Abstract
Fasciolosis is of considerable economic and public health importance worldwide. Little information is available on the ovicidal effects of anthelminthic drugs. The use of ovicidal anthelmintics can be effective in disease control. In this study, the effectiveness of the methanolic extract of ginger (Zingiber officinale) on the eggs of Fasciola hepatica is investigated. Fasciola hepatica eggs were obtained from the gall bladders of naturally infected sheep and kept at 4 °C until use. The eggs were exposed to varying concentrations of ginger extract (1, 5, 10, 25 and 50 mg/mL) for 24, 48 and 72 h. To investigate the effect of the ginger extracts on the miracidial formation, the treated eggs were incubated at 28 °C for 14 days. The results indicated that F. hepatica eggs are susceptible to the methanolic extract of Z. officinale. The ovicidal effect of ginger extract at a concentration of 1 mg/mL with 24, 48 and 72 h treatment time was 46.08, 51.53 and 69.09 % respectively (compared with 22.70 % for control group). The ovicidal effect of ginger extract at a concentration of 5 mg/mL after 24 h was 98.84 %. One hundred percent ovicidal efficacy was obtained through application of ginger extract at concentrations of 5 and 10 mg/mL with a 48 and 24 h treatment time respectively. The in vitro ovicidal effect of the methanolic extract of Z. officinale was satisfactory in this study, however, in vivo efficacy of this extract, remains for further investigation. To the best of our knowledge, this is the first report on the ovicidal effect of Z. officinale against F. hepatica eggs.
Keywords: Fasciola hepatica, Ovicidal, Methanolic extract, Ginger, Zingiber officinale
Introduction
Fascioliasis is a parasitic disease caused by liver fluke species of the genus Fasciola, with Fasciola hepatica as the main cause because of its very wide distribution in Europe, Africa, Asia, Oceania and the Americas. The disease is of well-known veterinary importance and is an increasing human health problem, with reported cases in the five continents (Mas-Coma and Bargues 1997). Eradication of fasciolosis is rarely a practical option and control needs to be focused on reduction of the disease (Torgerson and Claxton 1999). Fasciolosis control is almost exclusively carried out by strategic applications of a wide number of effective anthelmintics (Sanchez-Andrade et al. 2001). Only triclabendazole is efficacious against both pre-adults in hepatic parenchyma and adults in the bile ducts (Boray et al. 1983; Perez et al. 2005). However, the effectiveness of triclabendazole represents problems of resistance (Fairweather and Boray 1999; Robinson et al. 2002; Fairweather 2005; Fairweather 2009). The risk of appearance of resistance to triclabendazole cannot be ignored, taking into account: (i) the long-term veterinary use of triclabendazole for livestock treatment in endemic areas; (ii) the tradition of human self treatments with triclabendazole owing to the very general availability of this drug; and (iii) the recent appearance of triclabendazole resistance in the Old World (Mas-Coma et al. 2005). New effective alternative treatment is extremely important in today’s climate where species are becoming resistant and there has been a resurgence in the use of natural alternative therapies instead of synthetic pharmaceuticals that often have severe side effects (Harris et al. 2000).
Zingiber officinale Roscoe, commonly called ginger, is a perennial plant with narrow, bright green, grass-like leaves and yellowish green flowers with purple markings. Ginger is cultivated in the tropics for its edible rhizome at approximately 10 months of age, with the root stocks serving a variety of purposes, including culinary and medicinal (Grant 2000). Z. officinale is widely distributed throughout the tropical and subtropical regions, particularly on the West coast, South, North and Central India. It is cultivated on a large scale in India, Bangladesh, Taiwan, Nigeria, Sri Lanka and East Asiatic countries. Rhizomes are aromatic, thick lobed and pale yellowish. The family Zingiberaceae contains a variety of compounds which have shown insecticidal, oviposition, antifeedant, growth regulating, reducing fecundity, development modifying properties and repellent activity against many tested insects (Abdul Rahuman et al. 2008).
Scientific reports show that Z. officinale has carminative, antipyretic, anticancer, cardio tonic, antispasmodic, antidiabetic, antioxidant and antihepatotoxic activities. It also ameliorates motion sickness and is a known thromboxane synthesis and platelet aggregation inhibitor and diaphoretic agent (Lakshmi and Sudhakar 2010). Except for albendazole, which showed excellent ovicidal activity against F.hepatica (Alvarez et al. 2009), ivermectin and artemether (Diab et al. 2009) have been shown to have some ovicidal activity against F. hepatica eggs, but to our knowledge, no data on the ovicidal effect of triclabendazole and other antihelmintic drugs against F.hepatica eggs is available. The main goal of the current work was to evaluate the potential of the methanolic extract of this herbal plant to inhibit the miracidial development inside F. hepatica eggs.
Materials and methods
Bioassay
Fasciola hepatica eggs were exposed to ginger extract for 24, 48 and 72 h. Five concentrations (1, 5, 10, 25 and 50 mg/mL) of the ginger extract were used in this study. The experiments were performed at 37 °C (close to the normal body temperature for sheep). To determine the ovicidal activity of ginger extract, the treated eggs were incubated at 28 °C for 14 days and miracidial formation was investigated under a light microscope.
Preparation of F. hepatica eggs
Gall bladders of sheep naturally infected with F. hepatica were obtained from local abattoirs. The gall bladders were taken to the laboratory within 2 h in the sterile glass containers. The bile was aseptically transferred into glass cylinders and left to set for 30 min. The eggs settled at the bottom of the cylinders. The supernatant was then removed and the yielded eggs were washed several times using normal saline; the eggs were finally transferred into a dark glass container containing normal saline and stored at 4 °C for further use.
Preparation of ginger extract
The fresh rhizomes of ginger were purchased from a local herbal market in Shiraz (Iran). The rhizomes were sliced, dried under shade for one week and powdered mechanically using a commercial electric blender. To obtain the methanolic extract, 100 g of dry ginger powder was added to 400 mL of pure methanol and mixed gently for 1 h using a magnetic stirrer. The obtained solution was left at room temperature for 24 h. The solution was stirred again and filtered using filter paper (Grade 1 Whatman cellulose filter papers, Balstone, UK) and the solvent was then removed by evaporation in a rotary evaporator. The remaining semisolid material was then freeze-dried. The obtained residue (4 g) was placed into a sterile glass container and stored at 4 °C for further use (Moazeni and Nazer 2010). To prepare the ginger extract solution at 1, 5, 10, 25 and 50 mg/mL concentrations, 0.01, 0.05, 0.1, 0.25 and 0.5 g of dried extract was dissolved in 10 mL of distilled water, respectively.
Ovicidal test
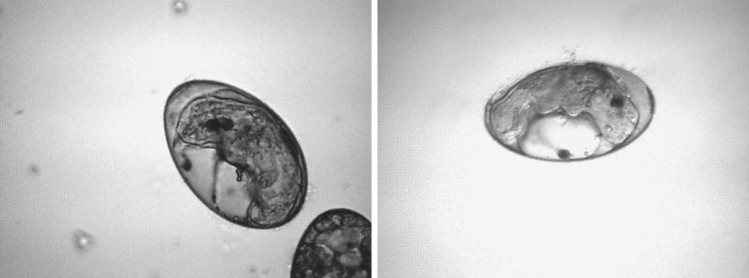
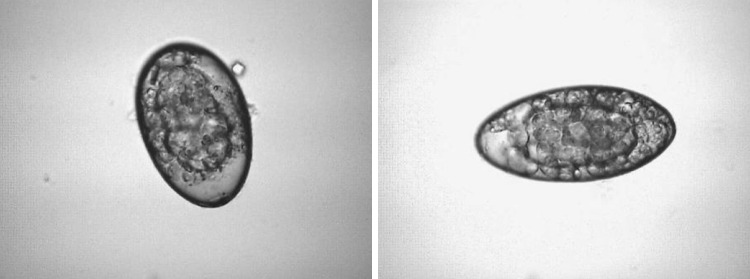
In this study, F.hepatica eggs were exposed to different concentrations (1, 5, 10, 25 and 50 mg/mL) of ginger extract at various times (24, 48 and 72 h). In each experiment a drop of egg rich sediment containing at least 1,500 eggs was added to a test tube containing 10 mL of ginger extract. The tubes were then incubated at 37 °C for 24, 48 and 72 h. Then, 9 mL of the upper part of the ginger solution was removed with a pipette, avoiding the settled eggs. Following several washes, the eggs of each tube were transferred into special small plastic containers (Supa Industries, Iran) containing 5 mL dechlorinated tap water. The containers were then incubated at 28 °C for 14 days. At the same time, one container at least 3,000 eggs with no exposure to ginger extract was also incubated at 28 °C as a control group. At the end of the incubation time, the eggs were smeared on a manually scaled glass slide, covered with a cover glass, and examined under a light microscope. The ovicidal activity of the ginger extract was determined by counting a minimum of 1,000 eggs (usually more than 1,500) in each experiment. The eggs were divided into three groups: containing live miracidia (Fig. 1), containing developing cells (Fig. 2) and the dead eggs (Fig. 3).
Fig. 1.
Eggs containing live miracidia
Fig. 2.
Developing eggs
Fig. 3.
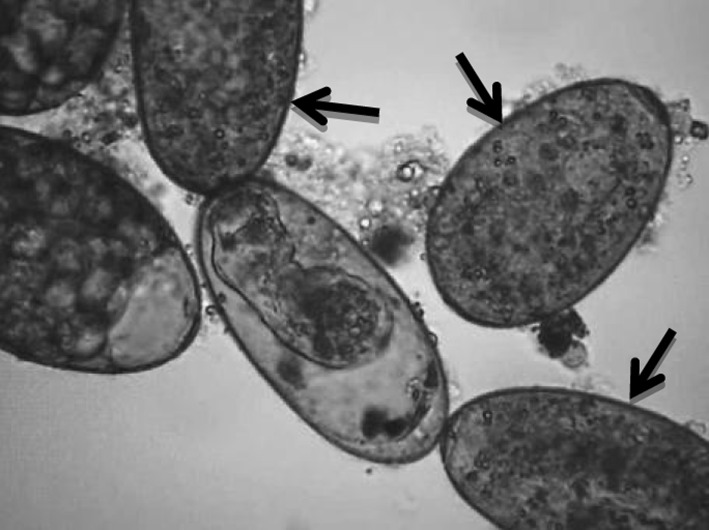
Dead eggs (arrows)
Statistical analysis
Differences between the rate of miracidial formation in the eggs treated with ginger extract and the untreated (control group) eggs were analyzed with χ2 test. Statistical analysis was performed with GraphPad InStat software and P values less than 0.01 were considered to be significant.
Results
The results of the microscopic observations of the eggs treated with ginger extract at different concentrations and various exposure times (24, 48 and 72 h) are presented in Tables 1, 2, 3 respectively. The miracidial formation obtained for the untreated eggs (control group) was 68.95 %. As shown in Table 1, following 24 h of exposure, the miracidial formation obtained for the eggs treated with ginger extract at concentrations of 1 and 5 mg/mL was 52.28 % and 1.16 %respectively. In this exposure time, the death rate in the eggs treated with ginger extract at concentrations of 10, 25 and 50 mg/mL was 100 %. In this exposure time (24 h), the ovicidal activity in the treated eggs, at all concentrations was increased significantly compared to the control group (P < 0.01). Following 48 h of exposure, the miracidial formation was slightly reduced (47.82 %) by ginger extract at a concentration of 1 mg/mL as compared with the control group (68.95 %) but no miracidial formation was observed in the eggs treated with ginger extract at concentrations of 5, 10, 25 and 50 mg mL−1(Table 2).
Table 1.
In vitro ovicidal effects of different concentrations of Zingiber officinale extract on F. hepatica eggs after 24 h of exposure
| Concentration of ginger extract (mg/ml) | Examined eggs | Miracidial formation after 14 days | ||
|---|---|---|---|---|
| Eggs containing live miracidia | Eggs containing no miracidia | |||
| Developing eggs | Dead eggs | |||
| 1 | 1,226 | 641 (52.28 %) | 20 (1.63 %) | 565 (46.08 %) |
| 5 | 1,546 | 18 (1.16 %) | 0 | 1,528 (98.84 %) |
| 10 | 1,472 | 0 | 0 | 1,472 (100 %) |
| 25 | 1,502 | 0 | 0 | 1,502 (100 %) |
| 50 | 1,125 | 0 | 0 | 1,125 (100 %) |
| 0 (Control) | 2,731 | 1,883 (68.95 %) | 228 (8.34 %) | 620 (22.70 %) |
Table 2.
In vitro ovicidal effects of different concentrations of Zingiber officinale extract on F. hepatica eggs after 48 h of exposure
| Concentration of ginger extract (mg/ml) | Examined eggs | Miracidial formation after 14 days | ||
|---|---|---|---|---|
| Eggs containing live miracidia | Eggs containing no miracidia | |||
| Developing eggs | Dead eggs | |||
| 1 | 1,240 | 593 (47.82 %) | 8 (0.64 %) | 639 (51.53 %) |
| 5 | 1,609 | 0 | 0 | 1,609 (100 %) |
| 10 | 1,703 | 0 | 0 | 1,703 (100 %) |
| 25 | 1,447 | 0 | 0 | 1,447 (100 %) |
| 50 | 1,532 | 0 | 0 | 1,532 (100 %) |
| 0 (Control) | 2,731 | 1,883 (68.95 %) | 228 (8.34 %) | 620 (22.70 %) |
Table 3.
In vitro ovicidal effects of different concentrations of Zingiber officinale extract on F. hepatica eggs after 72 h of exposure
| Concentration of ginger extract (mg/ml) | Examined eggs | Miracidial formation after 14 days | ||
|---|---|---|---|---|
| Eggs containing live miracidia | Eggs containing no miracidia | |||
| Developing eggs | Dead eggs | |||
| 1 | 1,330 | 411 (30.90 %) | 0 | 919 (69.09 %) |
| 5 | 1,733 | 0 | 0 | 1,733 (100 %) |
| 10 | 1,905 | 0 | 0 | 1,905 (100 %) |
| 25 | 1,940 | 0 | 0 | 1,940 (100 %) |
| 50 | 1,391 | 0 | 0 | 1,391 (100 %) |
| 0 (Control) | 2,731 | 1,883 (68.95 %) | 228 (8.34 %) | 620 (22.70 %) |
Following 72 h of exposure, the miracidial formation obtained for the treated eggs at a concentration of 1 mg/mL was 30.90 %, but no miracidial formation was observed in the eggs treated with ginger extract at concentrations of 5, 10, 25 and 50 mg mL−1 (Table 3). In this exposure time (72 h), the difference in the rate of miracidial formation between the eggs treated with ginger extract at a concentration of 1 mg/mL and the untreated eggs (control group) was extremely significant (P < 0.01).
Discussion
Several studies have been conducted to evaluate the ovicidal activity of essential oils or the major components of herbal plants against many insect species (Shaaya et al. 1993; Ho et al. 1997; Huang et al. 1997; Obeng-Ofori et al. 1997; Obeng-Ofori and Reichmuth 1997; Soliman and Tewfieck 1999; Tunc et al. 2000). Some authors have described the ovicidal activity of various herbal plants against Haemonchus contortus. In an in vivo study of gastrointestinal nematode-infected sheep, the papaya seed extracts induced 80 % of egg hatching reduction on H. contortus (Hounzangbe-Adote et al. 2001). Costa et al. (2002) have reported that the seed ethanol extract of Mangifera indica revealed 91 % effective inhibition of egg hatching at 10 mg/mL on H. contortus. The seed aqueous and hydroalcoholic crude extracts of Melia azedarach showed 100 % inhibition of egg hatching at 12.5 mg/mL against H. contortus (Kamaraj and Abdul Rahuman 2011). To the best of our knowledge, no data on the ovicidal effect of herbal plants against F. hepatica eggs is available. In this study, the effectiveness of the methanolic extract of Z. officinale on F. hepatica eggs was investigated.
According to the results of the present study, the ginger extract at a concentration of 1 mg/mL, after 24, 48 and 72 h, reduced the miracidil formation rate of F. hepatica eggs to 52.28, 47.82 and 30.90 % respectively (compared with 68.95 % for control group). 5 mg/mL concentration of the extract reduced the miracidial formation rate of the eggs to 1.16 % with 24 h treatment time. However, with this exposure time, the 10 mg/mL concentration of ginger extract reduced the miracidial formation rate to 0 %. The same efficacy with a concentration of 5 mg/mL was obtained with 48 h exposure time. Our results indicated that F. hepatica eggs are susceptible to the methanolic extract of Z. officinale, and the ovicidal effect of ginger extract at a concentration of 1 mg/mL after 24, 48 and 72 h was 46.08, 51.53 and 69.09 % respectively (compared with 22.70 % for control group).The ovicidal effect of ginger extract at a concentration of 5 mg/mL and 24 h treatment time was 98.84 %. One hundred percent ovicidal efficacy was obtained through application of ginger extract at concentrations of 10 and 5 mg/mL with 24 and 48 h treatment time respectively.
Further chemical studies are needed to isolate the organic compounds responsible for ovicidal activities presented in the rhizomes of Z. officinale. However, in vivo ovicidal efficacy of ginger extract requires further investigation as well. Based on the results presented in this work, Z. officinale offers an opportunity for new compounds. In fact, the potential flukicidal activity of the methanolic extract of Z. officinale has not yet been investigated, and this compound probably has some pharmacological activity against adult F. hepatica. Further studies should also focus on lower concentrations of this extract.
Acknowledgments
The authors are thankful to Shiraz University for providing financial assistance (Grant number 87-GR-VT-24). We kindly thank Mr. A. Mootabi Alavi for his kind assistance in performing this study.
Contributor Information
Mohammad Moazeni, Phone: 0098 711 2286950, Email: moazeni@shirazu.ac.ir.
Ali Asghar Khademolhoseini, Email: alikhdem67kh@gmail.com.
References
- Abdul Rahuman A, Gopalakrishnan G, Venkatesan P, Kannappan G, Bagavan A. Mosquito larvicidal activity of isolated compounds from the rhizome of Zingiber officinale. Phytother Res. 2008;22:1035–1039. doi: 10.1002/ptr.2423. [DOI] [PubMed] [Google Scholar]
- Alvarez L, Moreno G, Moreno L, Ceballos L, Shaw L, Fairweather I, Lanusse C. Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet Parasitol. 2009;164:211–216. doi: 10.1016/j.vetpar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Boray JC, Crowfoot PD, Strong MB, Allison JR, Shellenbaum M, Van Orelli M, Sarasin G. Treatment of immature and mature Fasciola hepatica infections in sheep with triclabendazole. Vet Rec. 1983;113:315–317. doi: 10.1136/vr.113.14.315. [DOI] [PubMed] [Google Scholar]
- Costa CTC, Morais SM, Bivilaqua CML, Souza MMC, Leite FKA (2002) Ovicidal effect of Mangifera indica L. seeds extracts on Haemonchus contortus. Brazil J Vet Parasitol 11:57–60
- Diab TM, Mansour HH, Mahmoud SS. Fasciola gigantica: parasitological and scanning electron microscopy study of the in vitro effects of ivermectin and/or artemether. Exp Parasitol. 2009;124(3):279–284. doi: 10.1016/j.exppara.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Fairweather I. Triclabendazole: new skills to unravel an old (ish) enigma. J Helminthol. 2005;79:227–234. doi: 10.1079/JOH2005298. [DOI] [PubMed] [Google Scholar]
- Fairweather I. Triclabendazole progress report 2005–2009: an advancement of learning? J Helminthol. 2009;83:139–150. doi: 10.1017/S0022149X09321173. [DOI] [PubMed] [Google Scholar]
- Fairweather I, Boray JC. Fasciolicides: efficacy actions resistance and its management. Vet J. 1999;158:81–112. doi: 10.1053/tvjl.1999.0377. [DOI] [PubMed] [Google Scholar]
- Grant KI. Ginger. Am J Health-Syst Pharm. 2000;57:945–947. doi: 10.1093/ajhp/57.10.945. [DOI] [PubMed] [Google Scholar]
- Harris JC, Plummer S, Turner MP, Lloyd D. The microaerophilic flagellate Giardia intestinalis: allium sativum (garlic) is an effective antigiardial. Microbiology. 2000;146:3119–3127. doi: 10.1099/00221287-146-12-3119. [DOI] [PubMed] [Google Scholar]
- Ho SH, Ma Y, Huang Y. Anethole a potential insecticide from Illicium verum Hook F against two stored-product insects. Int Pest Control. 1997;39:50–51. [Google Scholar]
- Hounzangbe-Adote MS, Zinsou FE, Affognon KJ, Koutinhouin B, Adamou-N’Diaye M, Moutaeirou K. Efficacite antiparasitaire de la poudre des graines de papaye (Carica papaya) surles strongles gastro intestinaux des moutons Djallonke au sud du Benin. Rev Elev Med Vet Pays Trop. 2001;54:225–229. doi: 10.19182/remvt.9778. [DOI] [Google Scholar]
- Huang Y, Tan JMWL, Kini RM, Ho SH. Toxic and antifeedant action of nutmeg oil against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J Stored Prod Res. 1997;33:289–298. doi: 10.1016/S0022-474X(97)00009-X. [DOI] [Google Scholar]
- Kamaraj C, Abdul Rahuman A. Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus. Res Vet Sci. 2011;91:400–404. doi: 10.1016/j.rvsc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Lakshmi BVS, Sudhakar M. Attenuation of acute and chronic restraint stress-induced perturbations in experimental animals by Zingiber officinale Roscoe. Food Chem Toxicol. 2010;48:530–535. doi: 10.1016/j.fct.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Bargues MD. Human liver flukes: a review. Re Rev Parasitol. 1997;57:145–218. [Google Scholar]
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Moazeni M, Nazer A. In vitro Effectiveness of Garlic (Allium sativum) Extract on Scolices of Hydatid Cyst. World J Surg. 2010;34:2677–2681. doi: 10.1007/s00268-010-0718-7. [DOI] [PubMed] [Google Scholar]
- Obeng-Ofori D, Reichmuth Ch. Bioactivity of eugenol a major component of Ocimum suave (Wild) against four species of stored product Coleoptera. Int J Pest Manage. 1997;43:89–94. doi: 10.1080/096708797229040. [DOI] [Google Scholar]
- Obeng-Ofori D, Reichmuth Ch, Bekele J, Hassanali A. Biological activity of 18 cineole a major component of essential oil of Ocimum kenyense (Ayobaugira) against stored product beetles. Int J Pest Manage. 1997;121:237–243. [Google Scholar]
- Perez J, Ortega J, Baravo A, Diez-Banos P, Morrondo P, Moreno T, Martinez-Moreno A. Phenotype of hepatic infiltrates and hepatic lymph nodes of lambs primarily and challenge infected with Fasciola hepatica with and without triclabendozole treatment. Vet Res. 2005;36:1–12. doi: 10.1051/vetres:2004047. [DOI] [PubMed] [Google Scholar]
- Robinson MW, Trudgett A, Hoey EM, Fairweather I. Triclabendazole resistant Fasciola hepatica: β-tubulin and response to in vitro treatment with triclabendazol. Parasitology. 2002;124:325–338. doi: 10.1017/S003118200100124X. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade R, Paz-Silva A, Suarez JL, Panadero R, Pedreira I, Diez-Banos P, Morrondo P. Effect of Fasciolicides on the antigenaemia in sheep naturally infected with Fasciola hepatica. Parasitol Res. 2001;87:609–614. doi: 10.1007/s004360100425. [DOI] [PubMed] [Google Scholar]
- Shaaya E, Ravid U, Paster N, Kostjukovsky M, Menasherov M, Plotkin S. Essential oils and their components as active fumigants against several species of stored product insects and fungi. Acta Horticulturae. 1993;344:131–137. doi: 10.17660/ActaHortic.1993.344.16. [DOI] [Google Scholar]
- Soliman BA, Tewfieck MA. Activity and efficacy of azadirachtin (Neem product) on the eggs of the filarial vector Culex pipiens (Diptera: culicidae) J Union Arab Biol Cairo. 1999;12:33–41. [Google Scholar]
- Torgerson P, Claxton J (1999) Epidemiology and control In: Dalton EJP (Ed) Fasciolosis. CABI Publishing, Wallingford, pp 113–149
- Tunc IB, Berger BM, Erler F, Dagli F. Ovicidal activity of essential oils from five plants against two stored-product insects. J Stored Prod Res. 2000;36:161–168. doi: 10.1016/S0022-474X(99)00036-3. [DOI] [Google Scholar]