Abstract
A large number of medicinal plants are used as herbal remedy for the treatment of helminthic diseases in the developing countries, however, far too little attention has been paid to assess the anthelmintic potentials of chemical compounds that are present in these plants. This study was carried out to assess the in vitro anthelmintic effects of biochanin A, ursolic acid, betulinic acid and beta-sitosterol which are the major phytochemicals of Trifolium repens, Houttuynia cordata and Lasia spinosa, the traditionally used anthelmintic plants of Northeast India. The in vitro anthelmintic testing of these phytochemicals was undertaken against Hymenolepis diminuta, a zoonotic tapeworm, and their efficacy was compared with a reference drug, praziquantel. The results revealed that except beta-sitosterol, which showed a very weak anthelmintic effect, remaining all other tested compounds possess highly significant (p ≤ 0.001) and dose-dependent anthelmintic effects. Upon exposure to 0.25, 0.50 and 1 mg/ml concentrations of biochanin A, ursolic acid and betulinic acid, the test parasite H. diminuta, at first, showed a paralyzed state which later culminated into their mortality after short time periods. Of all the phytochemicals tested, betulinic acid (1 mg/ml) showed the best anthelmintic effect and caused the mortality of test parasites at 3.4 ± 0.66 h. In conclusion, the results of this study demonstrate for the first time that betulinic acid, biochanin A and ursolic acid possess significant in vitro anthelmintic effects against H. diminuta, a zoonotic tapeworm, and, therefore, these compounds may be exploited further for anthelmintic drug development.
Keywords: Anthelmintics, Beta-sitosterol, Betulinic acid, Biochanin A, Hymenolepis diminuta, Ursolic acid, Phytochemicals
Introduction
According to the World Health Organization (WHO), over two billion people in the world are chronically infected with at least one or other gastro-intestinal helminth parasite, many suffer from severe morbidity, and others from more hidden manifestations of disease (WHO 2005). Considering that intestinal parasitic infections are among the most common infections worldwide and affect the poorest and most deprived communities, the WHO has also recognized them under ‘Neglected Tropical Diseases’ which have always ranked low on national and international health agendas (Hotez et al. 2007).
Although, there are some effective anthelmintics available for the treatment of intestinal helminth infections but, by and large, they still remain out of reach to the majority of people, particularly in remote and rural areas in the endemic regions. And to further add to this problem, a continuous and long-term dependence on a small range of compounds to treat intestinal helminth infections also poses a threat of drug-resistance in many helminth strains in near future (Hoste and Torres-Acosta 2011). Thus, over the last two decades, workers around the world have shown a keen interest in assessing the potentials of medicinal plants for the development of new anthelmintics (for a review see Tagboto and Townson 2001; Abdel-Ghaffar et al. 2011; Klimpel et al. 2011). As per the estimates of WHO, about 80 % of the world’s population relies on plant-based medicines for their primary health needs (WHO 2000). Similarly, a close to about 80 % of the drugs used globally at present also happen to come either from natural products or some derivatives inspired by natural precursors (Li and Vederas 2009). Therefore, plants constitute a very rich source of bioactive chemical compounds against many diseases and ailments (Newman and Cragg 2007). In this connection, particularly those plants that are used in the traditional systems of medicine in different indigenous societies often provide a very rich reservoir of new lead molecules for the development of new drugs (Liu 2011). Thus, bioprospection of phytoconstituents in the background of some relevant information about their presence in ethnopharmacologically used plants offers an alternative approach to explore these compounds for antiparasitic drug development.
Plants produce a high diversity of low molecular weight compounds and in recent years some of these phytocompounds have also shown good antiparasitic effects in laboratory trials (Anthony et al. 2005; Kumar et al. 2012; Nagajyothi et al. 2012; Teichmann et al. 2012; Zahir et al. 2012; González-Coloma et al. 2012; Koné et al. 2012). The Northeast region of India is blessed with a rich wealth of medicinal plants, whose decoctions, infusions, syrups, etc. are commonly employed as traditional anthelmintics by local tribes. To date, the crude extracts of many such plants, namely Trifolium repens, Houttuynia cordata and Lasia spinosa, etc. have shown promising anthelmintic effects in experimental animal models (Tangpu and Temjenmongla 2004; Yadav and Temjenmongla 2011, 2012a, b). However, no attention has been paid to explore the anthelmintic potentials of various chemical compounds that are present in these plants. Therefore, this study was conducted to assess the in vitro anthelmintic efficacy of four major phytoconstituents, i.e. biochanin A, ursolic acid, betulinic acid and beta-sitosterol that are found in T. repens, H. cordata and L. spinosa, the traditionally used anthelmintic plants of Northeast India.
Materials and methods
Drugs and chemicals
The reference drug, praziquantel (PZQ) with the trade name Distocide® (composed of 600 mg praziquantel) was a product of Chandra Bhagat Pharma Pvt. Ltd., Mumbai, India. The tested phytochemicals biochanin A, ursolic acid, betulinic acid and beta-sitosterol were of analytical grade and procured from Sigma-Aldrich, Bangalore, India.
In vitro anthelmintic assay
In vitro testing of phytochemicals was performed on adult Hymenolepis diminuta (Cestoda: Hymenolepididae) that were obtained from freshly necropsied Wistar rats carrying experimentally induced H. diminuta infections (Yadav and Tangpu 2012). For in vitro testing, the adult tapeworms were washed several times in 0.9 % phosphate buffered saline (PBS) at 37 ± 1 °C. The test worms (n = 5) were then maintained in petridishes inside an incubator at 37 ± 1 °C containing 0.25, 0.50 and 1 mg/ml concentrations of each phytochemical and 1 mg/ml concentration of PZQ. One set of worms (n = 5), maintained alone in PBS, were kept as controls. The anthelmintic efficacy of compounds was adjudged in terms of physical motility of test worms, as evidenced by their paralysis or mortality, and the same was monitored at every half an hour interval. In order to confirm the mortality of test parasites, two types of PBS solutions, i.e. one warm (37 ± 1 °C) and the other slightly more warm (40 ± 1 °C) were employed. Worms not showing any physical movements on gentle stimulation by a soft brush were picked up and transferred first to the warm saline solution. Total loss of noticeable movements in the parasite during exposure to warm solution was considered as a sign of paralysis and the same was recorded for each set of experiment. Likewise, when the parasites showed no movements in this warm saline for about 5 min, they were then immediately transferred to the slightly more warm saline solution so as to finally confirm their mortality. The mortality of parasites was assumed to have occurred when all signs of movements had ceased even after exposing to slightly warmer saline solution for 30 min. Accordingly, the mortality time of test parasites was also recorded for each experiment. The experiments were repeated thrice with five number of test worms per concentration of each test agent/control.
Statistical analysis
All the experimental data are represented as mean ± standard error of the mean (SEM). Origin version 8.0 SR6 was used for graphical representation of data. All data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Results with p < 0.05 were considered to be statistically significant.
Results and discussion
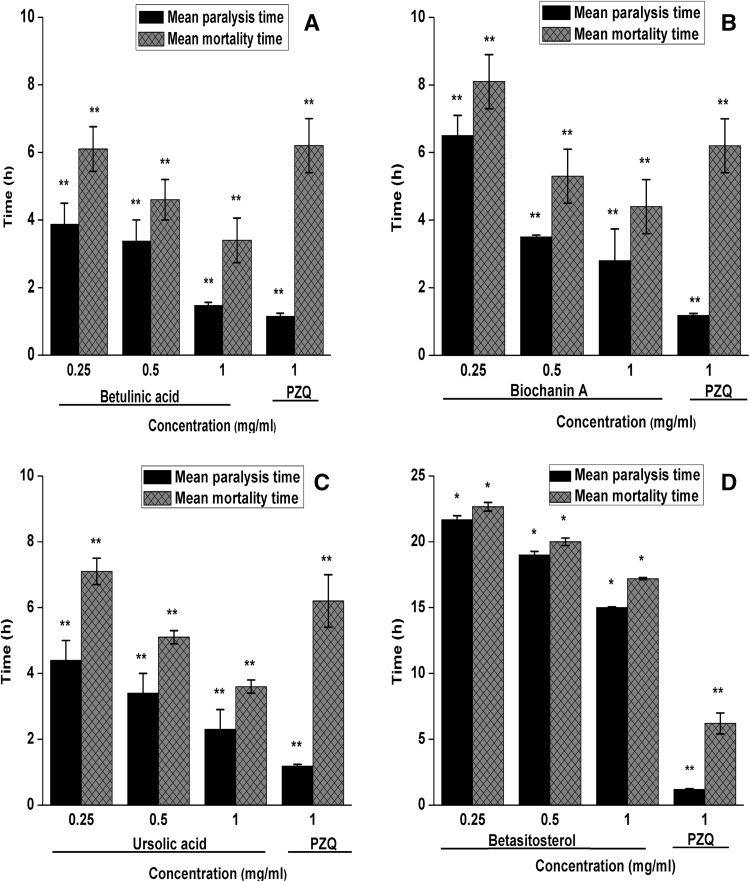
The results of this study revealed that after exposure of H. diminuta worms to different concentrations of four phytochemicals, except betasitosterol, remaining all three test compounds, i.e. biochanin A, ursolic acid and betulinic acid, bring out an early paralysis and mortality in test parasites (Fig. 1). The paralysis and mortality time of phytochemical-treated worms were recorded to be dose-dependent and highly significant (p ≤ 0.001) when compared to control. Of all the test compounds, betulinic acid showed the best anthelmintic effects. At 1 mg/ml concentration, betulinic acid caused the mortality of test worms in 3.4 ± 0.66 h. On the other hand, the reference drug, praziquantel (PZQ) at 1 mg/ml concentration caused the mortality of test worms in 6.2 ± 0.80 h (Fig. 1). This study also revealed that ursolic acid possesses the second highest anthelmintic efficacy among all the compounds tested. At 1 mg/ml concentration, ursolic acid caused the mortality of test worms in 3.6 ± 0.2 h. Similarly, the testing of biochanin also showed significant anthelmintic effects on H. diminuta. At 1 mg/ml concentration, it caused the mortality of test worms in 4.4 ± 0.8 h. Unlike the above three test compounds, the beta-sitosterol showed very weak anthelminthic effects. In case of beta-sitosterol (1 mg/ml), the test worms showed mortality in 17.2 ± 0.08, which was about three times higher when compared to the reference drug PZQ (Fig. 1).
Fig. 1.
In-vitro anthelmintic effects of a Betulinic acid, b Biochanin A, c Ursolic acid, d Betasitosterol against Hymenolepis diminuta. The physical activity of worms maintained in the control medium was observed till 53.33 ± 0.35 h. All data are presented as mean ± S.E.M. (n = 5). *Significant at p ≤ 0.05 level; **Significant at p ≤ 0.001
Plants and their extracts, which are rich in plant secondary metabolites, like terpenoids alkaloids, tannins, steroids, etc., have been used to combat parasitism for many centuries, and in many parts of the world such products are still used for this purpose (Athanasiadou and Kyriazakis 2004; Anthony et al. 2005). In addition, these products have also served as the most significant source of new leads for pharmaceutical development (Wink 2012). Although, there are many evidences of the antiparasitic effects of these phytoconstituents, however, their purported antiparasitic effects have also been the cause of controversy amongst the scientific community (Athanasiadou and Kyriazakis 2004). A perusal of literature reveals that majority of scientific evidences supporting the antiparasitic properties of phytoconstituents have come from in vitro studies (Athanasiadou and Kyriazakis 2004; Nagajyothi et al. 2012; Teichmann et al. 2012; Zahir et al. 2012), because employing this method the phytoconstituents can be reliably quantified without the interference of other plant components or nutrients. Therefore, in this study we employed a suitable in vitro assay to test the anthelmintic efficacy of four selected phytochemicals on H. diminuta.
Phytochemicals possess a wide range of chemical structures, and in experimental studies, different phytocompounds have also shown an array of biological activities, which have often been found to vary from compound to compound (Newman and Cragg 2007; Teichmann et al. 2012; Wink 2012). This may explain, in part, as to why, in the present study, different tested phytochemicals revealed different levels of anthelmintic activity. In the present study, betulinic acid, ursolic acid and biochanin A showed good anthelmintic effects, however, the anthelmintic efficacy of betasitosterol was recorded to be very weak. It may be mentioned here that anthelmintic agents, in general, either bring out their actions by inhibiting the machinery of energy metabolism in the parasite or they also work through neuromuscular or transtegumental mode of actions, etc. (Martin 1997). In the context of present study, we believe that perhaps beta-sitosterol, which showed a very anthelmintic activity, does not have any such potentials to bring out it anthelmintic effects on test parasite. In the literature, too, we did not find any convincing evidence about anthelmintic effects of beta-sitosterol.
Betulinic acid is a naturally pentacyclic triterpenoid which is reported to be present in a number of plant species (Yogeeswari and Sriram 2005). Many plants that are rich in betulinic contents have also long been used in different societies for curing a variety of human ailments, such as diarrhea, dysentery and other intestinal problems (Yogeeswari and Sriram 2005). Experimentally, it has also been shown to exhibit a variety of biological activities, including antibacterial and antimalarial properties (Yogeeswari and Sriram 2005). Like in the present study, Enwerem et al. (2001) have also reported a strong anthelmintic activity of betulinic acid, but against a free-living nematode Caenorhabditis elegans. In another study as well, betulinic acid has demonstrated concentration-dependent anthelmintic activities against human intestinal worms, Fasciola gigantica and Taenia solium at 10–100 mg/ml (Lasisi and Idowu 2012). Ursolic acid is a triterpenoid found in a variety of medicinal herbs (Liu 2011) and some related biological studies have revealed that it possesses significant trypanocidal (Cunha et al. 2003) and antimalarial effects (Innocente et al. 2012). Other in vitro studies have also demonstrated that purified terpenoids from several legumes reduce the mobility and consequent migration ability of ovine nematode larvae (Molan et al. 2003). Likewise, Biochanin A belongs to the class of isoflavones and has also been reported to be present in many medicinal plants (Sartorelli et al. 2009). Isoflavones, such as genistein and daidzein, are found in a number of plants, including Flemingia vestita which has shown significant anthelmintic effects against many intestinal helminth parasites (Tandon et al. 2011). Silva et al. (2008) have studied the anthelmintic effects of biochanin A purified from the roots of Andira anthelmia in mice naturally infected by Aspiculuris tetraptera. In that study 2.0 mg/kg dose of biochanin A was found to be quite effective in the removal of the total number of A. tetraptera from mice. Therefore, the findings of the current study are also consistent with those of Silva et al. (2008) who found significant anthelmintic effects in Biochanin A. In addition, Naguleswaran et al. (2006) have also opined that synthetic isoflavones exhibit distinct in vitro effects on Echinococcus metacestodes and protoscoleces, which could potentially be exploited further for the development of novel chemotherapeutical tools against larval-stage Echinococcus infection.
In conclusion, the results of this study suggests that phytochemicals betulinic acid, ursolic acid and biochanin A possess significant anthelmintic effects against H. diminuta, a zoonotic tapeworm. Therefore, further in vivo testing of these compounds against representative mammalian parasitic groups will be valued from a therapeutic point of view.
Acknowledgments
Facilities for research work was provided by the Department of Zoology, NEHU, Shillong. Vijaya was recipient of a non-net fellowship from North-Eastern Hill University, Shillong.
References
- Abdel-Ghaffar F, Semmler M, Al-Rasheid KAS, Strassen B, Fischer K, Aksu G, Klimpel S, Mehlhorn H. The effects of different plant extracts on intestinal cestodes and on trematodes. Parasitol Res. 2011;108:979–984. doi: 10.1007/s00436-010-2167-5. [DOI] [PubMed] [Google Scholar]
- Anthony JP, Fyfe L, Smith H. Plant active components—a resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Athanasiadou S, Kyriazakis I. Plant secondary metabolites: antiparasitic effects and their role in ruminant production systems. Proc Nut Soc. 2004;63:631–639. doi: 10.1079/PNS2004396. [DOI] [PubMed] [Google Scholar]
- Cunha WR, Martins C, Ferreira DS, Crotti AEM, Lopes NP, Albuquerque S. In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med. 2003;69:470–472. doi: 10.1055/s-2003-39719. [DOI] [PubMed] [Google Scholar]
- Enwerem NM, Okogun JJ, Wambebe CO, Okorie DA, Akah PA. Anthelmintic activity of the stem bark extracts of Berlina grandiflora and one of its active principles, betulinic acid. Phytomed. 2001;8:112–114. doi: 10.1078/0944-7113-00023. [DOI] [PubMed] [Google Scholar]
- González-Coloma A, Reina M, Sáenz C, Lacret R, Ruiz-Mesia L, Arán VJ, Sanz J, Martínez-Díaz RA. Antileishmanial, antitrypanosomal, and cytotoxic screening of ethnopharmacologically selected Peruvian plants. Parasitol Res. 2012;110:1381–1392. doi: 10.1007/s00436-011-2638-3. [DOI] [PubMed] [Google Scholar]
- Hoste H, Torres-Acosta JFJ. Non chemical control of helminths in ruminants: adapting solutions for changing worms in a changing world. Vet Parasitol. 2011;180:144–154. doi: 10.1016/j.vetpar.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Innocente AM, Silva GNS, Cruz LN, Moraes MS, Sonnet MNP, Gosmann G, Garcia CRSG, Gnoatto SCB. Synthesis and antiplasmodial activity of betulinic acid and ursolic acid analogues. Molecules. 2012;17:12003–12014. doi: 10.3390/molecules171012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel S, Abdel-Ghaffar F, Al-Rasheid KAS, Aksu G, Fischer K, Strassen B, Mehlhorn H. The effects of different plant extracts on nematodes. Parasitol Res. 2011;108:1047–1054. doi: 10.1007/s00436-010-2168-4. [DOI] [PubMed] [Google Scholar]
- Koné WM, Vargas M, Keiser J. Anthelmintic activity of medicinal plants used in Côte d’Ivoire for treating parasitic diseases. Parasitol Res. 2012;110:2351–2362. doi: 10.1007/s00436-011-2771-z. [DOI] [PubMed] [Google Scholar]
- Kumar S, Raman RP, Kumar K, Pandey PK, Kumar N, Mohanty S, Kumar A. In vitro and in vivo antiparasitic activity of Azadirachtin against Argulus spp. in Carassius auratus (Linn. 1758) Parasitol Res. 2012;110:1795–1800. doi: 10.1007/s00436-011-2701-0. [DOI] [PubMed] [Google Scholar]
- Lasisi A, Idowu O. In vitro anthelmintic and cytotoxic activities of extracts from the stem barks of Berlinia confusa (C. Hoyle) and identification of its active constituents. J Saudi Chem Soc. 2012 doi: 10.1016/j.jscs.2011.11.016. [DOI] [Google Scholar]
- Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- Liu WJH. Introduction to traditional herbal medicines and their study. In: Liu WJH, editor. Traditional herbal medicine research methods: identification, analysis, bioassay, and pharmaceutical and clinical studies. New York: Wiley; 2011. pp. 1–26. [Google Scholar]
- Martin RJ. Modes of action of anthelmintic drugs. Vet J. 1997;154:11–34. doi: 10.1016/S1090-0233(05)80005-X. [DOI] [PubMed] [Google Scholar]
- Molan AP, Duncan A, Barry TN, McNabb WC. Effects of condensed tannins and sesquiterpene lactones extracted from chicory on the viability of deer lungworm larvae. Proc New Zeal Soc An. 2003;60:26–29. doi: 10.1016/s1383-5769(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Nagajyothi F, Zhao D, Weiss LM, Tanowitz HB. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol Res. 2012;110:2491–2499. doi: 10.1007/s00436-011-2790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguleswaran A, Spicher M, Vonlaufen N, Ortega-Mora LM, Torgerson P, Gottstein B, Hemphill A. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob Agents Chemother. 2006;50:3770–3778. doi: 10.1128/AAC.00578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Sartorelli P, Carvalho CS, Reimao JQ, Ferreira MJ, Tempone AG. Antiparasitic activity of biochanin A, an isolated isoflavone from fruits of Cassia fistula (Leguminosae) Parasitol Res. 2009;104:311–314. doi: 10.1007/s00436-008-1193-z. [DOI] [PubMed] [Google Scholar]
- Silva VC, Carvalho MG, Borba HR, Silva SLC. Atividade anti-helmíntica dos fl avonóides isolados das raízes de Andira anthelmia (Leguminosae) Rev Bras Farmacogn. 2008;18:573–576. doi: 10.1590/S0102-695X2008000400013. [DOI] [Google Scholar]
- Tagboto S, Townson S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasit. 2001;50:199–295. doi: 10.1016/S0065-308X(01)50032-9. [DOI] [PubMed] [Google Scholar]
- Tandon V, Yadav AK, Roy B, Das B. Phytochemicals as cure of worm infections in traditional medicine systems. In: Srivastava UC, Kumar S, editors. Emerging trends in zoology. New Delhi: Narendra Publishing House; 2011. pp. 351–378. [Google Scholar]
- Tangpu V, Temjenmongla Yadav AK. Anticestodal activity of Trifolium repens extract. Pharm Biol. 2004;42:656–658. doi: 10.1080/13880200490902617. [DOI] [Google Scholar]
- Teichmann K, Kuliberda M, Schatzmayr G, Hadacek F, Joachim A. In vitro determination of anticryptosporidial activity of phytogenic extracts and compounds. Parasitol Res. 2012;111:231–240. doi: 10.1007/s00436-012-2824-y. [DOI] [PubMed] [Google Scholar]
- WHO (2000) Promoting the role of traditional medicine in health systems: a strategy for the African region. Report of the Regional Director. AFR/RC50/9, p 5
- WHO (2005) Report of the third global meeting of the partners for parasite control: deworming for health and development. Geneva, 29–30 November 2004. WHO/CDS/CPE/PVC/2005.14, Geneva, p 64
- Wink M. Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Tangpu V. Anthelmintic activity of ripe fruit extract of Solanum myriacanthum Dunal (Solanaceae) against experimentally induced Hymenolepis diminuta (Cestoda) infections in rats. Parasitol Res. 2012;110:1047–1053. doi: 10.1007/s00436-011-2596-9. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Temjenmongla Anticestodal activity of Houttuynia cordata leaf extract against Hymenolepis diminuta in experimentally infected rats. J Parasit Dis. 2011;35:190–194. doi: 10.1007/s12639-011-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Temjenmongla Efficacy of Lasia spinosa leaf extract in treating mice infected with Trichinella spiralis. Parasitol Res. 2012;110:439–498. doi: 10.1007/s00436-011-2551-9. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Temjenmongla In vivo anthelmintic activity of Clerodendrum colebrookianum Walp., a traditionally used taenicidal plant in Northeast India. Parasitol Res. 2012;111:1841–1846. doi: 10.1007/s00436-012-2908-8. [DOI] [PubMed] [Google Scholar]
- Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- Zahir AA, Rahuman AA, Bagavan A, Geetha K, Kamaraj C, Elango G. Evaluation of medicinal plant extracts and isolated compound epicatechin from Ricinus communis against Paramphistomum cervi. Parasitol Res. 2012;111:1629–1635. doi: 10.1007/s00436-011-2589-8. [DOI] [PubMed] [Google Scholar]