Abstract
The aim of the present study was to determine the effects of Toxoplasma gondii (T. gondii) infection on anxiety, depression and ghrelin level in male rats. Twenty four male rats were assessed in two equal groups. T. gondii tachyzoite (ip) were injected in infected group and control group received (2 ml) physiological serum (ip). Elevated plus Maze and swimming tests were used to assess anxiety and depression in rats respectively. The ghrelin and T. gondii IgG serum levels were measured by enzyme immunoassay kits. The Student’s t test and Pearson correlation coefficient were used. The ghrelin serum level was significantly lower in the infected rats than control (P = 0.03). There were no significant differences in the depression and anxiety behavior between two groups. However, here were no significant correlations between ghrelin level and anxiety or depression in rats. It seems that latent T. gondii infection decreases the ghrelin serum level but does not change anxiety and depression like behaviors.
Keywords: Ghrelin, Toxoplasma, Anxiety, Depression
Introduction
Toxoplasma gondii (T. gondii) is one of the world’s most common parasites that infects approximately one third of the world’s population (Halonen and Weiss 2013; Shaddel et al. 2007). It is an intracellular obligatory protozoan which belongs to coccidian order. Toxoplasmosis can be transmitted to humans by ingestion of cysts in raw or inadequately cooked infected meat, by ingestion of cat feces or transplacentally (Shaddel et al. 2014). The parasite rarely causes significant clinical disease. However, certain individuals are at high risk for severe or life-threatening toxoplasmosis. Individuals at risk for toxoplasmosis include fetuses, newborns, and immunocompromised patients (Tenter et al. 2000; Okome-Nkoumou et al. 2014).
Recent studies demonstrated that T. gondii can modify brain function and intermediate host’s behavior (Hermes et al. 2008; Vyas et al. 2007; Kannan et al. 2010), and also increase the chances of serious psychological disturbances such as schizophrenia and bipolar disorders in human (Pearce et al. 2012; Mortensen et al. 2007). In addition there is correlation between T. gondii infection and mood disorders. Pregnant women with serological evidence of exposure to T. gondii showed positive correlations between IgG levels and depression and anxiety (Groër et al. 2011). It has been reported a depressed patient with toxoplasma seropositivity improved only after treatment by anti- toxoplasma drugs (Kar and Misra 2004). Furthermore, it has been shown that T. gondii IgG antibody titer is higher in suicide attempter patients than non-suicide attempter individuals (Arling et al. 2009). Therefore, all above studies show there is association between T. gondii infection and onset of anxiety and depression.
Rises in ghrelin as an appetite-stimulating hormone occur not only in response to states of energy insufficiency but also following stress (Kristenssson et al. 2006). It has been shown that ghrelin has anxiolytic and antidepressant effects. Calorie-restricted wild-type mice showed robust anxiolytic and antidepressant-like behavior in the elevated plus maze and forced swim test (Lutter et al. 2008).
However, to the best of our knowledge there is no report about effect of T. gondii infection on the ghrelin serum level and its association with anxiety and depression behavior. Therefore, the aim of the present study was to determine the effects of Toxoplasma gondii infection on the anxiety, depression and ghrelin levels in male rat.
Method
Animals
Twenty-four adult Wistar rats (290–350 gr) were used. The animals were maintained under standard condition (temperature 22 ± 2 °C, lights on at 6:00–18:00 h) with free access to food and water. After one week of acclimatization, the rats were randomly divided into two groups (n = 12 for each group): group1, infected group received T. gondii tachyzoite. group2, control group received (2 ml) physiological serum (ip).
All experimental procedures were done in accordance with AJA University of Medical Sciences Ethic committee.
Toxoplasma gondii culture and infection
T. gondii RH strain was obtained from Parasitology department of Tarbiat Modares University in Tehran, Iran. Experimental chronic Toxoplasmosis was initiated by injection of up to 10 (Vyas et al. 2007) alive Toxoplasma gondii tachyzoite (ip) in infected group.
Infection determination
After 3 weeks, all rats were evaluated for serology tests. Briefly, sera were collected and stored at −70 °C. Antibodies serum levels against T. gondii were evaluated in IgG classes using ELISA kits (Meddens Diagnostics BV, The Netherlands) according to the manufacturers’ instructions.
Behavioral assessments
Behavioral assessments were done by a video camera and an observer unaware of experimental groups. Elevated plus Maze and swimming test were used respectively to assess anxiety and depression in rats. In elevated plus maze, the rats were placed in the central area of the maze facing an open arm and their behavior recorded during 5-min. After 5 min, rats were removed from the maze and returned to their home cage. The maze was then cleaned with a solution of 70 % ethyl alcohol and permitted to dry between tests.
Behaviors scored included
Open arms entries Frequency with which the animal entered the open arms. All four of the mouse’s paws were required to be in the arm to be counted as an entry.
Closed arm entries Frequency with which the animal entered the closed arms. All four of the mouse’s paws were required to be in the arm to be counted as an entry.
Open arm duration: Length of time the animal spent in the open arms.
Closed arm duration Length of time the animal spent in the closed arms.
Center square entries Frequency with which the animal entered the central square with all four paws.
Central square duration Length of time the animal spent in the central square.
Head dipping Frequency with which the animal lowered its head over the sides of the open arm toward the floor.
Rearing Frequency with which the animal stands on hind legs or leans against walls of the maze with front paws.
Grooming Frequency with which the animal spent licking or scratching itself while Stationary.
Urination Number of puddles or streaks of urine.
Defecation Number of fecal boli produced.
The Index open arms avoidance was determined as [100 − [% time on open arms + % entries into the open arms]/2] (Trullas and Skolnick 1993).
In force swimming test rats were placed individually in a transplant glass cylinder (50 cm height and 30 cm in diameter) filled with tap water. The temperature of water was +25 °C. The immobility time (seconds spent floating without moving) was recorded. At the end of the swimming period rats were dried with a towel.
Hormonal assay
Trunk blood was collected between the hours of 10:00 and 12:00 a.m. and serum samples were obtained by centrifugation of blood at 3,000×g for 10 min. Serum was frozen until the day of the analysis. Measurement of ghrelin serum level was done with a commercial rat ghrelin ELISA kit (Ck-E90442 HANG2H04 East Bio Pharm Co.LTD).
Statistical analysis
All values were expressed as Mean ± S.E.M. Statistical analyses were performed using spss16 software. Student’s unpaired t test was used to analyze hormonal and behavior data. Differences were considered significant if P values were less than 0.05. Associations between ghrelin concentration and behavior scores (anxiety and depression level) were assessed by liner correlation (Pearson).
Result
Hormonal assay
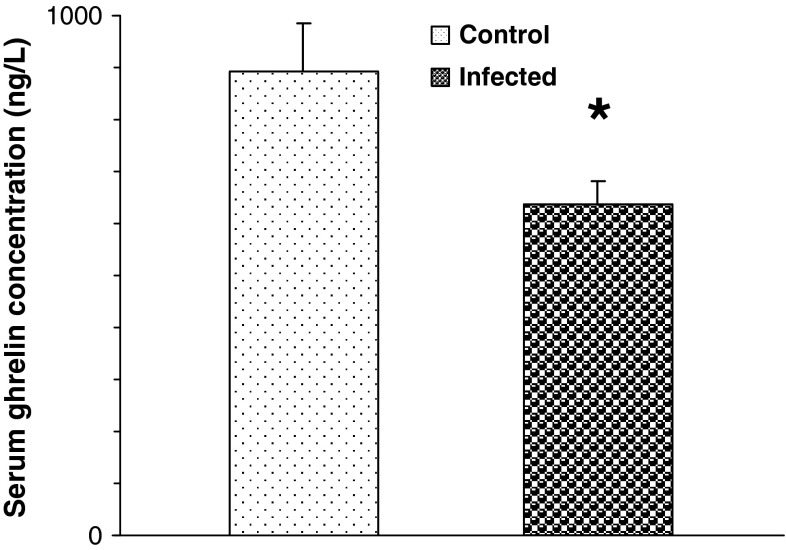
As indicated in Fig. 1, the ghrelin serum level was significantly lower in infected rats than healthy rats (P = 0.03).
Fig. 1.
The effect of T. gondii infection on the ghrelin serum level in rats. [Data are represented as mean ± S.E.M] *P = 0.03
The elevated plus maze
There were no significant differences between two groups in exploration levels, the number of open and closed arms entries, the time spent on closed or open arm, rearing, head dipping and grooming behaviors. However, defecation and the time spent on the central platform were significantly more in healthy rats as compared to infected rats (Table 1).
Table 1.
Behavior of rats in the elevated plus maze; data expressed as Mean ± S.E.M
| Behavior | Control | Infected | P value |
|---|---|---|---|
| Number of open arm entries | 0.58 ± 0.28 | 0.5 ± 0.23 | 0.8 |
| Time spent in open arms[s] | 5.7 ± 2.7 | 7 ± 3.5 | 0.7 |
| open arm avoidance index | 98.9 ± 0.5 | 98.7 ± 0.6 | 0.8 |
| Number of closed arm entries | 3.1 ± 1.4 | 1.2 ± 0.13 | 0.1 |
| Time spent in closed arms[s] | 281.6 ± 5 | 290.9 ± 3.5 | 0.1 |
| Number of center entries | 3.4 ± 1.7 | 0.9 ± 0.25 | 0.1 |
| Time spent in center[s] | 12.4 ± 3.1 | 2.3 ± 0.8 | 0.009 |
| Number of head dips | 3 ± 0.8 | 1.1 ± 0.5 | 0.06 |
| Number of rears | 9.5 ± 0.7 | 8.5 ± 1.1 | 0.4 |
| Number of grooming | 3.5 ± 0.7 | 3.4 ± 0.5 | 0.9 |
| Duration of grooming[s] | 31.9 ± 5.3 | 47.7 ± 11.5 | 0.2 |
| Urination | 2.5 ± 0.3 | 3.2 ± 0.4 | 0.1 |
| Defecation | 2.3 ± 0.5 | 0.3 ± 0.1 | 0.005 |
The forced swimming test [FST]
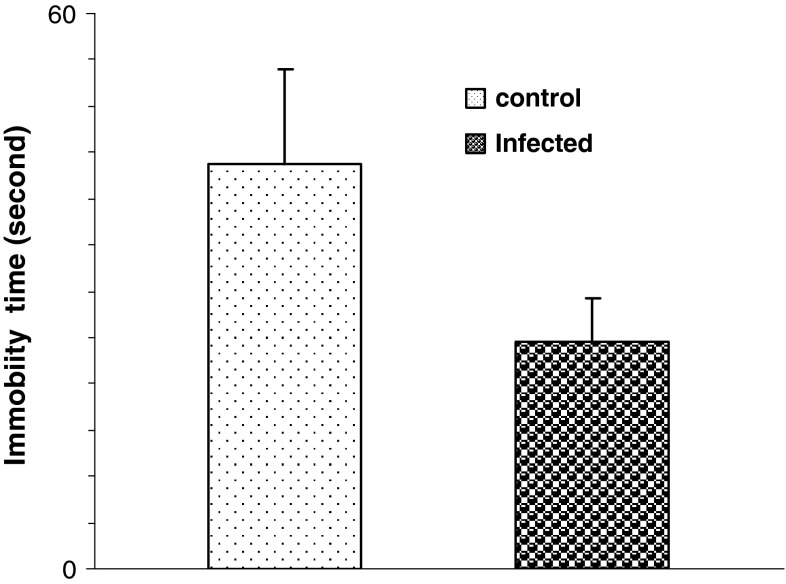
There was no difference in immobility time between infected and non infected rats (Fig. 2).
Fig. 2.
The effect of T. gondii infection in Forced Swimming Test and on immobility time in rats. [Data are represented as mean ± S.E.M]
The ghrelin serum level was not correlated with time spent in open arms (r = 0.27, P = 0.2), open arm avoidance index (r = 0.26, P = 0.2) and immobility time (r = 0.39, P = 0.09).
Discussion
T. gondii can alter brain function and induce changes in the behavior of its intermediate hosts (Mortensen et al. 2007; Kar and Misra 2004; Arling et al. 2009). In male Long-Evans rats, Latent Toxoplasma infection resulted to reduced the normal aversion to cat odor and converted it into a mild attraction (Vyas et al. 2007).
Flegr et al. (1996) reported personality profiles such as, superego strength (the willingness to accept group moral standards) were significantly different between Toxoplasma-infected and uninfected subjects. In a population-based study, association of Toxoplasma-specific immunoglobulin G results with mood disorder outcomes in 7,440 respondents was assessed. There were no statistically significant associations between T. gondii seroprevalence and a history of major depression or severe major depression (Pearce et al. 2012).
In a case report, patient with depression and positive serological test for the toxoplasma showed poor response to antidepressants. The response to antidepressant treatment improved only after adequate treatment for toxoplasma (Kar and Misra 2004).
Patients with recurrent mood disorders with history of suicide attempt had higher T. gondii antibody titers than patients with recurrent mood disorders without history of suicide attempt (Arling et al. 2009). In a case–control study, the association between latent toxoplasmosis and cognitive performance with a battery of standardized, widely employed neuropsychological tests were assessed. There were no statistically significant association between latent toxoplasmosis and cognitive performance (Guenter et al. 2012).
In present study, possible effects of T. gondii infection on the anxiety, depression and ghrelin level in rats were evaluated.
Anxiety is the body’s normal response to stressful situations. Anxiety involves a wide range of physical and psychological symptoms (Huffman et al. 2010).
In the elevated plus maze, the behaviors that exemplify exploration include open arm activity and head dipping (Brown et al. 1999) and the behaviors that exemplify fear include time spent in the closed arms, stretch attend postures, grooming, defecation and urination (Lister 1990). Cole and Rodgers (1994) reported, decreased open arm activities and increased risk assessment behaviors [stretch attend postures, head dips and closed arm returns] without concomitant changes in general locomotion and exploration indicate increased anxiety in the Elevated Plus-Maze. The index of open arm avoidance also gives a measure of anxiety (Trullas and Skolnick 1993).
Our result about elevated plus maze showed that the behaviors that exemplify exploration include open arm activity and head dipping, and behaviors that exemplify fear include time spent in the closed arms, grooming and urination were not significantly differ between two groups. In addition, there was not significantly difference between the indexes of open arm avoidance between two groups. Therefore it seems there is no significant difference in anxiety level between infected and healthy rats. In this regard the results of this study are in consistent with a previous study. Gulinello et al. (2010) reported normal cognitive function, anxiety level, social behavior and the motivation to explore novel objects in mice after infection with T gondii.
In the force swimming test there was no difference in immobility time between infected and uninfected rats. In this test, the time spent immobile is considered an index of depression-like behavior in rodents.
Although we did not find significant difference in anxiety and depression behavior between infected and healthy rats, some previous studies have demonstrated T. gondii infection influences the host behavior. This difference may be related in used parasite strain (Kannan et al. 2010) rat strain (Gonzalez et al. 2007) and host variations (Groër et al. 2011, 2012).
Kannan et al. (2010) compared the effects of infection by two Type II strains of T. gondii, Prugniaud (PRU) and ME49, on mouse behavior. PRU infected mice showed increased locomotor activity in the open field, In contrast; only ME49-infected mice exhibited decreased spontaneous alternations in the Y-maze, indicative of impaired spatial working memory. Infection with 100 and 1,000 tachyzoites increased plus-maze open arm exploration in a dose-related manner in Wistar Rats (Gonzalez et al. 2007).
In another study, pregnant women with serological evidence of exposure to T. gondii showed positive correlations between IgG levels and the depression scale scores and anxiety subscales (Groër et al. 2011).
Ghrelin is a growth-hormone-releasing peptide, first isolated from the rat stomach. This hormone increases the appetite, decreases metabolism and therefore participates in long term food intake (Kojima et al. 1999).
A number of previous studies showed that ghrelin has anxiogenic-like actions. In one of these studies, i.c.v. administration of ghrelin or its direct microinjection into the hippocampus, amygdale, or dorsal raphe nucleus induced anxiety-like behaviors in certain rat strains in the elevated plus maze, open field test and step-down/inhibitory avoidance test (Carlini et al. 2002, 2004). Also, both intra-third cerebroventricular and intraperitoneal administration of ghrelin to mice decreased duration of time in and number of entries into the open arms of an elevated plus maze when assessed ten minutes after injection (Mortensen et al. 2007).Finally, a recent study demonstrated that intracerebroventricularly administration of ghrelin to neonatal chicks in a dose-dependent manner increased the latency to ambulate but decreased ambulation activity in the Open Field test, indicating an anxiogenic effect (Carvajal et al. 2009).
These findings of anxiogenic-like effects of raised ghrelin level differ from the results of several other studies. Calorie-restricted wild-type mice showed anxiolytic- and antidepressant-like behavior in the elevated plus maze and forced swim test, respectively, as compared to wild-type mice (Lutter et al. 2008). Also, high-anxiety WKY rats had lower circulating ghrelin than SPD rats in both the fasted and fed state (Kristensson et al. 2007). Masayuki Kanehisa reported that in forced swimming tests, rats that received antisense DNA for ghrelin, immobilization time decreased. Also, Ghrelin antisense oligonucleotides produced an anxiolytic-like effects in the elevated plus maze test, black and white test, or conditioned fear tests (Kanehisa et al. 2006).
In present study, T. gondii infection resulted to decrease in ghrelin serum level. Similar to our study, Erensoy et al. (2010) reported serum concentrations of acylated and desacylated ghrelin were markedly decreased in subjects with intestinal parasitic infections. As the level of IL-5, IL-6 and IL-10 were high in T. gondii infected patients (Matowicka-Karna et al. 2009) and TNF-α and IL-6 suppresses the ghrelin expression in a dose-dependant manner (Lao et al. 2013). It seems that decrease in ghrelin level in our study might be related to increase in interleukin concentrations. We did not investigate serum level of interleukins in this study, thus, future studies should verify the relation between ghrelin and cytokines in T. gondii infection. However, as ghrelin is an appetite-stimulating hormone and ghrelin is decreased following T. gondii infection, it is possible T. gondii infection result to appétit supression. This issue also requires further evaluation.
Conclusion
It seems that latent T. gondii infection decreases the ghrelin serum level but does not change anxiety and depression like behaviors.
Acknowledgments
we are grateful to Dr. Mohammad Reza Ibrahimi Dep. Of Psychology, School of Medicine, AJA University of Medical Sciences, Tehran, Iran for providing Elevated plus maze for anxiety measurement. This research was supported by the research project of AJA University of Medical Sciences (591107).
References
- Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. 2009;197(12):905–908. doi: 10.1097/NMD.0b013e3181c29a23. [DOI] [PubMed] [Google Scholar]
- Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H2K mice. Behav Genet. 1999;26:263–271. doi: 10.1023/A:1021694307672. [DOI] [Google Scholar]
- Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299(5):739–743. doi: 10.1016/S0006-291X(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313(3):635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- Carvajal P, Carlini VP, Schiöth HB, de Barioglio SR, Salvatierra NA. Salvatierra, Central ghrelin increases anxiety in the open field test and impairs retention memory in a passive avoidance task in neonatal chicks. Neurobiol Learn Mem. 2009;91(4):402–407. doi: 10.1016/j.nlm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. Ethological evaluation of the effects of acute and chronic buspirone treatment in the murine elevated plus-maze test: comparison with haloperidol. Psychopharmacology [Berl] 1994;114(2):288–296. doi: 10.1007/BF02244851. [DOI] [PubMed] [Google Scholar]
- Erensoy A, Aydin S, Kelestimur N, Kirbag S, Kuk S. The change of ghrelin levels in intestinal parasitic infections. JMB. 2010;29(1):34–38. [Google Scholar]
- Flegr J, Zitková S, Kodym P, Frynta D. Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii. Parasitology. 1996;113(Pt 1):49–54. doi: 10.1017/S0031182000066269. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, Colasante C, et al. Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats. A behavioral analysis. Behav Brain Res. 2007;177(1):70–79. doi: 10.1016/j.bbr.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Groër Maureen W., Yolken Robert H., Xiao J.-C., Beckstead Jason W., Fuchs Dietmar, Mohapatra Shyam S., Seyfang Andreas, Postolache Teodor T. Prenatal depression and anxiety in Toxoplasma gondii–positive women. American Journal of Obstetrics and Gynecology. 2011;204(5):433.e1-433.e7. doi: 10.1016/j.ajog.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groër MW, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biol Psychiatry. 2012;72(4):290–295. doi: 10.1016/j.biopsych.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenter W, Bieliński M, Deptuła A, Zalas-Wiecek P, Piskunowicz M, Szwed K et al (2012) Does Toxoplasma gondii infection affect cognitive function? A case control study. Folia Parasitol (Praha). 2012 Jun; 59(2):93–98. Erratum in: Folia Parasitol (Praha) 59(4):253–254 [DOI] [PubMed]
- Gulinello M, Acquarone M, Kim JH, Spray DC, Barbosa HS, Sellers R, et al. Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes Infect. 2010;12(7):528–537. doi: 10.1016/j.micinf.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen SK, Weiss LM. Toxoplasmosis. Handb Clin Neurol. 2013;114:125–145. doi: 10.1016/B978-0-444-53490-3.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Celano CM, Januzzi JL. The relationship between depression, anxiety and cardiovascular outcomes in patients with acute coronary syndromes. Neuropsychiatr Dis Treat. 2010;6:123–136. doi: 10.2147/NDT.S6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Akiyoshi J, Kitaichi T, Matsushita H, Tanaka E, Kodama K, et al. Administration of antisense DNA for ghrelin causes an antidepressant and anxiolytic response in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1403–1407. doi: 10.1016/j.pnpbp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Kannan G, Moldovan K, Xiao JC, Yolken RH, Jones-Brando L, Pletnikov MV. Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol [Praha] 2010;57(2):151–155. doi: 10.14411/fp.2010.019. [DOI] [PubMed] [Google Scholar]
- Kar N, Misra B. Toxoplasma seropositivity and depression: a case report. BMC Psychiatry. 2004;4:1. doi: 10.1186/1471-244X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kristensson E, Sundqvist M, Håkanson R, Lindström E. High gastrin cell activity and low ghrelin cell activity in high-anxiety Wistar Kyoto rats. J Endocrinol. 2007;193(2):245–250. doi: 10.1677/JOE-07-0028. [DOI] [PubMed] [Google Scholar]
- Kristenssson Elin, Sundqvist Monika, Astin Maria, Kjerling Marita, Mattsson Hillevi, Dornonville de la Cour Charlotta, Håkanson Rolf, Lindström Erik. Acute psychological stress raises plasma ghrelin in the rat. Regulatory Peptides. 2006;134(2-3):114–117. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Lao KM, Lim WS, Ng DL, Tengku-Muhammad TS, Choo OC, Chew H. Molecular regulation of ghrelin expression by pro-inflammatory cytokines TNF-α and IL-6 in rat pancreatic AR42J cell line. J Biol Life Sci. 2013;4(1):32–40. [Google Scholar]
- Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46(3):321–340. doi: 10.1016/0163-7258(90)90021-S. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matowicka-Karna J, Dymicka-Piekarska V, Kemona H. Does Toxoplasma gondii infection affect the levels of IgE and cytokines [IL-5, IL-6, IL-10, IL- 12, and TNF-alpha]? Clin Dev Immunol. 2009;2009:374696. doi: 10.1155/2009/374696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Torrey EF, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61(5):688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Okome-Nkoumou M, Guiyedi V, Ondounda M, Efire N, Clevenbergh P, Dibo M, Dzeing-Ella A. Opportunistic diseases in HIV-Infected patients in Gabon following the administration of highly active antiretroviral therapy: a retrospective study. Am J Trop Med Hyg. 2014;90(2):211–215. doi: 10.4269/ajtmh.12-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biol Psychiatry. 2012;72(4):290–295. doi: 10.1016/j.biopsych.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddel M, Mehbod ASA, Karamy M. Toxoplasma gondii infection in neonates. Iran J Parasitol. 2007;2(3):34–37. [Google Scholar]
- Shaddel M, Mirzaii-Dizgah I, Hoshangi M. Anti-Toxoplasma gondii antibody levels in blood supply of Shiraz blood transfusion institute, Iran. Iran J Parasitol. 2014;9(1):120–124. [PMC free article] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12-13):1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology [Berl] 1993;111(3):323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. 2007;104(15):6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]