Abstract
Leishmania infantum is a causative agent of visceral leishmaniasis or kala-azar, which is endemic in some part of Iran. Azarshahr city located in East Azerbaijan province, North West of Iran, which is endemic for visceral leishmaniasis. This study aimed to investigate the possible reservoir role of cats for visceral leishmaniasis in the Azarshahr area. Totally 65 cats have been trapped alive from villages of Azarshahr county and their serum samples subjected to direct agglutination test (DAT) for L. infantum antibodies. Giemsa stained impression smears have been prepared for parasitological examination of spleen and liver tissue. Also liver and spleen samples of the cats have been cultured in Novy-MacNeal-Nicolle (NNN) medium and also used for PCR. None from 65 samples was positive in NNN culture, PCR and microscopic examination. Fifteen (23.07 %) out of 65 serum samples showed Leishmania specific antibody agglutination at 1:320 dilution or above, but all considered as negative because none of them confirmed by Giemsa stained smears, PCR and NNN culture. According to the findings of the present study, cats are not a reservoir for visceral leishmaniasis in the Azarshahr area.
Keywords: Cat, Visceral leishmaniasis, Leishmania infantum, Iran
Introduction
Leishmaniasis is a disease caused by a genus of protozoan parasite called Leishmania (Kinetoplastida, Trypanosomatidae). The members of the genus are transmitted between female sand flies (Diptera, Psychodidae) and vertebrate hosts (Ready 2014; World Health Organization 2010a.). Mammals can be infected by almost 20 species of Leishmania and many cause human leishmaniasis. Leishmania donovani is causative agent of Indian visceral leishmaniasis (VL), which is considered as anthroponotic (Ready 2013). Also L. infantum causes VL in Mediterranean basin, which is regarded as zoonotic leishmaniasis (ZCL) (Mohebali 2013; Ready 2010). In most parts of the old world Phlebotomus spp. is responsible for transmission of the ZCL (Ready 2013).
Various Leishmania species can cause VL, which is also called kala-azar (World Health Organization 2010b). Domestic dogs (Canis familiaris) and also wild Canids are the main reservoir hosts for L. infantum in Iran (Mohebali 2013 ). It is estimated that each year about 0.2–0.4 million VL occurs worldwide. Six countries are subjected to the most of the cases of VL, including: India, Sudan, Bangladesh, South Sudan, Brazil and Ethiopia. Altogether, it is estimated that leishmaniasis is responsible for about 20,000–40,000 deaths each year (Alvar et al. 2012).
In Iran, both cutaneous leishmaniasis (CL) and VL are present. CL is accounts for about 20,000 new cases each year, but VL has been sporadically reported throughout Iran. Also we should keep mined that VL is endemic in some areas in Iran such as northwest and south part of the country, account for 100–300 new cases per year (Edrissian et al. 1998; Mohebali 2013).
In Iran infection with L. infantum was reported in desert rodents (gerbils), cats and also in a wolf in northwest of Iran, which is an endemic for canine and human VL (Hatam et al. 2010; Mohebali 2013). L. infantum can infect jackals, wolves and foxes and consequently they may play as secondary reservoirs for human infection in endemic areas, especially in mountainous regions where the sylvatic cycle of VL present (Mohebali 2013).
Cats are very close animals to human population, and most people keep them as pets. The first reports of leishmaniasis of cats (Felis catus) have been described in Algeria in 1912 (Maia and Campino 2011). Also cats are reported to be infected by L. infantum causing VL in Brazil, Middle East and Europe (da Silva et al. 2008; Maia and Campino 2011; Maia et al. 2010; Maia et al. 2008; Martin-Sanchez et al. 2007; Savani et al. 2004; Silva Rde et al. 2014; Solano-Gallego et al. 2007). The role of cat for being reservoir of VL is not completely clear and it is referred as a potential reservoir (Maia and Campino 2011).
As far as the authors knowledge, in Iran the first and only report of L. infantum in cats reported from two endemic areas of VL, Azarshahr district in northwestern and Fars Province in southern Iran (Hatam et al. 2010). Azarshahr is a city located in the East Azerbaijan province, north west of Iran, where VL is endemic (Mirsamadi et al. 2003). Considering the fact that there is a large number of stray cats in or around the cities and villages, it seems necessary to determine the epidemiology of feline VL and its role in transmission of VL to human population in each endemic areas of the country (Mohebali 2013).
The present study aimed to investigate the prevalence of feline VL among cats trapped from Azarshahr district, East Azerbaijan province, north west of Iran in 2013.
Materials and Method
Studied population and sampling
In this cross sectional study, 65 cats from Azarshahr city and nearby villages have been trapped alive and were anaesthetized using chloroform. Then autopsies performed in sterile condition. During autopsies, blood sample were collected directly from their heart, transferred to tubes and centrifuged at 1500 rpm for 5 min. The Sera were kept at -20 °C for serological examination.
Microscopic examination
Tissue samples from spleen and liver of the cats have been taken for microscopic examination using impression technique. The air dried impression smears fixed by methanol and then stained with Giemsa’s stain. The prepared microscopic slides examined by light microscope for presence of Leishmania parasite.
Leishmania culture in NNN
A piece of liver and spleen of the cats were excised; homogenized and cultured on Novy-MacNeal-Nicolle (NNN) medium. The cultured media were incubated at 24 ± 1 °C for 48 h. After 48 h of incubation the media have been examined under light microscope using 40 × objective for presence of mobile promastigotes. In negative cultures incubation process have been continued for two weeks and every 48 h they have been checked for the presence of parasite growth. The culture results were considered as negative when the last examination of media confirmed the absence of parasite growth.
Antigen preparation
In the present study L. infantum antigens has been produced in the Protozoology Laboratory of School of Public Health in Tehran University of Medical Sciences. The DAT antigen was prepared by mass production of L. infantum LON-49 promastigotes in RPMI1640 plus 10 % fetal bovine serum medium. The brief procedure of antigen preparation is as following; The promastigotes were treated with trypsin and after 5 times of washing process of the parasites, they fixed with 2 % formaldehyde, stained with comassie brilliant blue (0.1 % [wt/vol]) and finally washed two times in citrate-saline. The prepared antigen kept at 4 °C until it needed (el Harith et al. 1989).
Direct agglutination test (DAT)
In this study, because no standardized DAT for feline VL was available, we used the protocol of DAT for canine VL. The serum samples of cats have been underwent DAT using the method described by el Harith et al. (1989). The test performed as the following procedure; the sera were treated with 2-ME for 1 h at 37 °C, twofold serial dilutions were prepared using gelatin (50 µl per each well) with initial dilution at 1:10. Then 50 µl of antigen added to each wells and incubated at room temperature for 18 h and then the results were recorded (el Harith et al. 1989). A positive result of Leishmania antibody in DAT test for canine VL suggested at 1:320 and above (el Harith et al. 1989; Mohebali et al. 2004), but as far as authors' knowledge no data is available for feline VL.
DNA extraction
The spleen tissue samples subjected to DNA extraction for PCR. DNA extraction has been performed by BL DNEX 5050 kit (Pak Gen Yakhteh Co LTD). The extraction procedure has done according to the manufacturers’ instruction. Extraction mechanism of the kit is based on the lysis of the cells and precipitation of DNA by mineral salts. Proteinase K is used for removing proteins from the mixture.
Polymerase Chain Reaction (PCR)
The region of L. infantum mitochondrial DNA was amplified by simple PCR using pair of following primers, F 5′-CGCAGAACGCCCCTA-3′, R 5′-GGGGTTGGTCTAAAATAGG-3′ (Bioneer® Korea). The primers that used in this study were obtained from the report of Mahboudi et al. (2001); (Mahboudi et al. 2001) with some modifications in order to detect both L. infantum and L. tropica in the tissue samples.
The PCR procedure performed with master mix containing 10X PCR buffer (10 µl), MgCl2 (5 µl), forward and reveres primers (2 µl), dNTPs MIX(2 Mm) (2 µl), DNA template (3 µl), Taq DNA polymerase 2 U/µl (1.3 µl), DNase/RNase-Free distilled water (38 µl). PCR reaction performed under the following thermal cycling condition: 5 min of initial DNA denaturation at 94 °C, 35 cycles of denaturation for 1 min at 94 °C, 50 s of annealing at 65 °C, 1 min of extension at 72 °C and 4 min of final extension at 72 °C.
The products of PCR, 50 bp DNA ladder (Fermentas, Korea), positive (L. tropica and L. infantum) and negative controls underwent electrophoresis for 2 h (80 volts) in 1 % agarose gel. Ethidium bromide was used for staining of the gels. The stained gels studied by transilluminator device under UV light (Fig. 1).
Fig. 1.
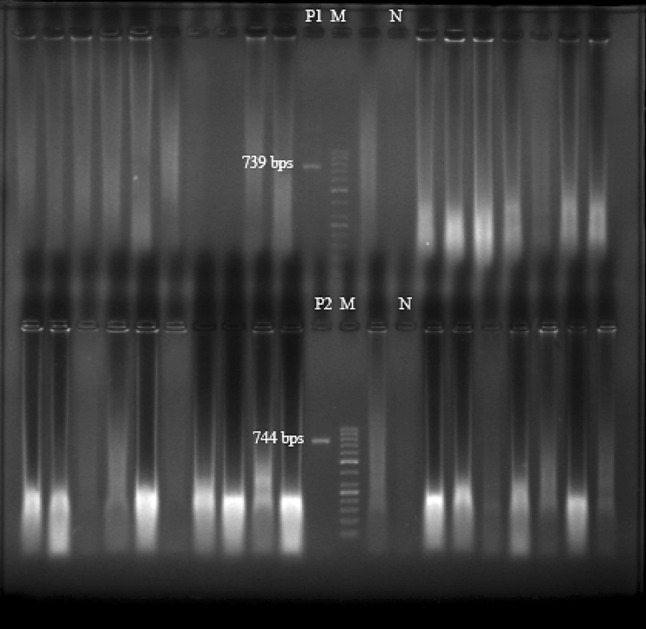
Gel electrophoresis of the liver and spleen samples of the studied cats with L. infantum and L. tropica positive controls. M: 50 bp DNA ladder; N: negative control; P1: L. infantum positive control; P2: L. tropica positive control. The other lanes are the spleen and liver sample of the cats
Results
Totally 65 cats were hunted and studied for L. infantum infection using four methods, namely: PCR, DAT, NNN culture and tissue touch smear for microscopic examination. None of the Giemsa stained microscopic slides of spleen and liver of the 65 cats were positive for the Leishmania spp. Also no leishmanial growth has been observed in NNN media (Table 1). Furthermore, L. infantum DNA has not been detected in the spleen samples of the cats. Altogether, none of the 65 studied cats were positive about L. infantum infection by PCR, NNN culture and microscopic methods.
Table 1.
Detected L. infantum infection among studied cats by 4 used methods
| NNN culture | Microscopy | PCR | DAT agglutination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cats | + | − | + | − | + | − | 1:80 | 1:160 | 1:320 | 1:640 | No agglutination |
| Frequency | 0 | 65 | 0 | 65 | 0 | 65 | 4 | 9 | 14 | 1 | 37 |
Fifteen (23.07 %) out of 65 serum samples showed Leishmania specific antibody agglutination at 1:320 dilution or above. If we consider agglutination at 1:320 titer or higher as a positive result as described by Harith et al. (1988) for canine VL (el Harith et al. 1988), these 15 cat should be regarded as infected animals, but none of them confirmed by Giemsa stained smears, PCR and NNN culture. The cutoff for canine VL is agglutination at 1:320, but based on the results it is not suitable for feline VL. Therefore, we considered all as negative results.
Discussion
PCR has been suggested to be more sensitive and specific method for diagnosis of VL (Ashford et al. 1995; Piarroux et al. 1994). In canine VL agglutination at 1:320 dilution and above is indicative of positive results (el Harith et al. 1989). Based on the authors’ knowledge and searching in the most of the databases, the test has not been standardized to feline VL and the accurate cut off needs to be defined. Cats and dogs are different animals and based on findings of this study, DAT may interpreted differently in these animals and the cutoffs may also be different.
Of all 65 cats from Azarshahr city and nearby villages, none has been fined to be infected by L. infantum. According to the results of this study, cats are not, or are at least importance to be a reservoir for VL of humans in the studied area. To our knowledge, in Iran just one report is available about feline VL, which is carried out in Kaleybar area, northern part of East Azerbaijan province. Hatam et al. (2010) studied 40 cats and reported that 1 (10 %) out of 10 and 3 (30 %) out of 30 cats from Kaleybar region and Fars province were infected by L. infantum, respectively. The parasite has been isolated from spleen and liver of the cat from Kaleybar area by NNN culture, but Giemsa-stained touch smears of liver and spleen reported to be negative. Also the infected animal manifested some skin lesions (Hatam et al. 2010).
Cats are reported to be infected by L. infantum in Brazil, Middle East and Europe (da Silva et al. 2008; Maia and Campino 2011; Maia et al. 2010; Maia et al. 2008; Martin-Sanchez et al. 2007; Savani et al. 2004; Silva Rde et al. 2014; Solano-Gallego et al. 2007). The role of cat for being reservoir of VL is not completely clear and it is referred as a potential reservoir (Maia and Campino 2011). Maia et al. (2010) reported feline Leishmania infection in 20.3 % of cats from a canine leishmaniasis endemic region from Portugal. They propose that healthy cats without any immunosuppressive diseases are susceptible to the Leishmania infection. Also, they used serological test using indirect immunofluorescence antibody test (IFAT), which adapted from the technique used for canine VL. They concluded that Leishmania specific antibodies are not sensitive enough for diagnosis of feline leishmaniasis (Maia et al. 2010). In the present study results of DAT on feline leishmaniasis cannot be interpreted based on the method used for dogs. As it seems, the positive results of DAT might be a titer >1:640, because none of the agglutinations at 1:80, 1:160, 1:320 and 1:640 delusions confirmed to be positive by PCR. It seems necessary to standardize and evaluate DAT for diagnosis of feline leishmaniasis and or evaluate the validity of DAT for diagnosis of VL of cats.
Moshfe et al. (2009), evaluated the DAT, Dipstick rK39 and PCR for diagnosis of canine VL in Meshkinshahr area, north west of Iran, where human and canine VL are endemic. They suggested DAT as a suitable method for diagnosis of the infection in symptomatic and asymptomatic dogs (Moshfe et al. 2009). According to the results of the present study the used cutoff point of DAT for canine VL is not suitable for the test in feline VL, so DAT for feline VL is need to be standardize.
According to the five criteria suggested by Bray (1982), a good reservoir of leishmaniasis should have the following characteristics: contact with man, good presentation of the disease organism, chronic susceptibility, intimate contact with the sand fly vector and major source of blood meals for the vector (Bray 1982). However the feline leishmaniasis has been described in one cat from Kaleybar region, Iran, but in our study with a larger sample size and different location, none of the cats were infected. So, based on the results of the present study we cannot account cats as a reservoir for L. infantum in Azarshahr area.
L. infantum can infect jackals, wolves and foxes and consequently they may play as secondary reservoirs for human infection in endemic areas, especially in mountainous regions where the sylvatic cycle of VL present. In Iran infection with L. infantum was reported in desert rodents (gerbils) and also in a wolf in northwest of Iran, which is endemic for canine and human VL (Mohebali 2013).
Fallah et al. (2007) studied 265 rodents belonging to 7 genera/species for presence of anti-leishmanial antibodies using direct DAT, IFA and microscopic examination in Azarshahr County. Of all 256 rodents 15 (5.3 %) were seropositive, and 224 (84.5 %) were seronegative. They used PCR for identification of the Leishmania spp. of the infected rodents and reported the first L. infantum infection in Meriones persicus, Cricetalus migratorius and Mesocricetus auratus from Azarshahr area. They also assumed infected rodents as potential reservoirs for VL in the area (Fallah et al. 2007).
Considering the fact that the present study and study of Fallah et al. (2007) carried out in the same region, the results of the present study indicates that cats are not a reservoir for L. infantum in the Azarshahr area. On the other hand, further studies are necessary to be done in order to clarify the role of cats in the transmission of human VL in different regions of East Azerbaijan, Iran.
Conclusion
According to the findings of the present study, cats are not reservoirs for VL in the Azarshahr area, north west of Iran.
Acknowledgments
This work was supported fully by Tabriz Infectious and Tropical Disease Research Center (grant no: 92-02), Tabriz University of Medical Sciences, Tabriz, Iran. This is a report of database from thesis of MSc degree entitled Survey of feline visceral leishmaniasis in Azarshahr area, north west of Iran, 2013, registered in Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of interest
The authors clarify to have no conflict of interests.
References
- Alvar Jorge, Vélez Iván D., Bern Caryn, Herrero Mercé, Desjeux Philippe, Cano Jorge, Jannin Jean, Boer Margriet den. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford David A., Degrave Wim, Eulalio Conceicao, Badaro Roberto, Lopes Ulisses, Miranda Jose Carlos, David John R., Sherlock Italo, Bozza Marcelo, Barker Robert H., Freire Miralba, Fernandes Octavio. Comparison of the Polymerase Chain Reaction and Serology for the Detection of Canine Visceral Leishmaniasis. The American Journal of Tropical Medicine and Hygiene. 1995;53(3):251–255. doi: 10.4269/ajtmh.1995.53.251. [DOI] [PubMed] [Google Scholar]
- Bray RS. The zoonotic potential of reservoirs of leishmaniasis in the old world. Ecol Dis. 1982;1:257–267. [PubMed] [Google Scholar]
- da Silva AV, de Souza Candido CD, de Pita Pereira D, Brazil RP, Carreira JC. The first record of American visceral leishmaniasis in domestic cats from Rio de Janeiro. Brazil Acta tropica. 2008;105:92–94. doi: 10.1016/j.actatropica.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Edrissian GH, Nadim A, Alborzi A, Ardehali S. Visceral leishmaniasis; the Iranian experience. Arch Iran Med. 1998;1:22–26. [Google Scholar]
- el Harith A, Kolk AH, Leeuwenburg J, Muigai R, Huigen E, Jelsma T, Kager PA. Improvement of a direct agglutination test for field studies of visceral leishmaniasis. J Clin Microbiol. 1988;26:1321–1325. doi: 10.1128/jcm.26.7.1321-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Harith A, Slappendel RJ, Reiter I, van Knapen F, de Korte P, Huigen E, Kolk AH. Application of a direct agglutination test for detection of specific anti-Leishmania antibodies in the canine reservoir. J Clin Microbiol. 1989;27:2252–2257. doi: 10.1128/jcm.27.10.2252-2257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah E, Farshchian M, Mazlomi A, Majidi J, Kusha A, Mardi A, Mahdipoorzareh N. Study on the prevalence of visceral Leishmaniasis in rodent’s of Azarshahr district (new focus), Northwest of Iran. Arch. Razi. Inst. 2007;61:33–40. [Google Scholar]
- Hatam GR, Adnani SJ, Asgari Q, Fallah E, Motazedian MH, Sadjjadi SM, Sarkari B. First report of natural infection in cats with Leishmania infantum in Iran. Vector Borne Zoonotic Dis. 2010;10:313–316. doi: 10.1089/vbz.2009.0023. [DOI] [PubMed] [Google Scholar]
- Mahboudi F, Abolhassan M, Yaran M, Mobtaker H, Azizi M. Identification and differentiation of Iranian Leishmania species by PCR amplification of kDNA. Scand J Infect Dis. 2001;33:596–598. doi: 10.1080/00365540110026746. [DOI] [PubMed] [Google Scholar]
- Maia C, Campino L. Can domestic cats be considered reservoir hosts of zoonotic leishmaniasis? Trends Parasitol. 2011;27:341–344. doi: 10.1016/j.pt.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Maia C, Nunes M, Campino L. Importance of cats in zoonotic leishmaniasis in Portugal. Vector Borne Zoonotic Dis. 2008;8:555–559. doi: 10.1089/vbz.2007.0247. [DOI] [PubMed] [Google Scholar]
- Maia C, Gomes J, Cristovao J, Nunes M, Martins A, Rebelo E, Campino L. Feline Leishmania infection in a canine leishmaniasis endemic region. Portugal Vet Parasitol. 2010;174:336–340. doi: 10.1016/j.vetpar.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez J, Acedo C, Munoz-Perez M, Pesson B, Marchal O, Morillas-Marquez F. Infection by Leishmania infantum in cats: epidemiological study in Spain. Vet Parasitol. 2007;145:267–273. doi: 10.1016/j.vetpar.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Mirsamadi N, Mohebali M, Atari M, Edrisian GH (2003) Serological survey of Visceral leishmaniasis (kala-azar) in Azarshahr, Azarbaijan province, Northwest of Iran HAKIM 6:17–25
- Mohebali M. Visceral leishmaniasis in Iran: Review of the epidemiological and clinical features. Iranian J Parasitol. 2013;8:348–358. [PMC free article] [PubMed] [Google Scholar]
- Mohebali M, Taran M, Zarei Z. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick rk39 test and direct agglutination. Vet Parasitol. 2004;121:239–245. doi: 10.1016/j.vetpar.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Moshfe A, Zarei Z, Akhoundi B, Edrissian GH, Kazemi B, Jamshidi SH, Mahmoudi M, Bandehpour M, Mohebali M (2009) Comparison between serology and PCR methods for the diagnosis of visceral leishmaniasis. Armaghan Danesh 14:31–42 Persian
- Piarroux R, Gambarelli F, Dumon H, Fontes M, Dunan S, Mary C, Toga B, Quilici M (1994) Comparison of PCR with direct examination of bone marrow aspiration, myeloculture, and serology for diagnosis of visceral Leishmaniasis in immunocompromised patients. J Clin Microbiol 32:746–749 [DOI] [PMC free article] [PubMed]
- Ready PD (2010) Leishmaniasis emergence in Europe Euro surveillance : Bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 15:19505 [PubMed]
- Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–154. doi: 10.2147/clep.s44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani Elisa San Martin Mouriz, de Oliveira Camargo Maria Cecília Gibrail, de Carvalho Marília Russi, Zampieri Ricardo Andrade, dos Santos Marcos Gonzaga, D’Áuria Sandra Regina Nicoletti, Shaw Jeffrey J, Floeter-Winter Lucile Maria. The first record in the Americas of an autochthonous case of Leishmania (Leishmania) infantum chagasi in a domestic cat (Felix catus) from Cotia County, São Paulo State, Brazil. Veterinary Parasitology. 2004;120(3):229–233. doi: 10.1016/j.vetpar.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Silva Rita de Cássia Nascimento, Ramos Rafael Antonio Nascimento, Pimentel Danillo de Souza, Oliveira Gênova Maria de Azevedo, Carvalho Gílcia Aparecida de, Santana Marília de Andrade, Faustino Maria Aparecida da Glória, Alves Leucio Câmara. Detection of antibodies against Leishmania infantum in cats (Felis catus) from the State of Pernambuco, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2014;47(1):108–109. doi: 10.1590/0037-8682-0005-2012. [DOI] [PubMed] [Google Scholar]
- SOLANO-GALLEGO LAIA, INIESTA LAURA, RODRÍGUEZ-CORTÉS ALHELÍ, PASTOR JOSEPH, QUINTANA JOSEFINA, ESPADA YVONNE, ALBEROLA JORDI, PORTÚS MONTSERRAT. CROSS-SECTIONAL SEROSURVEY OF FELINE LEISHMANIASIS IN ECOREGIONS AROUND THE NORTHWESTERN MEDITERRANEAN. The American Journal of Tropical Medicine and Hygiene. 2007;76(4):676–680. doi: 10.4269/ajtmh.2007.76.676. [DOI] [PubMed] [Google Scholar]
- World Health Organization Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, March 22–26, 2010. World Health Organ Tech Rep Ser. 2010;949:1–186. [PubMed] [Google Scholar]
- World Health Organization (2010 b) Control of leishmaniases; Geneva: Technical report series 793 of WHO Expert Committee [PubMed]


