with cirrhosis, waiting for approval of new therapies may be prudent, as it is unclear if an unsuccessful course of SOF/RBV or SOF/DCV will affect responses and/or access to future therapies.
Among patients with cirrhosis, low baseline albumin and low platelet count were associated with much lower odds of achieving SVR12. Only 20% of patients with albumin levels <3.2 g/dL achieved SVR, whereas 39% with baseline platelet count <105 cells/µL achieved SVR12 with SOF/RBV. Of the 18 patients with albumin <3.2 g/dL and platelets <105 cells/µL, only 4 (22%) achieved SVR, suggesting that for such individuals, waiting for availability of new multi-DAA regimens would be advisable.
This study has some important limitations. The lack of randomization limits the ability to directly compare treatment groups, which is further compounded by the small number of patients who received SOF/peg-IFN/RBV therapy. However, the results clearly demonstrate the limitations of SOF/RBV in patients with GT3 HCV infection and cirrhosis. In addition, by including a broader population of patients, many of whom may not have qualified for clinical trials, the safety and effectiveness profile provide very useful data for clinicians and patients.
CONCLUSIONS
This large observational, real-world study demonstrated that GT3 remains a clinical challenge. SOF/RBV for 24 weeks resulted in low SVR rates in patients with cirrhosis, particularly those with low baseline albumin and platelet levels. Although high efficacy and acceptable tolerability were seen in patients treated with SOF/peg-IFN/RBV, the extremely low uptake of this regimen highlights the poor acceptance of peg-IFN among patients and clinicians. Therapies in development will hopefully improve outcomes for this difficult-to-cure population.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author contributions. Data analysis, drafting and editing of manuscript: J. J. F., R. M. Study concept, data collection, editing of manuscript: M. W. F., D. R. N. Editing of manuscript: S. Z., A. K., A. M. D. B., M. M., K. S., L. M. F., R. S.
Financial support. Hepatitis C Virus Therapeutic Registry and Research Network is an investigator-initiated study jointly sponsored by the University of Florida, Gainesville (principal investigator [PI]: D. R. N.) and the University of North Carolina at Chapel Hill (PI: M. W. F.). It was funded in part by AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, and Vertex, and by the Clinical and Translational Science Award, University of Florida (UL1TR000064). M. W. F. was funded in part by a National Institutes of Health Mid-Career Mentoring Award (number K24 DK066144).
Potential conflicts of interest. J. J. F. reports receiving honoraria and/or research funds from AbbVie, Bristol-Myers Squibb (BMS), Gilead, Janssen, Merck, and Theravance. R. M. received financial compensation for consultancy from AbbVie. S. Z. reports honoraria for speaking/consultancy from AbbVie, BMS, Gilead, Janssen, and Merck. A. K. reports grant funding from Gilead. D. R. N. reports grant funding from AbbVie, BMS, Gilead, GSK, Janssen, and Merck. A. M. D. B. reports grant and consultancy fees from AbbVie, BMS, and Gilead. M. P. M. reports speaking, research, or consultancy fees from Achillion, Biotest, BI, BMS, Gilead, Idenix, Janssen, Merck, Novartis, and Roche. K. S. reports research grants from AbbVie, BMS, Gilead, Innovia, Medimmune, and Merck and consultancy fees from Medimmune and ViiV. L. M. F. reports research or consultancy fees from AbbVie, Gilead, Janssen, and Merck. R. S. reports grants support from AbbVie, BMS, Gilead, Merck, and Roche and consultancy fees from AbbVie, Baxter, Bayer, Gilead, GSK, Janssen, and Salix. M. S. reports funding from Merck. M. W. F. reports grant funding from AbbVie, BMS, Gilead, GSK, Janssen, Merck, Roche, and Vertex and consultancy fees from AbbVie, BMS, Gilead, Janssen, Merck, Novartis, and Roche. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61:S45–57. [DOI] [PubMed] [Google Scholar]
- 2.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10:553–62. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F, Hoang T, Kramer JR et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011; 140:1182–1188.e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lok AS, Seeff LB, Morgan TR et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009; 136:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Probst A, Dang T, Bochud M, Egger M, Negro F, Bochud PY. Role of hepatitis C virus genotype 3 in liver fibrosis progression—a systematic review and meta-analysis. J Viral Hepat 2011; 18:745–59. [DOI] [PubMed] [Google Scholar]
- 6.van der Meer AJ, Hansen BE, Fattovich G et al. Reliable prediction of clinical outcome in patients with chronic HCV infection and compensated advanced hepatic fibrosis: a validated model using objective and readily available clinical parameters. Gut 2015; 64:322–31. [DOI] [PubMed] [Google Scholar]
- 7.Vezali E, Aghemo A, Colombo M. A review of the treatment of chronic hepatitis C virus infection in cirrhosis. Clin Ther 2010; 32:2117–38. [DOI] [PubMed] [Google Scholar]
- 8.Duarte-Rojo A, Heathcote EJ, Feld JJ. ‘Easy to treat’ genotypes were not created equal: can rapid virological response (RVR) level the playing field? J Hepatol 2011; 55:466–73. [DOI] [PubMed] [Google Scholar]
- 9.Feld JJ. Interferon-free strategies with a nucleoside/nucleotide analogue. Semin Liver Dis 2014; 34:37–46. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson IM, Gordon SC, Kowdley KV et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013; 368:1867–77. [DOI] [PubMed] [Google Scholar]
- 11.Lawitz E, Mangia A, Wyles D et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–87. [DOI] [PubMed] [Google Scholar]
- 12.Zeuzem S, Dusheiko GM, Salupere R et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370:1993–2001. [DOI] [PubMed] [Google Scholar]
- 13.Lawitz E, Poordad F, Brainard DM et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology 2015; 61:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster GR, Pianko S, Brown A et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 2015; 149:1462–70. [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015; 63:199–236. [DOI] [PubMed] [Google Scholar]
- 16.American Association for the Study of Liver Diseases/Infectious Diseases Society of America HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62:932–54. [DOI] [PubMed] [Google Scholar]
- 17.Sulkowski MS, Vargas HE, Di Bisceglie AM et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology 2016; 150:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon SC, Muir AJ, Lim JK et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol 2015; 62:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. [DOI] [PubMed] [Google Scholar]
- 20.Al Marzooqi SH, Feld JJ. Sorting out cirrhosis: mechanisms of non-response to hepatitis C therapy. Liver Int 2015; 35:1923–33. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM, Stepanova M, Estep M et al. Dysregulation of distal cholesterol biosynthesis in association with relapse and advanced disease in CHC genotype 2 and 3 treated with sofosbuvir and ribavirin. J Hepatol 2015; 64:29–36. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DR, Cooper JN, Lalezari JP et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015; 61:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroy V, Angus P, Bronowicki JP et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+). Hepatology 2016; 63:1430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hezode C, de Ledinghen V, Fontaine H et al. Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV genotype 3 infection: interim analysis of a French multicenter compassionate use program. International Liver Congress, European Association for the Study of the Liver, Vienna, Austria, 2015. [Google Scholar]
- 25.Gane EJ, Hyland RH, An D et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015; 149:1454–61. [DOI] [PubMed] [Google Scholar]
- 26.Foster GR, Afdhal N, Roberts SK et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 27.Poordad F, Landis CS, Asatryan A et al. High antiviral activity of NS5A inhibitor ABT-530 with paritaprevir/ritonavir and ribavirin against hepatitis C virus genotype 3 infection. Liver Int 2016; doi:10.1111/liv.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.