Abstract
Protozoal diseases are prevalent globally and especially in developing countries that have relatively lower socioeconomic populations such as Egypt. Direct microscopic examination (DME) is used for the detection and identification of protozoa but lacks sufficient reliability, and thus may be detrimental in obtaining accurate diagnostic or epidemiological data. In this study, we determine the prevalence of infections by Giardia intestinalis, Cryptosporidium sp., and Entamoeba histolytica in humans in Egypt. Furthermore, we determine the reliability of DME in determining infections caused by these protozoa and compare the results to enzyme linked Immunosorbent assays (ELISA). Our results indicate that the prevalence of giardiasis, cryptosporidiosis, and entamoebiasis is 38, 22, and 16 %, respectively. The sensitivity and specificity of DME for detection of G. intestinalis is 45 and 99 %, for Cryptosporidium 66 and 99 %, and for Entamoeba 45 and 100 %, respectively. Our findings demonstrate that ELISA is more reliable for diagnostic and epidemiologic study purposes.
Keywords: Prevalence, Diagnosis, Entamoeba, Cryptosporidium, Giardia, ELISA
Introduction
Intestinal protozoal diseases are known to occur frequently in Egypt (Smith-Palmer and Cowden 2009; Smith-Palmer and Locking 2011). Among the most frequent are giardiasis, cryptosporidiosis, and entamoebiasis (Hegab et al. 2003; Zaki 2009). While few studies have attempted to investigate the prevalence and distribution of these infections in Egypt, these studies are incomplete, apply to different geographical locations (El-Shazly et al. 2006; Ibrahium 2011; Sabry et al. 2009), or involve distinct populations that do not represent the general population in Egypt (Antonios et al. 2010; El-Mahallawy et al. 2013; El-Sherbini et al. 2008). In addition, studies are often performed using variable, inaccurate, or inconsistent identification methods and few studies have addressed the reliability of these methods (Feng and Xiao 2011; Gaafar 2011; Selim et al. 2009). Furthermore, many studies have investigated Giardia, Cryptosporidium, and Entamoeba infections in combination with other parasitic infections and have therefore not focused sufficiently on these intestinal protozoa.
Symptoms of intestinal parasitic infections are usually general symptoms of gastrointestinal ailment including diarrhea, abdominal pain, nausea, vomiting, flatulence, anorexia, and fever (Katz et al. 2006). Entamoeba histolytica, G. Intestinalisand Cryptosporidium spp. are three of the most important and common diarrhea-causing parasitic protozoa, and often have a similar clinical presentations (Haque et al. 2007). However, the common symptoms and clinical presentations of these infections have not been sufficiently investigated. Examination of the literature on giardiasis revealed that only few contained information on symptoms or clinical presentations of the disease in Egypt (Muhsen and Levine 2012). This information is required for the study of these diseases and for the ability of healthcare providers to identify the protozoa and perform critical differential diagnoses onpatients.
Direct microscopic examination (DME) remains the most commonly used method for detecting and identifying intestinal parasites as DME is relatively inexpensive and appropriate for resource-limited developing countries (Utzinger et al. 2010). However, DME is prone to a number of limitations such as requiring trained personneland considerable effort for preparing, staining, and examining smears. In addition, there is a frequent need to examine multiple fecal samples to find a suspected organism (Johnston et al. 2003; Palmieri et al. 2011). Misdiagnosis by DME can significantly impact patient care (Newman et al. 1993; Palmieri et al. 2011). When DME was used, infection rates appeared 51.3 % lower when compared to rates detected by enzyme linked Immunosorbent assays (ELISA) and 17 % lower when compared to rates detected by polymerase chain reaction (PCR) (Feng and Xiao 2011). Immunoassays for the detection of stool copro-antigens have replaced DME as the routine diagnostic procedure in many laboratories worldwide (Garcia and Shimizu 1997), providing adequate sensitivity and specificity. The assays have also been used in the study of the epidemiology of asymptomatic disease (Gonzalez et al. 1995). However, the routine use of immunoassays for diagnosis of protozoal diseases is not common in Egypt. Furthermore, few studies have investigated the reliability of microscopic examination compared to serological or molecular based methods. There are no reported studies comparing Giardia, Cryptosporidium, and Entamoeba infections in Egypt.
In this study, we determine the prevalence of G. intestinalis, Cryptosporidium, and E.histolytica in humans. We investigate the prevalence of symptoms associated with each infection and their association with human disease. We also determine the reliability of DME in identifying these organisms from clinical samples compared to ELISA-based assays using commercially available diagnostic test kits.
Materials and methods
Study populations
Human stool samples from 185 patients at Mansoura University Hospital out-patient clinics were collected as part of their clinical evaluation. Patients were visiting the hospital complaining of a variety of gastrointestinal and non-gastrointestinal symptoms. Gastrointestinal symptoms at the time of sample submission were recorded for each patient. Sample collection and clinical information followed ethical medical research guidelines of Mansoura University, Egypt. Patients were between 2 and 58 years old and included 86 females and 99 males. Samples were tested for the presence of G. intestinalis, Cryptosporidium, and E. histolytica.
Microscopy
For determining the reliability of DME in identification of organisms, fecal samples were processed as per the standard procedures in the Mansoura University clinical diagnostic laboratory. Samples were preserved in 10 % buffered neutral formalin and concentrated by centrifugation at 500×g for 10 min and the resulting supernatant located directly above the concentrated stool sediment was discarded. For identification of G. intestinalis or Entamoeba spp., microscopic examination consisted of evaluating two wet mount preparations for each fecal specimen; one non-stained and the other stained with iodine. For identification of Cryptosporidium spp. concentrated samples were stained using Modified Ziehl-Neelson acid fast stain (Garcia 2001) before microscopic examination. All samples were examined at 1000× for the presence of trophozoites or cysts. Only findings of Giardia, Cryptosporidium, or Entamoeba Spp. Were included in the results and analysis of this study.
ELISA based testing
TechLab’s GIARDIA II, CRYPTOSPORIDIUM II, and E. HISTOLYTICA II ELISA-based diagnostic kits (TechLab, Blacksburg, VA, USA) were used to identify G. intestinalis, Cryptosporidium spp., or E. histolytica antigens, respectively. The GIARDIA II test relies on monoclonal antibodies for detection of Giardia cyst antigen and the CRYPTOSPORIDIUM II test detects Cryptosporidiumoocysts. The E. HISTOLYTICA II test relies on monoclonal antibodies for detection of E. histolyticaadhesin and does not cross react with Entamoeba dispar. The specificity of each kit is determined by the manufacturer to be 100 % and no cross reactivity was found. All kits were used according to the manufacturer’s instructions. Only non-preserved samples were used for ELISA testing.
Statistical analysis
To determine the reliability of DME, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated as described by Altman and Bland (1994a, 1994b). Sensitivity was determined by calculating the proportion of positive samples as determined by ELISA that are correctly identified by DME, and specificity is determined by calculating the portion of negative samples using ELISA that are also negative using DME. PPV = (sensitivity × prevalence)/(sensitivity × prevalence + (1 − specificity) × (1 − prevalence). NPV = (specificity × (1 − prevalence)/(1 − sensitivity) × prevalence + specificity × (1 − prevalence).
The findings of DME were considered the test results and the findings of the ELISA-based kits were considered the correct diagnostic results. To determine whether infection with G. intestinalis, Cryptosporidium, or E. histolytica is associated with absence of symptoms the Chi Square test (χ2) test was used. Significance was defined as P < 0.001.
Results
Disease prevalence and clinical presentation
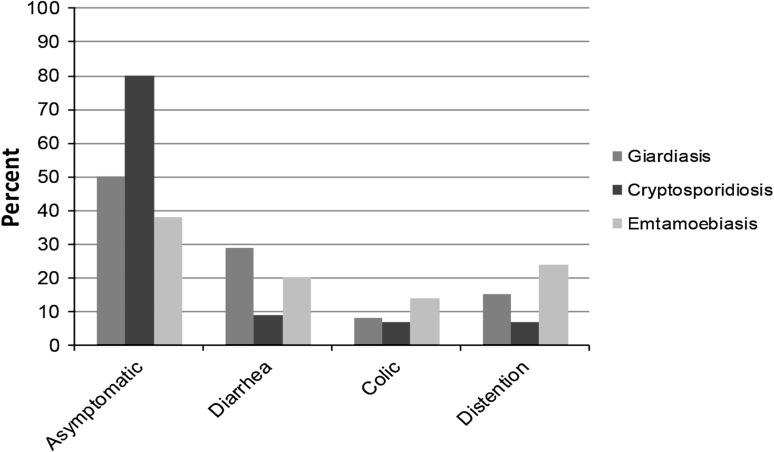
The prevalence of giardiasis, cryptosporidiosis, and entamoebiasis using ELISA assays was 38 % (71 cases), 22 % (41 cases) and 16 % (29 cases) respectively. The prevalence of giardiasis, cryptosporidiosis, and entamoebiasis using DME was 18 % (33 cases), 15 % (28 cases), and 7 % (13 cases), respectively. In the majority of cases diagnosed with each disease, patients were asymptomatic. Diarrhea, abdominal distention, and colic were the most frequently reported symptoms while nausea and constipation were less frequently reported (Fig. 1). To determine whether infection with each of the organisms tended to present with gastrointestinal symptoms and whether diarrhea was as an important symptom of each infection, the association between infection and the presence of symptoms, or specifically diarrhea, were determined for infection with each organism. In patients with giardiasis, there was an association between infection and asymptomatic presentation (Table 1), suggesting that infections tended to be asymptomatic. In patients who presented with gastrointestinal symptoms, there was an association with diarrhea (Table 2), suggesting that in patients who presented with gastrointestinal symptoms, diarrhea tended to occur.
Fig. 1.
Clinical symptoms associated with intestinal protozoal infection. Asymptomatic presentation was more frequent than each of the individual symptoms and diarrhea was the most common presenting symptom
Table 1.
Association of asymptomatic gastrointestinal presentation with giardiasis, cryptosporidiosis, or entamoebiasis
| Number of patients | Percent infected | χ2 | P | |
|---|---|---|---|---|
| Asymptomatic presentation (vs. giardiasis) | 13.890 | <0.001 | ||
| Yes | 124 | 29 | ||
| No | 61 | 57 | ||
| Asymptomatic presentation (vs. cryptosporidiosis) | 0.321 | 0.567 | ||
| Yes | 124 | 23 | ||
| No | 61 | 20 | ||
| Asymptomatic presentation (vs. entamoebiasis) | 13.174 | <0.001 | ||
| Yes | 124 | 9 | ||
| No | 61 | 29 |
Table 2.
Association of diarrhea with giardiasis, cryptosporidiosis, or entamoebiasis
| Number of patients | Percent infected | χ2 | P | |
|---|---|---|---|---|
| Diarrhea (vs. giardiasis) | 22.734 | <0.001 | ||
| Yes | 26 | 81 | ||
| No | 159 | 31 | ||
| Diarrhea (vs. cryptosporidiosis) | 0.805 | 0.369 | ||
| Yes | 26 | 15 | ||
| No | 159 | 23 | ||
| Diarrhea (vs. entamoebiasis) | 1.220 | 0.269 | ||
| Yes | 26 | |||
| No | 159 | 14 |
There was an association between infection with E. histolytica and absence of gastrointestinal symptoms (Table 1). However, there was no association between the infection and the presence of diarrhea when symptoms were present (Table 2), suggesting that infections tended to be asymptomatic and diarrhea was not especially present when symptoms occurred.
Reliability of microscopic examination for diagnosis
Enzyme linked Immunosorbent assays results were considered as the reference method when determining, the specificity, sensitivity, PPV, NPV of microscopical examination for diagnosis of giardiasis, cryptosporidiosis, or entamoebiasis. Results are shown in Table 3. No cross-reactivity with other parasites was detected.
Table 3.
Reliability of microscopic examination for identification of intestinal protozoa in fecal samples
| Organism | Sensitivity (%) | Specificity (%) | PPV (%)a | NPV (%)b |
|---|---|---|---|---|
| G. intestinalis | 45 | 99 | 97 | 74 |
| Cryptosporidium | 66 | 99 | 96 | 91 |
| E. histolytica | 45 | 100 | 100 | 91 |
aPositive predictive value
bNegative predictive value
Discussion
Intestinal protozoal infections are a public health problem in developing countries where they cause high morbidity and mortality (Dorny et al. 2009; Kenny and Kelly 2009).The prevalence of these infections and the extent of their public health effect in Egypt are not clearly understood. Lack of knowledge on their epidemiologic status is most likely due to incomplete understanding of the diseases’ symptoms, the variation and overlap of those symptoms, and the ineffectiveness of DME as a commonly used diagnostic tool. Therefore, there is a need to study the prevalence of intestinal protozoal infections, determine theirmost common symptoms, and determine the efficiency of DME in their identification in clinical samples.
In our study, the prevalence of G. intestinalis, Cryptosporidium, and E. histolyticainfections was 38, 22 and 16 %, respectively, when determinedby ELISA-based diagnostic kits. Giardia intestinalis infections had the highest prevalence among the examined protozoans, which is in-line with previous studies that demonstrate that giardiasis is the most common intestinal parasitic disease of humans in developing countries (Feng and Xiao 2011; Smith-Palmer and Locking 2011). The prevalence of giardiasis in our study (38 %) was higher than that reported in other studies from Egypt, which varied between 14.8 and 30.8 % (El-Kadi et al. 2006; Sabry et al. 2009). Cryptosporidiosis is mainly reported in animals in Egypt(Mahran and Taher 2010; Samaha et al. 2012). However, cryptosporidiosis is one of the least studied infectious disease in Egypt and, as with other parasitic diseases, its prevalence is thought to be underreported (Palmieri et al. 2011). The prevalence of Cryptosporidium infections obtained in our study by ELISA was high (22 %) in comparison with studies using the same method in other countries [1.7 % in Italy (Cirak and Bauer 2004) and 7.4 % in Canada (Rinaldi et al. 2008)] and approximates that of Germany 23 % (Shukla et al. 2006). Few studies have reported the prevalence of Entamoeba infections in Egypt. In these reports, DME was often used and the organisms were reported as either E. histolytica or E. dispar. However, DME alone is not capable of distinguishing the two species (Stauffer et al. 2006) and these reports are likely to include false negative and false positive results. In a study by El-Shazly et al. (2006)carried out in Dakahlia, Egypt among 3,180 patients, the authors reported a prevalence of 19.6, 19.0, and 14.3 % for Giardia, Entamoeba and Cryptosporidium, respectively, using microscopy with staining. These percentages are lower than our findings and this may be due to the use of different microscopic methods in other studies compared to ours, resulting in an increase in false negative cases.
In this study, a variety of symptoms were reported by patients with giardiasis. Symptoms of giardiasis may be typical or atypical. Typical symptoms include diarrhea, loose stools, malaise, abdominal cramps and weight loss. Atypical symptoms vary and patients may be asymptomaticorbemildlysymptomatic (Meyer and Radulescu 1979). However, common symptoms associated with giardiasis in Egypt have not been previously reported. Our findings demonstrate that 57.3 % of Giardia positive cases are asymptomatic while diarrhea is the main symptom in 29 % of symptomatic cases. Most cases of Cryptosporidium infection (80 %) were asymptomatic and diarrhea was the main complaintin symptomatic cases. This finding is consistent with that of previous studies (Huang and White 2006; Raccurt 2007). In our study the majority of entamoebiasis cases were symptomatic (29.5 %) while distension and diarrhea were the main symptoms. Only 8.9 % cases were asymptomatic. Our findings suggest that in our population, giardiasis tends to be asymptomatic but causes diarrhea if symptoms exist. Entamoebiasis also tends to be asymptomatic, but presenting with diarrhea was not associated with its symptoms. The variation of symptoms associated with each of the diseases we examined may be due to their intermittent nature or due to the presence of an undetermined underlying condition. The endemic nature of these diseases in Egypt may also contribute to the presence of variable and atypical symptoms. The variety of symptoms and their atypical nature may also contribute to the diseases being commonly under-diagnosed or misdiagnosed by healthcare providers.
In this study routine microscopic examination of stool samples did not reveal as many positive specimens as the ELISA tests. We also demonstrated that the sensitivity of DME was 45, 66, and 45 % while the specificity was 99, 99, and 100 % for detection of G. intestinalis, E. histolytica, and Cryptosporidium, respectively. The specificity for detection and identification of each of the organisms was acceptable. However, the sensitivity was not acceptable. The sensitivity for identification of cryptosporidiosis was higher than that for giardiasis and entamoebiasis. This difference may be due to the use of staining for the routine identification of Cryptosporidium using DME, which enhances the ability to detect and correctly identify the organism. Our results demonstrate that ELISA is more sensitive than DME in detection of G. intestinalis, E. histolytica, and Cryptosporidiumas ELISA is able to detect minimal amounts of antigen and canshow a positive result even when the parasite load is low. These findings are in accordance with reports that indicate that TechLab ELISA for detection of E. histolytica antigen in stool specimens have excellent correlation with PCR (Haque et al. 1998). Other studies have also found that ELISA is more sensitive (80–94 %) and more specific (94–100 %) than microscopy and culture (Haque et al. 2000) and DME was less effective in detection and identification of Giardia (Shatla et al. 2004). Other enzyme immune assays (EIA) have also been used in detection and identification of these protozoans. In one study in Egypt, microscopic examination detected Giardia in 19 %, Cryptosporidium in 4 %, and E. histolytica/E. dispar in 1 % of examined stool samples, while an EIA kit detected Giardia in 23 %, Cryptosporidium in 5 %, and E. histolytica/E. dispar in 2 % (Gaafar 2011). In another study, a Giardia EIA identified the organism in at least 30 % more specimens than microscopic examination (Rosoff et al. 1989). These studies also indicate that the immune assays are more reliable than microscopic examination.
Enzyme linked Immunosorbent assays has many significant advantages for the diagnosis of intestinal protozoa. The technique is able to differentiate E. histolytica from E. dispar and has excellent sensitivity and specificity. When used as commercial kits, the technique does not require a higher level of training and experience as often required for DME. Enzyme linked Immunosorbent assays can outperform microscopy in its potential as a large-scale diagnostic tool in epidemiological studies (Gaafar 2011). Most studies of Giardia, Entamoeba, and Cryptosporidium infections in Egypt use DME for diagnosis of the diseases and as the standard when determining the sensitivity and specificity of other tests (Antonios et al. 2010; El-Shazly et al. 2004). However, microscopy appears to be an unsuitable reference standard; techniques that have more sensitivity and specificity should be adopted instead. Enzyme linked Immunosorbent assays-based tools are more specific and sensitive, and are more affordable and thus appear to be the most suitable tests. Microscopy may continue to be used as a confirmatory test.
Overall, our results demonstrate that G. intestinalis, E. histolytica, and Cryptosporidium infections are prevalent in Egypt, indicating that a management strategy is needed to prevent and control infections. In our study, many infections were asymptomatic, necessitating more efficient methods of detection and identification of their causative organisms. Our findings suggest that DME is not adequate for identification of these organisms for the purpose of clinical diagnosis or epidemiological studies and should not be used as a reference technique to evaluate other diagnostic methods. Enzyme linked Immunosorbent assays-based or other serological detection and identification tests are more reliable. These tests, or other molecular testes, should be used for identification and diagnosis of these intestinal parasitic infections and as the reference tests when evaluating other commonly used techniques.
Acknowledgment
The authors acknowledge Dr. Joel Herbein, TechLab, for providing the ELISA kits and Mrs. Jennifer Cacciola, TechLab, for technical support.
Contributor Information
Shaadi F. Elswaifi, Email: selswaifi@vcom.vt.edu
James R. Palmieri, Email: jpalmieri@vcom.vt.edu
Nora El-Tantawy, Email: noratantawy@yahoo.com.
Ekbal Abohashem, Email: dr_ekbalabohashem@yahoo.com.
References
- Altman D G, Bland J M. Statistics Notes: Diagnostic tests 1: sensitivity and specificity. BMJ. 1994;308(6943):1552–1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D. G, Bland J M. Statistics Notes: Diagnostic tests 2: predictive values. BMJ. 1994;309(6947):102–102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonios SN, Tolba OA, Othman AA, Saad MA. A preliminary study on the prevalence of parasitic infections in immunocompromised children. J Egypt Soc Parasitol. 2010;40:617–630. [PubMed] [Google Scholar]
- Cirak VY, Bauer C. Comparison of conventional coproscopical methods and commercial coproantigen ELISA kits for the detection of Giardia and Cryptosporidium infections in dogs and cats. Berl Munch Tierarztl Wochenschr. 2004;117:410–413. [PubMed] [Google Scholar]
- Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Vet Parasitol. 2009;163:196–206. doi: 10.1016/j.vetpar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- El-Kadi MA, Dorrah AO, Shoukry NM. Patients with gastrointestinal complaints due to enteric parasites, with reference to Entamoeba histolytica/dispar as dected by ELISA E. histolytica adhesion in stool. J Egypt Soc Parasitol. 2006;36:53–64. [PubMed] [Google Scholar]
- El-Mahallawy Hadir, El Basha Noussa R., Zaki Mayssa M., El-Arousy Maha, Elswaifi Shaadi F., Abo-hashem E. M. A comparative study on enteric parasitic infections in immunocompetent and immunosuppressed children in Egypt. Comparative Clinical Pathology. 2013;23(5):1509–1514. doi: 10.1007/s00580-013-1814-5. [DOI] [Google Scholar]
- El-Shazly AM, Mowafy N, Soliman M, El-Bendary M, Morsy ATA, Ramadan NII, Arafa WAS. Egyptian genotyping of Giardia lamblia. J Egypt Soc Parasitol. 2004;34:265–280. [PubMed] [Google Scholar]
- El-Shazly AM, Awad SE, Sultan DM, Sadek GS, Khalil HHM, Morsy TA. Intestinal parasites in Dakahlia Governorate, with different techniques in diagnosing protozoa. J Egypt Soc Parasitol. 2006;36:1023–1034. [PubMed] [Google Scholar]
- El-Sherbini GT, Noor MFA, Hegazi MM. Parasitiosis in handicapped children in an Egyptian blind asylum. J Egypt Soc Parasitol. 2008;38:319–326. [PubMed] [Google Scholar]
- Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaafar MR. Evaluation of enzyme immunoassay techniques for diagnosis of the most common intestinal protozoa in fecal samples. Int J Infect Dis. 2011;15:e541–e544. doi: 10.1016/j.ijid.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Garcia LS. Diagnostic medical parasitology. 4. Washington, DC: American Society for Microbiology; 2001. [Google Scholar]
- Garcia LS, Shimizu RY. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997;35:1526–1529. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CR, et al. Prevalence of antibodies against Entamoeba histolytica in Mexico measured by ELISA. Epidemiol Infect. 1995;115:535–543. doi: 10.1017/S0950268800058702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Ali IK, Akther S, Petri WA., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Mollah NU, Ali IK, Alam K, Eubanks A, Lyerly D, Petri WA., Jr Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J Clin Microbiol. 2000;38:3235–3239. doi: 10.1128/jcm.38.9.3235-3239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, et al. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am J Trop Med Hyg. 2007;76:713–717. doi: 10.4269/ajtmh.2007.76.713. [DOI] [PubMed] [Google Scholar]
- Hegab MHA, Zamzam SM, Khater NM, Tawfeek DM, Abdel-Rahman HM. Opportunistic intestinal parasites among children with chronic liver disease. J Egypt Soc Parasitol. 2003;33:969–977. [PubMed] [Google Scholar]
- Huang DB, White AC. An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am. 2006;35:291–314. doi: 10.1016/j.gtc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ibrahium FAA. Prevalence and predisposing factors regarding intestinal parasitic infections among rural primary school pupils at Minia Governorate, Egypt. J Public Health Afr. 2011;2:e29. doi: 10.4081/jphia.2011.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol. 2003;41:623–626. doi: 10.1128/JCM.41.2.623-626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DE, Heisey-Grove D, Beach M, Dicker RC, Matyas BT. Prolonged outbreak of giardiasis with two modes of transmission. Epidemiol Infect. 2006;134:935–941. doi: 10.1017/S0950268805005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny JM, Kelly P. Protozoal gastrointestinal infections. Medicine. 2009;37:599–602. doi: 10.1016/j.mpmed.2009.08.001. [DOI] [Google Scholar]
- Mahran OM, Taher GA. Survey of Cryptosporidium and Giardia infection and trials of treatment in sheep and goats at the triangular area (Shalatin-Abu-Ramaid-Halaeeb) Red Sea Governorate, Egypt. Assiut Vet Med J. 2010;56:200–217. [Google Scholar]
- Meyer EA, Radulescu S. Giardia and giardiasis. Adv Parasitol. 1979;17:1–47. doi: 10.1016/S0065-308X(08)60548-5. [DOI] [PubMed] [Google Scholar]
- Muhsen K, Levine MM. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis. 2012;55(Suppl 4):S271–S293. doi: 10.1093/cid/cis762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RD, Jaeger KL, Wuhib T, Lima AA, Guerrant RL, Sears CL. Evaluation of an antigen capture enzyme-linked immunosorbent assay for detection of Cryptosporidium oocysts. J Clin Microbiol. 1993;31:2080–2084. doi: 10.1128/jcm.31.8.2080-2084.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri JR, Elswaifi SF, Fried KK. Emerging need for parasitology education: training to identify and diagnose parasitic infections. Am J Trop Med Hyg. 2011;84:845–846. doi: 10.4269/ajtmh.2011.10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccurt CP. Worldwide human zoonotic cryptosporidiosis caused by Cryptosporidium felis. Parasite. 2007;14:15–20. doi: 10.1051/parasite/2007141015. [DOI] [PubMed] [Google Scholar]
- Rinaldi L, et al. Giardia and Cryptosporidium in canine faecal samples contaminating an urban area. Res Vet Sci. 2008;84:413–415. doi: 10.1016/j.rvsc.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Rosoff JD, et al. Stool diagnosis of giardiasis using a commercially available enzyme immunoassay to detect Giardia-specific antigen 65 (GSA 65) J Clin Microbiol. 1989;27:1997–2002. doi: 10.1128/jcm.27.9.1997-2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry MA, Taher ES, Meabed EMH. Prevalence and genotyping of zoonotic Giardia from Fayoum Governorate, Egypt. Res J Parasitol. 2009;4:105–114. doi: 10.3923/jp.2009.105.114. [DOI] [Google Scholar]
- Samaha HA, Haggag YN, Nossair MA, El-Radda I. Role of calves and lambs in transmitting cryptosporidiosis to children in west Delta region of Egypt. Assiut Vet Med J. 2012;58:16–30. [Google Scholar]
- Selim S, Nassef N, Sharaf S, Badra G, Abdel-Atty D. Copro-antigen detection versus direct methods for the diagnosis of Giardia lamblia in patients from the National Liver Institute. J Egypt Soc Parasitol. 2009;39:575–583. [PubMed] [Google Scholar]
- Shatla HM, El-Hodhod MTA, Mohsen DM, El-Din MYS. Potential diagnosis of Giardia lamblia infection through specific antibody detection in saliva. J Egypt Soc Parasitol. 2004;34:621–630. [PubMed] [Google Scholar]
- Shukla R, Giraldo P, Kraliz A, Finnigan M, Sanchez AL. Cryptosporidium spp. and other zoonotic enteric parasites in a sample of domestic dogs and cats in the Niagara region of Ontario. Can Vet J. 2006;47:1179–1184. [PMC free article] [PubMed] [Google Scholar]
- Smith-Palmer A, Cowden JM. Overseas outbreaks of infectious intestinal disease identified in Scotland, 2003 to 2007. J Travel Med. 2009;16:322–327. doi: 10.1111/j.1708-8305.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- Smith-Palmer A, Locking M. Gastro-intestinal and foodborne infections: overseas outbreaks of infectious intestinal disease. HPS Wkly Rep. 2011;45:230–231. [Google Scholar]
- Stauffer W, Abd-Alla M, Ravdin JI. Prevalence and incidence of Entamoeba histolytica infection in South Africa and Egypt. Arch Med Res. 2006;37:266–269. doi: 10.1016/j.arcmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Utzinger J, et al. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin Microbiol Infect. 2010;16:267–273. doi: 10.1111/j.1469-0691.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- Zaki ME. Study of enteropathogens associated with paediatric gastroenteritis. Asian Pac J Trop Med. 2009;2:63–69. [Google Scholar]