Abstract
Because of developing resistance to the existing anthelmintic drugs, there is a need for new anthelmintic agents. Tobacco plant has alkaloid materials that have antiparasitic effect. We investigated the in vitro anthelminthic effect of aqueous and alcoholic extract of Tobacco (Nicotiana tabacum) against M. marshalli. For investigating this effect, we prepared three dilutions of aqueous and alcoholic extract of Tobacco (25, 50 and 75 mg/ml). The worms exposed to extracts for 10 h at 25–30 °C. The buffer PBS used as negative control and 50 mg/ml dilution of Levamisole used as standard reference. In each group, 50 worms were examined. We used an inhibition mobility test for our study. Survival analysis with Cox proportional hazard model was used for data analysis. The result showed that compared with Levamisole 50 mg/ml, dilution of 25 and 50 mg/ml of the aqueous extract had the same anthelminthic effects (P > 0.05), but 75 mg/ml dilution of the aqueous extract and dilution of 25, 50 and 75 mg/ml of alcoholic extract had more anthelminthic effect (P < 0.05). Overall, extracts of Tobacco possess considerable anthelminthic activity and more potent effects were observed with the highest concentrations. Therefore, the in vivo study on Tobocco in animal models is recommended.
Keywords: Tobacco, Marshallagia marshalli, Levamisole, Anthelmintic
Introduction
Gastrointestinal parasite infection is one of the main causes of impaired production in small ruminants worldwide and can cause significant production losses and lead to death. Helminthic parasites impair health by causing inappetance, diarrhoea, anaemia and, in severe cases, death (Coop and Kyriazakis 1999).
Species of Marshallagia (Orloff 1933) are typical abomasal nematodes in sheep, goats and wild ruminants in tropical and sub-tropical climates throughout the world (Soulsby 1982, Hoberg et al. 2001). In Iran, among different species of Ostertagiinae that can infect sheep, Marshallagia marshalli is one of the most important species because of its high prevalence and pathogenicity (Eslami et al. 1979).
The principle of a parasite control program is on the strategic use of anthelmintic drugs. However, resistance to these chemical drugs is now a widespread problem in some countries. The cost of chemical drugs in developing countries is another additional limitation on their use. For these reasons, another plan for nematode control is required (Athanasiadou et al. 2007).
Evidence of the anthelmintic properties of plants and plant extracts is derived primarily from ethno-veterinary sources (Hammond et al. 1997; Akhtar et al. 2000; Waller et al. 2001). The use medicinal plant preparations have been accepted in different parts of the world (Tabuti et al. 2003; Mathias 2004; Githiori et al. 2006; Lans 2011).
A number of plants with anthelmintic properties have also been recognized in Iran.
In many centuries, medicinal plants have been used against parasites, and are still used for this purpose. In ethno-veterinary medicine, there seems to be a range of plants or plant extract suitable for treating parasitic disease of livestock (International Institute of Rural Reconstruction (IIRR) 1994).
Studies show that plant species can effectively reduce the degree of parasite infestation in livestock (Hammond et al. 1997; Akhtar et al. 2000; Waller et al. 2001; Zenner et al. 2003; Athanasiadou et al. 2007; Souto et al. 2011). Because of developing resistance to the existing anthelmintic drugs (Prichard 1990), there is a need for new anthelmintic agents. The anthelmintic activity of plant extracts can arise either from a direct action of a drug on the worms or through induction of gastrointestinal irritation and diarrhea (Lorimer et al. 1996).
Nicotiana tabacum L., containing nicotine whose pharmacological action includes promoting evacuation of the bowel, the probable basis of its anthelmintic action (Budavari 1989).
Nicotiana tabacum (family Solanaceae), commonly known as tobacco, is a world popular plant used for its narcotic properties. Tobacco refers to the more than 70 plant species within the genus Nicotiana of the Solanaceae (nightshade) family. It was originally native to the Americas but is now cultivated in the north of Iran where it is locally known as “Tootoon”. A large number of alkaloids have been identified in the plant. Nicotine is known as the main alkaloid of tobacco, isolated in 1828 from the tobacco leaf, accounting for over 90 % of the total alkaloidal content (Bowman and Rand 1980). Nicotine has synonyms of 3-(1-methyl-2-pyrrolidinyl) pyridine, (S)-(−)-nicotine and high formula C10H14N. Apart from nicotine, the other known alkaloids are nicotyrine, nornicotine, anabasine, myosmine, anatabine, nicotelline and isonicoteine (Kuhn 1965). Dried leaves, stalks and the whole herb of tobacco are widely used as an anti inflammatory, antirheumatic and anthelmintic agent (Nadkarni 1976; Swerdlow and Johnson 2000; Iqbal et al. 2006).
To the best of our knowledge, there is no evidence to consider the effect of tobacco extract on M. marshalli, so we did this study to explore the in vitro anthelmintic properties aqueous and alcoholic extract of tobacco against M. marshalli.
Materials and methods
Plant material and extract preparation
Collection and identification of plant material was made under the supervision of botanists. The plant material was dried in a 50 °C oven and stored at room temperature until extraction. Dried leaves were ground to a powder using a Warring commercial Blender and stored at 4 °C until use. A 25 g sample was mixed in 250 mL of distillated water for 5 min, and then soaked over-night in an incubator shaker at 37 °C at a low speed shake. Following incubation, the herb-water mix was filtered using a porcelain Buchner funnel. To accelerate the filtration process, the filter was connected to a vacuum. To preparation of alchoholic extract, the powdered plant material was thoroughly extracted with methanol in a Soxhlet apparatus (Asuzu and Onu 1994). This solvent was chosen because it will extract with a wide range of polarities, is volatile, and has relatively low toxicity (Farnsworth 1990). Then the plant extracts were filtered through Whatman No. 1 filter paper. The methanol extract was evaporated to dryness and stored at 4 °C until used.
In vitro anthelmintic activity
The in vitro assay for anthelmintic activity of aqueous and alcoholic extract was performed on adult live M. marshalli of sheep as described previously for other helminth parasites (Lal et al. 1976).
Because of easy availability, M. marshalli have been used in this study. Briefly, the mature worms were collected from the abomasums of slaughtered sheep in abattoir. The worms were washed and suspended in PBS. Ten worms were exposed for 10 h in the following treatments in separate Petri dishes at room temperature (25–30 °C). In each group, 50 worms were examined. Aqueous and alcoholic extract of Tobacco (25, 50 and 75 mg/ml), Levamisole (5, 50 and 500 mg/ml), Levamisole in 5 mg/ml concentration was used as standard reference and PBS as negative control. The inhibition of motility of the worms was used as the criterion for anthelmintic activity which involved the determination of time of paralysis and time of death of the worm. The mobility of the adult worms was noted by careful observation under a stereomicroscope at magnification 40× for paralysis was noted when no movement of any sort could be observed except when the worms were shaken vigorously. Death was concluded when the worms lost their motility. For each treatment (plant extract and dose), the number of immobile worms was recorded with time.
We used survival analysis with Cox proportional hazard model for data analysis using Stata 10.1.
Results
Based on our data, the aqueous and alcoholic extracts of N. tabacum exhibited activity against M. marshalli. All the worms exposed to levamisole, a standard anthelmintic agent (Edwards and Breckenridge 1988), were found dead at 8 h; whereas, none of the worms were found dead or paralysed in PBS which acted as the negative control.
Three concentrations (25, 50 and 75 mg/ml) of aqueous and alcoholic extract of Tobacco leaves were studied in this study, Tobacco extract exhibited significant anthelmintic activity at highest concentration of 75 mg/ml.
The result showed that compared with Levamisole 50 mg/ml, dilution of 25 and 50 mg/ml of aqueous extract had the same anthelminthic effects (P > 0.05), but 75 mg/ml dilution of the aqueous extract and dilution of 25, 50 and 75 mg/ml of alcoholic extract had more anthelminthic effect (P < 0.05).
Compare to Levamisol 50 mg/dl the anthelminthic effect of aqueous tobacco extract 25 and 50 was the same but aqueous tobacco extract 75 had a higher significant effect and the hazard of death was increased by 1.7×. Table 1 shows the total time, death rate and hazard ratio of aqueous and alcoholic tobacco extract compare to levamisole and PBS on M.marshalli.
Table 1.
Total time at risk, death rate and hazard ratio of different dilution of aqueous and alcoholic tobacco extract on Marshallagia marshalli in compare to levamisole and PBS
| Groups | Total time at risk (min) | Death rate | Hazard Ratio | Confidence level %95 | P Value |
|---|---|---|---|---|---|
| Levamisole 50 | 242 | 0.21 | 1 | – | – |
| Levamisole 5 | 274 | 0.18 | 0.68 | 0.45–0.99 | 0.049 |
| Levamisole 500 | 184 | 0.27 | 2.1 | 1.43–3.16 | <0.001 |
| Aqueous tobacco extract 25 | 269 | 0.19 | 0.69 | 0.46–1.02 | 0.07 |
| Aqueous tobbaco extract 50 | 239 | 0.20 | 0.99 | 0.66–1.46 | 0.94 |
| Aqueous tobacco extract 75 | 201 | 0.25 | 1.7 | 1.15–2.54 | 0.008 |
| Alcholic tobacco extract 25 | 177 | 0.28 | 2.3 | 1.23–3.4 | <0.001 |
| Alcholic tobacco exract 50 | 152 | 0.33 | 3.7 | 2.44–5.5 | <0.001 |
| Alcholic tobacco exract 75 | 114 | 0.44 | 11.1 | 7.09–17.35 | <0.001 |
| PBS | 500 | 0 | 0 | – | – |
The effect of all dilution of alcoholic tobacco extract was higher in compare to levamisole 50. The most effect was for alcoholic tobbaco extract 75 and this was almost higher than levamisole 500 and the effect of alcoholic tobacco extract 50 was similar to levamisole 500.
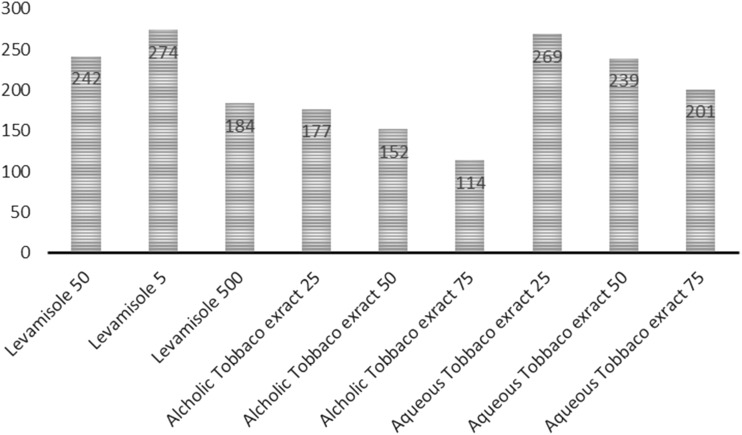
Graph 1 shows the total time at risk for different groups.
Graph 1.
Total time at risk for different aqueous and alcoholic dilution of tobacco extract and different dilution of levamisole
Discussion
Aqueous and alcoholic extracts of Tobacco (Nicotiana tabacum) were screened in vitro for potential anti-parasitic effects against M. marshalli.
The immobility of adult M. marshalli induced by the tobacco extracts appeared to be time dependent and dose dependent. Tobacco alcoholic extract (75 mg/ml) appeared to be the most effect on adult worms.
Tobacco leaf is known to contain nicotine, a ganglion stimulant (Bowman and Rand 1980), which is possibly responsible for the observed anthelmintic activity of the plant extract. Levamisol, used in this study as a standard agent, also stimulates the nicotinic receptors in exhibiting its anthelmintic activity (Neal 2002).Nematode muscles are known to contain excitatory neuromuscular junctions containing ganglion-type nicotinic receptors with acetylcholine as their neurotransmitter (Neal 2002). Any ganglion stimulant would tend to activate these neuromuscular junctions causing a spastic paralysis in the worms leading to their death and expulsion from the host.
We used inhibition of motility of the worms as the criterion for anthelmintic activity. The assay described by Rasoanaivo et al. (1993) where nematode mortality after addition of nematocidal compound or plant extract was assessed. The assay was first standardized using the anthelmintic drug levamisole in place of plant extract. A standard concentration of 5 mg/ml levamisole was used as a control in all subsequent experiments.
Iqbal et al. (2006) showed the aqueous and methanol extracts of Nicotiana tabacum exhibit dose-dependent anthelmintic activity both in vitro and in vivo against gastrointestinal nematodes of sheep.
The emergence of resistance to anthelmintic drugs, which is now a worldwide phenomenon (Jackson and Coop 2000), and the increased awareness about drug residues that potentially enter the food chain have encouraged study into alternatives to commercially anthelmintics, such as medicinal plants.
Although the majority of the evidence on antiparasitic activity of plants has been traditionally based on subjective observations, there are currently an increasing number of controlled experimental studies that aim to verify, validate and quantify in a scientific manner such plant activity.
Traditionally, there are two approaches that have been employed for this purpose. The first one is through offering plants or plant parts to naturally or experimentally infected animals. The second approach is through testing plant extracts and derived from medicinal plants via in vitro systems (Athanasiadou et al. 2007). In vitro assays for screening the anti-parasitic properties of the plants and plant extracts are low costs and rapid turnover, which allow screening large number of plants (Athanasiadou et al. 2007).
Extraction procedures used for this purpose vary from simple water extractions to very complicated ones, where a series of organic solvents is used (Waterman and Mole 1994).
In addition to the antiparasitic and antinutritional effects of medicinal plants on host performance and behaviour, the immunomodulatory effects of plants are another benefit of the use of medicinal plants.
The in vitro methods provide a means to screen rapidly for potential anthelmintic activities of different plant extracts and to analyse the possible mechanisms involved in the interactions between active compounds and parasites.
Overall, these in vitro our results clearly suggest that extracts of Nicotiana tabacum possess appreciable anthelmintic activity. Therefore, its use is justified in the ethno-veterinary medicine practice and this study may be a step forward towards evidence-based indigenous medicine. These effects remain to be confirmed through in vivo studies.
Acknowledgments
The authors would like to thank Mansour Aminzadeh for his technical support and assistance with this project.
References
- Akhtar MS, Iqbal Z, Khan MN, Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rumin. 2000;38(2):99–107. doi: 10.1016/S0921-4488(00)00163-2. [DOI] [Google Scholar]
- Asuzu IU, Onu OU. Anthelmintic activity of the ethanolic extract of Piliostigma thonningii bark in Ascaridia galli infected chickens. Fitoterapia. 1994;65:291–297. [Google Scholar]
- Athanasiadou S, Githiori J, Kyriazakis I. Medicinal plants for helminth parasite control: facts and fiction. Animal. 2007;1(9):1392–1400. doi: 10.1017/S1751731107000730. [DOI] [PubMed] [Google Scholar]
- Bowman WC, Rand MJ. Textbook of pharmacology. Oxford: Blackwell Scientific Publications; 1980. pp. 42.29–42.31. [Google Scholar]
- Budavari S. The Merck Index. 11. Rahway: Merck & Co., Inc.; 1989. [Google Scholar]
- Coop RL, Kyriazakis I. Nutrition-parasite interaction. Vet Parasitol. 1999;84:187–204. doi: 10.1016/S0304-4017(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Edwards G, Breckenridge AM. Clinical pharmacokinetics of anthelmintic drugs. Clin Pharmacokinet. 1988;15:67–93. doi: 10.2165/00003088-198815020-00001. [DOI] [PubMed] [Google Scholar]
- Eslami A, Meydani M, Maleki S, Zargarzadeh A. Gastrointestinal nematodes of wild sheep (Ovis orientalis) from Iran. J Wildl Dis. 1979;15(2):263–265. doi: 10.7589/0090-3558-15.2.263. [DOI] [PubMed] [Google Scholar]
- Farnsworth NR (1990) The role of ethnopharmacology in drug development. In: Bioactive compounds from plants; Ciba Foundation Symposium 154; Wiley, Chichester, pp 2–21 [DOI] [PubMed]
- Githiori JB, Athanasiadou S, Thamsborg SM. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet Parasitol. 2006;139:308–320. doi: 10.1016/j.vetpar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Hammond JA, Fielding D, Bishop SC. Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Commun. 1997;21:213–228. doi: 10.1023/A:1005884429253. [DOI] [PubMed] [Google Scholar]
- Hoberg EP, Kocan AA, Rickard LG. Gastrointestinal strongyles in wild ruminants. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wild mammals. Ames: Iowa State University Press; 2001. pp. 193–227. [Google Scholar]
- International Institute of Rural Reconstruction . Ethnoveterinary medicine in Asia: an information kit on traditional animal health care practices. 2. Cavite: International Institute of Rural Reconstruction (IIRR); 1994. [Google Scholar]
- Iqbal Z, Lateef M, Jabbar A, Ghayur MN, Gilani AH. In vitro and In vivo anthelmintic activity of Nicotiana tabacum L. leaves against gastrointestinal nematodes of sheep. Phytother Res. 2006;20:46–48. doi: 10.1002/ptr.1800. [DOI] [PubMed] [Google Scholar]
- Jackson F, Coop RL. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120(07):95–107. doi: 10.1017/S0031182099005740. [DOI] [PubMed] [Google Scholar]
- Kuhn H (1965) Tobacco alkaloids and their pyrolysis products in the smoke. In: Tobacco alkaloids and related compounds. Macmillan, Stockholm, pp 37–51
- Lal J, Chandra S, Prakash VR, Sabir M. In vitro anthelmintic action of some indigenous medicinal plants on Ascaridia galli worms. Indian J Physiol Pharmacol. 1976;20:64–68. [PubMed] [Google Scholar]
- Lans C. Validation of ethnoveterinary medicinal treatments. Vet Parasitol. 2011;178(3–4):389–390. doi: 10.1016/j.vetpar.2010.12.046. [DOI] [PubMed] [Google Scholar]
- Lorimer SD, Perry NB, Foster ML, Burgess EJ. A nematode larval motility inhibition assay for screening plant extracts and natural products. J Agric Food Chem. 1996;44:2842–2845. doi: 10.1021/jf9602176. [DOI] [Google Scholar]
- Mathias E. Ethnoveterinary medicine: harnessing its potential. Vet Bull. 2004;74(8):27N–37N. [Google Scholar]
- Nadkarni KM. Indian materia medica. Popular Prakashan: Bombay; 1976. pp. 850–854. [Google Scholar]
- Neal MJ. Medical pharmacology at a glance. Oxford: Blackwell Science; 2002. pp. 88–89. [Google Scholar]
- Orloff IW. Sur la reconstruction de la syste´matique du genre Ostertagia Ransom, 1907. Ann de Parasitol. 1933;11:96–111. doi: 10.1051/parasite/1933112096. [DOI] [Google Scholar]
- Prichard RK. Anthelmintic resistance in nematodes: extent, recent understanding and future directions for control and research. Int J Parasitol. 1990;20:515–523. doi: 10.1016/0020-7519(90)90199-W. [DOI] [PubMed] [Google Scholar]
- Rasoanaivo P, Ratsimamanga-Urverg S, Scott G (1993) Biological evaluation of plants with reference to the Malagasy flora. Napreca, Madagascar, pp 72–83
- Soulsby EJL. Helminths arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. pp. 231–250. [Google Scholar]
- Souto WMS, Mourão JS, Barboza RRD, Mendonça LET, Lucena RFP, Confessor MVA, Vieira WLS, Montenegro PFGP, Lopez LCS, Alves RRN. Medicinal animals used in ethnoveterinary practices of the ‘Cariri Paraibano’, NE Brazil. J Ethnobiol Ethnomed. 2011;7(30):1–46. doi: 10.1186/1746-4269-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow JL, Johnson L. Nature’s medicine, plants that heal. Willard: National Geographic Society, RR Donnelley and Sons; 2000. p. 400. [Google Scholar]
- Tabuti JR, Dhillion SS, Lye KA. Ethnoveterinary medicines for cattle (Bos indicus) in Bulamogi county, Uganda: plant species and mode of use. J Ethnopharmacol. 2003;88(2–3):279–286. doi: 10.1016/S0378-8741(03)00265-4. [DOI] [PubMed] [Google Scholar]
- Waller P, Bernes G, Thamsborg SM, Sukura A, Richter SH, Ingebrigsten K, Hoglund J. Plants as deworming agents of livestock in the Nordic countries: historical perspective, popular beliefs and prospects for the future. Acta Vet Scand. 2001;42:31–44. doi: 10.1186/1751-0147-42-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman PG, Mole S. Extraction and chemical quantification. In: Lawton JH, Likens GE, editors. Analysis of phenolic plant metabolites. Oxford: Blackwell Scientific Publication; 1994. pp. 66–103. [Google Scholar]
- Zenner L, Callait MP, Granier C, Chauve C. In vitro effect of essential oils from Cinnamomum aromaticum, citrus lemon and Allium sativum on two intestinal flagellates of poultry, Tetratrichomonas gallinarum and Histomonas meleagridis. Parasite. 2003;10:153–157. doi: 10.1051/parasite/2003102153. [DOI] [PubMed] [Google Scholar]