Abstract
Trichomonas vaginalis is a parasitic protozoan that is the aetiological agent of trichomoniasis, the most common non-viral sexually transmitted disease worldwide. Currently, the compound of choice for the treatment of T. vaginalis infections is metronidazole, however, it has many side effects and an increase in metronidazole-resistant trichomoniasis has been observed. Medicinal plants could be a source of new antiprotozoal drugs with high activity, low toxicity and lower price. The present work was carried out to investigate the therapeutic potential of Nigella sativa alcoholic extract and oil, as well as Phaseolus vulgaris (kidney bean) lectin and their in vitro activity on the ultrastructure of T. vaginalis trophozoites in comparison to metronidazole, as detected by transmission electron microscope. Both N. sativa oil and P. vulgaris lectin showed high toxic effect as evidenced by severe cell damage with cytoplasmic and nuclear destruction, while the effect of N. sativa alcoholic extract was moderate. Therefore, these two extracts could offer an effective, cheaper and more safe alternative for metronidazole in treatment of trichomoniasis.
Keywords: Trichomonas vaginalis, Nigella sativa extracts, Phaseolus vulgaris lectin, Transmission electron microscope
Introduction
Trichomonas vaginalis (T. vaginalis) is a parasitic protozoan that is the aetiological agent of trichomoniasis, the most common non-viral sexually transmitted disease worldwide (Rosa et al. 2011), with an estimation of 250 million cases worldwide (Mundodi et al. 2006).
Trichomonas vaginalis colonizes the female and male urogenital tract and it is capable of causing severe vaginal, ectocervical, prostatic and urethral inflammation and it is linked with sterility, pelvic inflammatory disease, adverse pregnancy outcomes, postnatal complications and cervical cancer (Schwebke and Burgess 2004; Nanda et al. 2006; Johnston and Mabey 2008). Moreover, infected individuals are predisposed to a higher transmission rate of HIV (Petrin et al. 1998), and, a recent study showed a relationship between trichomoniasis and prostate cancer (Sutcliffe et al. 2009).
Currently, the compound of choice for the treatment of T. vaginalis infections is metronidazole (MTZ), which has been effectively used since the 1960s (Durel et al. 1960). However, an increase in MTZ-resistant trichomoniasis has been observed (Lossick and M¨uller M and Gorrell TE, 1986). Furthermore, it is known to be carcinogenic in rats and mice and mutagenic in bacteria, thus it should not be used during pregnancy particularly during the first trimester. It is also known to be injurious to new born (World Health Organization 1995). Therefore alternative drugs are necessary for the treatment of trichomoniasis (Giordani et al. 2008).
Medicinal plants could be a source of new antiprotozoal drugs with high activity, low toxicity and lower price (Tagboto and Townson 2001). Nigella sativa (Family: Ranunculacea), commonly known as black seed, black cumin or habatul Barakah, is an annual herbaceous plant growing in Mediterranean countries and it is one of the native plants that are widely distributed in Egypt. It has been used for centuries as a spice, food preservative and curative or medicinal remedy for various ailments, including infectious diseases. It is one of the important medicines of Tibbe Nabawi (Prophetic Medicine) and identified as the curative black cumin in the Holy Bible. The seeds have been considered one of the potential natural sources in folk medicine (Randhawa and Al-Ghamdi 2002; Ali and Blunden 2003).
Crude alcoholic extract and essential oil of N. sativa were proved to have many therapeutic effects. The active principles extracted from N. sativa seeds are mostly from its essential (volatile) oil. The N. sativa alcoholic extract was found to be as effective as MTZ in the cure of giardiasis (Bishara and Masoud 1992). Moreover, aqueous extract has demonstrated inhibitory effect against candidiasis (Khan et al. 2003) and a potential therapeutic effect against Blastocystis hominis (El Wakil 2007).
Cell surface glycoconjugates of parasites have been postulated to play an important role in a variety of biological functions. Lectins are carbohydrate-binding proteins; a wide range of biological actions is mediated by lectin-glycoprotein interactions, including cellular differentiation, adherence and cytotoxicity to human cells (Sharon 1996). Lectins are very widely distributed in the plant kingdom, particularly among the legumes such as kidney bean and pea (Brinda et al. 2005). A number of diverse physiological roles have been proposed for these proteins, including mitogenic (Sharma et al. 2009), antifungal (Barrientos and Gronenborn 2005), and antitumor (Li et al. 2008) activities. The presence of lectin receptors on the surface membrane of T. vaginalis has been shown. It was suggested that the pathogenicity of T. vaginalis depends on a lectin specifically sensitive to N-acetyl-d-glucosamine (GlcNAc) (Roussel et al. 1991).
Therefore, since new alternatives for treatment of trichomoniasis, that are effective, safe and of low cost are needed, the present work was carried out to investigate the therapeutic potential of N. sativa alcoholic extract and oil, as well as Phaseolus vulgaris (kidney bean) lectin and their in vitro activity on the ultrastructure of T. vaginalis trophozoites in comparison to MTZ, as detected by transmission electron microscope (TEM).
Materials and methods
Parasites and culture
Trichomonas vaginalis was isolated from vaginal washouts of female patients attending the outpatient clinic, Gynecology and Obstetrics Hospital, Ain Shams University. One drop of vaginal washout sediment was examined microscopically for motile T. vaginalis trophozoites (Cheesbrough 1998). Few drops of sediment containing the trophozoites were inoculated into 9 ml of TYM medium (pH 6.0) at 37 °C, supplemented with 0.9 ml of heat inactivated horse serum (in a water bath at 56 °C for 30 min.), 0.1 ml penicillin G sodium (1,000,000 IU/ml) and 0.1 ml streptomycin sulfate (100,000 μg/ml) (Diamond 1957). Isolates were sub-cultured every 48 h in TYM medium and maintained in Parasitology Diagnostic and Research Unit, Faculty of Medicine, Ain Shams University.
Extracts of Nigella sativa
Plant materials and oil extract
Nigella sativa seeds and the oil were purchased from a local herb store. It was coded as N. sativa oil (NsO). Oil was diluted in incubation medium to yield 500 µg/ml.
Crude (alcoholic) extracts
Nigella sativa seeds were washed to remove any debris and air dried. Amount of 250 g seeds were ground to powder and soaked in 85 % aqueous-methanol (1/10, w/v) for 24 h. The extract is filtered through a Buchner funnel. The plant residue is re-extracted with 50 % methanol for additional 2 h. After filtration of the slurry, the two extracts are combined and concentrated under reduced pressure on a rotatory evaporator below 40 °C until most of the methanol has been removed (Houcher et al. 2007). The brownish black crude extract, yielding about 27 %, was coded as N. sativa crude extract (NsCr). The extract was then diluted in incubation medium to yield 1 mg/ml.
Isolation of Phaseolus vulgaris (kidney bean) lectin
Phaseolus vulgaris seeds were obtained from the Agriculture Research Center, Giza, Egypt. Kidney bean lectin was extracted using soaking method as described by Hou et al. (2010). Kidney beans were ground to a powder in an electric mill and filtered through 80 mesh grit. The powder (5 g) was mixed with 0.15 M NaCl (1:8, w/v) for 48 h at 4 °C, and filtered through 80 mesh grid. Subsequently, the filtrate was centrifuged at 9,168×g for 30 min, and the supernatant was fractionally precipitated with ammonium sulfate at 40, 50, 60, and 70 % saturation, respectively. The four pellets were combined, dissolved in a minimal volume of water, and dialyzed against distilled water at 4° C. The resulted extract was diluted in incubation medium yielding 500 µg/ml.
Experimental design
In the present work, three herbs were tested; NsO (500 µg/ml), NsCr extract (1 mg/ml) and P. vulgaris lectin (500 µg/ml). T. vaginalis trophozoites were incubated with each herb for 24 and 48 h. In addition, culture tubes containing T. vaginalis trophozoites without adding any herbs was used as control tubes.
Determination of protein concentration of P. vulgaris extract
Bradford’s method (Bradford 1974) was used for protein quantification, using bovine serum albumin (BSA) as the standard. The relative protein concentration of the eluted fractions was determined by measuring the absorbance with a Carl Zeiss Spekol Spectrophotometer at A595.
Electron microscopy for T. vaginalis
The ultrastructural changes of the parasite in culture were studied using TEM. Trophozoites were incubated for 24 and 48 h with each herb. Culture tubes containing T. vaginalis trophozoites without adding any drugs (control tubes), as well as the culture tubes after the incubation period with the drug were chilled in ice and centrifuged at 1000 g for 10 min, trophozoites were fixed with 2.5 % (v/v) glutaraldehyde in 0.1, cacodylate buffer, pH 7.2 for 1 h. Fixed samples were washed twice in PBS and post-fixed with 1 % (v/v) osmium tetroxide in 0.1 M cacodylate buffer for 30 min, pH 7.2, at room temperature and dehydrated in increasing concentrations of ethanol, followed by a final dehydration in 100 % propylene oxide. Samples were embedded in Epon 812. Thin sections were cut in the Reichert ultra microtome, mounted on copper grids, stained with uranyl acetate and lead citrate, and examined in a JOEL1200EXII electron microscope. For this study, 40–50 fields of each preparation were analyzed (Cedillo-Rivera et al. 2002).
Ethical consideration
An informed consent was taken from all patients before taking vaginal samples.
Results
Figure 1 shows the TEM of T. vaginalis trophozoite without adding any herb (control tube).
Fig. 1.
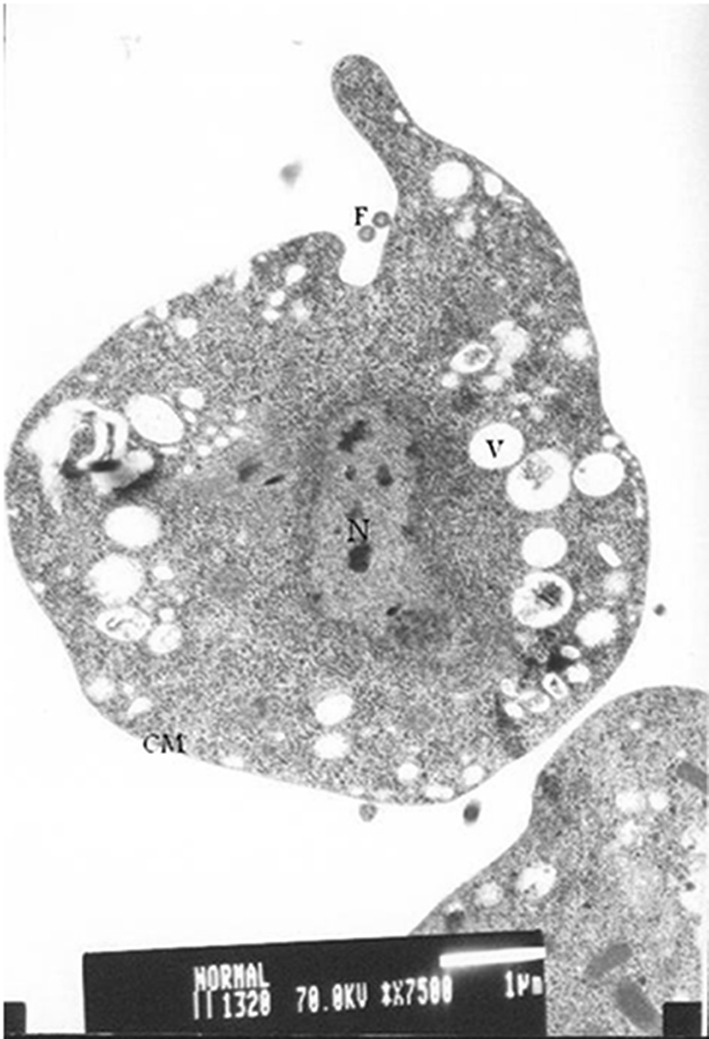
TEM of Trichomonas vaginalis trophozoites from control tubes (without adding any herb) showing intact cell membrane (CM), undulating membrane (UM), flagella (F) and few vacuoles (V). Scale bar 1 µm
The Figs. 2, 3, 4, 5, 6, 7 show the TEM of T. vaginalis trophozoite after 24 and 48 h incubation with N. sativa alcoholic extract and oil, as well as P. vulgaris lectin extract.
Fig. 2.

TEM of Trichomonas vaginalis trophozoites after incubation with Nigella sativa alcoholic extract: After 24 h incubation showing cell swelling with moderate destruction, cell membrane (CM) shows multiple defects (arrows), no undulating membrane or flagella and a small part of the axostyle (A) is still preserved. Scale bar 2 µm
Fig. 3.
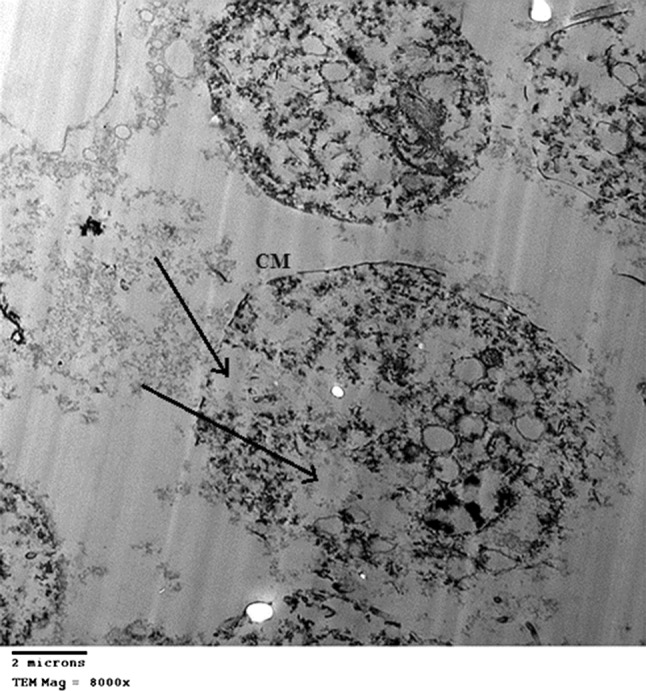
TEM of Trichomonas vaginalis trophozoites after incubation with Nigella sativa alcoholic extract: After 48 h incubation showing severe destruction, no undulating membrane or flagella, with moderate to severe nuclear destruction and large cavities (arrows). Cell membrane (CM) is partially destructed. Scale bar 2 μm
Fig. 4.
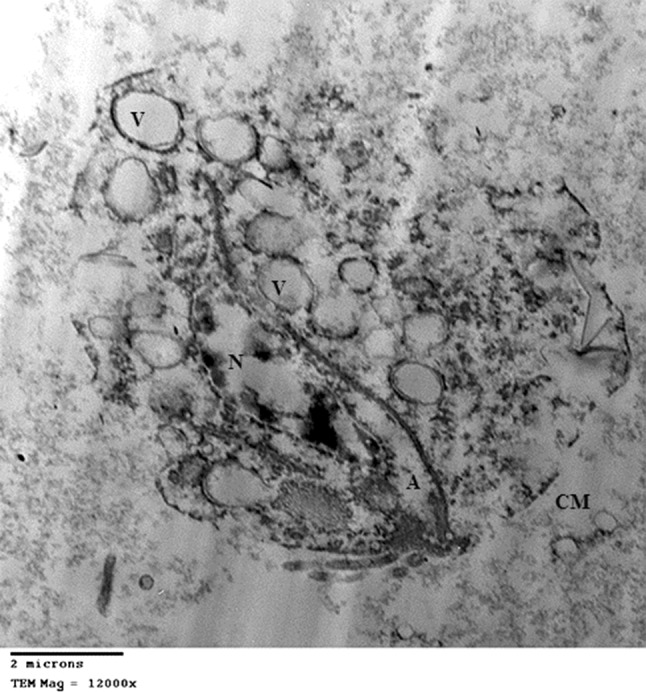
TEM of Trichomonas vaginalis trophozoites after incubation with Nigella sativa oil: After 24 h incubation showing cell swelling with moderate destruction, cell membrane (CM) is partially destructed. There are many vacuoles (V) with nuclear destruction (N), while the axostyle (A) is still preserved. Scale bar 2 µm
Fig. 5.

TEM of Trichomonas vaginalis trophozoites after incubation with Nigella sativa oil: After 48 h incubation showing severe cytoplasmic and nuclear destruction, with large cavities (arrows). Cell membrane (CM) is completely destructed. Scale bar 2 µm
Fig. 6.
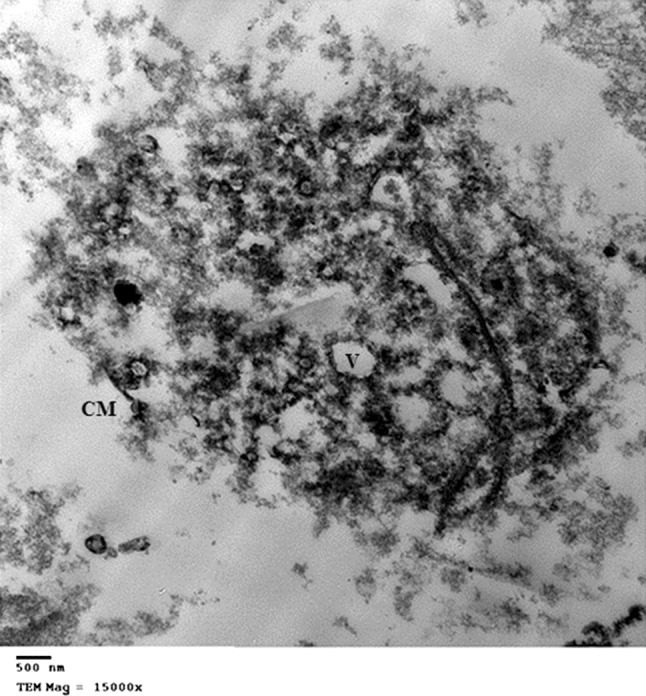
TEM of Trichomonas vaginalis trophozoite after incubation with Phaseolus vulgaris lectin extract: After 24 h incubation showing moderate cell destruction, with vacuoles (V). Cell membrane (CM) shows moderate destruction. No undulating membrane or flagella. Nucleus is severely destructed, while a part of the axostyle is still conserved. Scale bar 500 nm
Fig. 7.
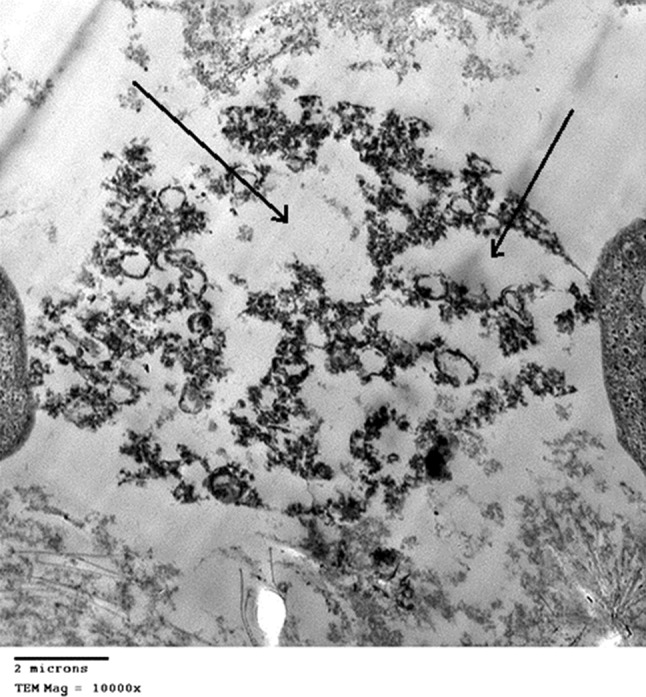
TEM of Trichomonas vaginalis trophozoite after incubation with Phaseolus vulgaris lectin extract: After 48 h incubation showing severe cell destruction with large cavities (arrows). Cell membrane is completely destructed, as well as the nucleus and the cytoplasm. Scale bar 2 µm
Total protein concentration of P. vulgaris extract was calculated by 162 mg with extraction rate estimated by 3.24 %.
Discussion
Trichomonas vaginalis which is the aetiological agent of trichomoniasis is a parasitic protozoan that colonizes the female and male urogenital tract. A typical T. vaginalis cell, grown in axenic medium, is characterized by a pear-shaped body, four anterior flagella and a recurrent flagellum adhered to the cell body that runs toward the posterior region of the cell, forming an undulating membrane (Rosa et al. 2011). By TEM, one nucleus, hydrogenosomes, an axostyle which runs the length of the parasite, a glycogen-rich cytoplasm, a variety of vacuoles (including lysosomes), and numerous micro-tubules with varied structures are observed (Warton and Honnigberg 1979).
Metronidazole is the drug of choice recommended for the treatment of human trichomoniasis (Fernando et al. 2007). However, it can lead to drug resistance and potential risks of mutagenesis and carcinogenicity (World Health Organization 2001). TEM revealed that T. vaginalis trophozoites treated with MTZ (1 and 3 µg/ml, for 48 h) are swollen and have many prominent alterations; redistribution of the pinocytotic and phagocytic vacuoles, with large empty areas in the cytoplasm, and disruption of the plasma membrane (Cedillo-Rivera et al. 2002) (Fig. 8).
Fig. 8.
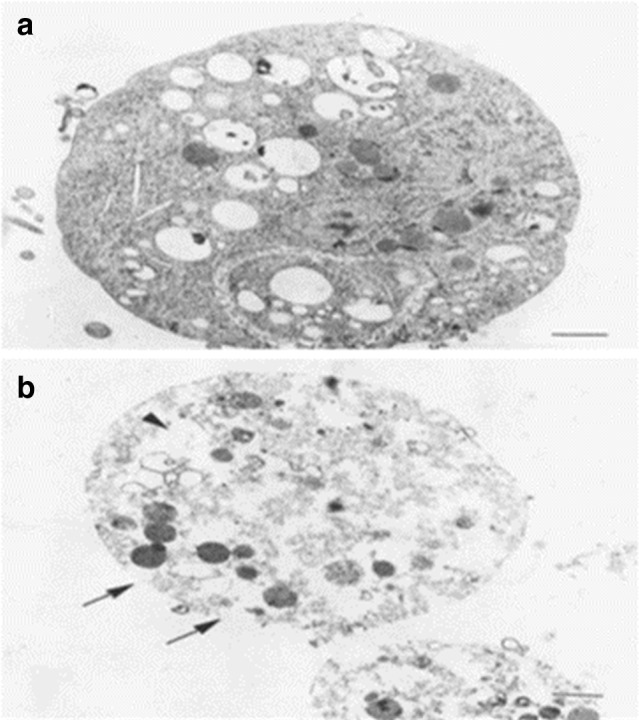
TEM of Trichomonas vaginalis trophozoites treated by metronidazole (1 µg/ml) for 48 h showing cell swelling and altered distribution of vacuoles (a). At higher concentration (3 µg/ml) (b) the effects were more pronounced and large empty areas and damage to the plasma membrane was evident (arrows). Bar scale 1 µm (Cedillo-Rivera et al. 2002)
Crude extracts and essential oil of N. sativa were proved to have many therapeutic effects (Salem 2005). It was found to exert an anti-Toxoplasma and anti-malarial effect as it significantly decreased the parasitaemia and increased the survival times of mice infected with Plasmodium berghei (Abdulelah and Zainal-Abidin 2007). Moreover, its alcoholic extract was found to be as effective as MTZ in the cure of giardiasis (Issa 2003). Nigella sativa oil was found to afford protection and prevent liver damage induced by Schistosoma mansoni infection in mice by modulating the immune response and reducing inflammation (Mahmoud 2002).
Concerning lectins, Rios-de Alvarez et al. (2012a) suggested that plant lectins can have an inhibitory effect of the feeding behavior of first stage larvae of ovine gastrointestinal nematodes in vitro. Similarly, another study performed by Rios-de Alvarez et al. (2012b) showed that phytohaemagglutinins extracted from P. vulgaris are the responsible for inhibiting the feeding of Trichostrongylus colubriformis larvae. Moreover, they reported that P. vulgaris has two possible modes of action in vivo with no adverse clinical effects; a direct anthelminthic effect on nematode fecundity and an indirect effect through enhancing local immune response in the host.
The purpose of this study is to investigate the therapeutic potential of N. sativa alcoholic extract and oil, as well as P. vulgaris (kidney bean) lectin and their activity on the ultrastructure of T. vaginalis in comparison to MTZ, as detected by TEM. TEM studies were performed on trophozoites following in vitro exposure to each plant extract for 24 and 48 h.
TEM demonstrated normal ultrastructure in control preparations, showing intact cell membrane, undulating membrane, flagella and nucleus (Fig. 1). After 24 h incubation with N. sativa alcoholic extract, T. vaginalis trophozoites show cell swelling with moderate destruction, cell membrane is still intact except for some defects, no undulating membrane or flagella but a small part of the axostyle is still preserved and the vacuoles are still small in size (Fig. 2). After 48 h incubation, the trophozoites show severe cytoplasmic and nuclear destruction with large cavities, cell membrane is partially destructed (Fig. 3).
Nigella sativa oil and Phaseolus vulgaris (kidney bean) lectin, both caused similar ultrastructural changes in T. vaginalis trophozoites. After 24 h incubation with NsO and P. vulgaris (Figs. 4 and 6, respectively), the trophozoites show cell swelling with moderate destruction, cell membrane is partially destructed with numerous defects. Many vacuoles are found, which are larger in size and number in comparison to those found after incubation with N. sativa alcoholic extract. Moreover, there is severe nuclear destruction but a part of the axostyle is still preserved. No undulating membrane or flagella.
After 48 h incubation with the same plant extracts; NsO and P. vulgaris lectin (Figs. 5 and 7, respectively), T. vaginalis trophozoites show severe cell damage, the cytoplasm and nucleus are severely destructed with large cavities. Cell membrane is completely destructed.
Many studies performed on T. vaginalis showed similar findings like that achieved by Cedillo-Rivera et al. (2002) Who found that nitazoxanide caused cell swelling and distorted cell shape, plasma membrane damage, and the formation of extensive empty areas in the cytoplasm of the protozoa as shown by TEM. Another TEM study was performed by Pan et al. (2009) where T. vaginalis was treated with three peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor. TEM showed that severe swelling preceded cell death and breakage of the outer membrane, and the intracellular inclusion was found to have effluxed extracellularly.
The remarkable effect of NsO may be attributed to the fact that it has a high content of poly-unsaturated fatty acids, the major constituent of which is linoleic acid (60.0–61.7 %) (Edris 2011), followed by oleic and palmitic acids (Ramadan and Mörsel 2002). Many other studies have proved the nematocidal activity of NsO like that performed by Shalaby and El-Moghazy (2013) on adult Toxocara vitulorum where NsO caused extensive and severe disorganization of the cuticle and body musculature. Abu El Ezz (2005) found that NsO has anthelminthic effect in the rats infected with Trichinella spiralis and that they increased the production of antibodies generated during life cycle of this parasite. Moreover, Ayaz et al. (2007) found that NsO has an antiparasitic effect against Hymenolepis nana through stimulating the immune system.
Zaoui et al. (2002) showed that the low toxicity of N. sativa fixed oil, evidenced by high LD50 values, key hepatic enzyme stability and organ integrity, suggests a wide margin of safety for therapeutic doses of N. sativa fixed oil.
Lectins are carbohydrate-binding proteins or glycoproteins of non-immune origin that can agglutinate cells or precipitate glycoconjugates and polysaccharides (Alizadeh et al. 1997). Because of their chemical properties lectins have become useful tools in several fields of biological research such as immunology, cell biology, membrane structure, cancer research and genetic engineering (Endriga et al. 2005). A literature survey revealed that plant lectins showed an inhibitory effect on nematodes and their larvae, with no side effects (Rios-de Alvarez et al. 2012b). But no study found to evaluate its effect on T. vaginalis.
The present study indicate that lectins may be exploited as potential chemotherapeutic agents against trichomoniasis and that it might be worthwhile looking into the potential activity of lectins against other pathological microorganisms.
Ultrastructural alterations in the T. vaginalis trophozoites as observed in the present work thus provide a clear evidence of the toxic effect of NsO and P. vulgaris lectin, and to a lesser extent N. sativa alcoholic extract, that could offer an effective, cheaper and safer alternative for MTZ.
References
- Abdulelah H, Zainal-Abidin B. In vivo anti-malarial tests of Nigella sativa (Black Seed) different extracts. Am J Pharmacol Toxicol. 2007;2(2):46–50. [Google Scholar]
- Abu El Ezz NM. Effect of Nigella sativa and Allium cepa oils on Trichinella spiralis in experimentally infected rats. J Egypt Soc Parasitol. 2005;35(2):511–523. [PubMed] [Google Scholar]
- Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, Silvany RE, Meyer DR, Dougherty JM, McCulley JP. In vitro amoebicidal activity of propamidine and pentamidine isethionate against Acanthamoeba species and toxicity to corneal tissues. Cornea. 1997;16:94–100. [PubMed] [Google Scholar]
- Ayaz E, Yilmaz H, Ozbek H, Tas Z, Orunc O. The effect of Nigella sativa oil against Aspiculuris tetraptera and Hymenolepis nana in naturally infected mice. Saudi Med J. 2007;28(11):1654–1657. [PubMed] [Google Scholar]
- Barrientos LG, Gro—nenborn AM. The highly specific carbohydrate—binding protein cyanovirin-N: structure, anti-HIV/Ebola activity and possibilities for therapy. Med Chem. 2005;5(1):21–31. doi: 10.2174/1389557053402783. [DOI] [PubMed] [Google Scholar]
- Bishara SA, Masoud SI. Effect of Nigella sativa extract on experimental giardiasis. The New Egypt J Med. 1992;7:1–3. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1974;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brinda KV, Surolia A, Vishveshwara S. Insights into the quaternary association of proteins through structure graphs: a case study of lectins. Biochem J. 2005;391(1):1–15. doi: 10.1042/BJ20050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedillo-Rivera R, Chávez B, González-Robles A, Tapia A, Yépez-Mulia L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J Eukaryot Microbiol. 2002;49(3):201–208. doi: 10.1111/j.1550-7408.2002.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Cheesbrough M. Parasitological tests. In: CheesbroughM, editor. District laboratory practice in tropical countries Part 1. Cambridge: Cambridge Low Price Editions, Cambridge University Press; 1998. pp. 178–310. [Google Scholar]
- Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- Durel P, Couture J, Collart P, Girot C. Flagyl (metronidazole) Br J Vener Dis. 1960;36:154–162. doi: 10.1136/sti.36.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edris Amr E. The Chemical Composition and the Content of Volatile Oil: Potential Factors that Can Contribute to the Oxidative Stability ofNigella sativaL. Crude Oil. Journal of Dietary Supplements. 2011;8(1):34–42. doi: 10.3109/19390211.2010.547242. [DOI] [PubMed] [Google Scholar]
- El Wakil HS. Evaluation of the in vitro effect of Nigella sativa aqueous extract on Blastocystis hominis isolates. J Egypt Soc Parasitol. 2007;37(3):801–813. [PubMed] [Google Scholar]
- Endriga MA, Mojica EE, Merca FE, Lacsamana MS, Deocaris CC. Evaluation of some lectins as anti-protozoal agents. J Med Sci. 2005;5:31–34. [Google Scholar]
- Fernando C, Lilian Y, Amparo T. Effect of Mexican medicinal plant used to treat trichomoniasis on Trichomonas vaginalis trophozoites. J Ethnopharmacol. 2007;113:248–251. doi: 10.1016/j.jep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Giordani RB, De Almeida MV, Fernandes E, França da Costa C, De Carli GA, Tasca T, Zuanazzi JA. Anti-Trichomonas vaginalis activity of synthetic lipophilic diamine and amino alcohol derivatives. Biomed Pharmacother. 2008;63(8):613–617. doi: 10.1016/j.biopha.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Hou Y, Hou Y, Yanyan L, Qin G, Li J. Extraction and purification of a lectin from red kidney bean and preliminary immune function studies of the lectin and four Chinese herbal polysaccharides. J Biomed Biotechnol. 2010 doi: 10.1155/2010/217342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houcher Z, Boudiaf K, Benboubetra M, Houcher B. Effects of methanolic extract and commercial oil of Nigella sativa on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines. 2007;18:8–18. [Google Scholar]
- Issa RM. Using Nigella sativa (Habbet El baraka) in treatment of some parasitic diseases. Egypt J Med Sci. 2003;24:435–446. [Google Scholar]
- Johnston VJ, Mabey DC. Global epidemiology and control of Trichomonas vaginalis. Curr Opin Infect Dis. 2008;21:56–64. doi: 10.1097/QCO.0b013e3282f3d999. [DOI] [PubMed] [Google Scholar]
- Khan MA, Ashfaq MK, Zuberi HS, Mhmood MS, Gilani AH. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother Res. 2003;17(2):183–186. doi: 10.1002/ptr.1146. [DOI] [PubMed] [Google Scholar]
- Li J, Wu H, Hong J, Xu X, Yang H, Wu B, Wang Y, Zhu J, Lai R, Jiang X, Lin D, Mark C, Rees HH. Odorranalectin is a small peptide lectin with potential for drug delivery and targeting. PLoS ONE. 2008 doi: 10.1371/journal.pone.0002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossick JG, M¨uller M, Gorrell TE. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis. 1986;153:948–955. doi: 10.1093/infdis/153.5.948. [DOI] [PubMed] [Google Scholar]
- Mahmoud MR, El-Abhar HS, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79:1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- Mundodi V, Kucknoor AS, Chang TH, Alderete JF. A novel surface protein of Trichomonas vaginalis is regulated independently by low iron and contact with vaginal epithelial cells. BMC Microbiol. 2006;6(1):6. doi: 10.1186/1471-2180-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda N, Michel RG, Kurdgelashvili G, Wendel KA. Trichomoniasis and its treatment. Expert Rev Anti Infect Ther. 2006;4:125–135. doi: 10.1586/14787210.4.1.125. [DOI] [PubMed] [Google Scholar]
- Pan CY, Chen JY, Lin TL, Lin CH. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides. 2009;30(6):1058–1068. doi: 10.1016/j.peptides.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan MF, Mörsel JT. Characterization of phospholipid composition of black cumin (Nigella sativa L.) seed oil. Nahrung. 2002;46(4):240–244. doi: 10.1002/1521-3803(20020701)46:4<240::AID-FOOD240>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Randhawa MA, Al-Ghamdi MJ. A review of pharmacotherapeutic effects of Nigella sativa. Pak J Med Res. 2002;41(2):77–83. [Google Scholar]
- Rios-de Alvarez L, Jackson F, Greer A, Bartley Y, Bartley D, Grant G, Huntley J. In vitro screening of plant lectins and tropical plant extracts for anthelminthic properties. Vet Parasitol. 2012;186(3–4):390–398. doi: 10.1016/j.vetpar.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Rios-de Alvarez L, Jackson F, Greer A, Grant G, Jackson E, Morrison A, Huntley J. Direct anthelminthic and immunostimulatory effects of oral dosing semi-purified phytohaemagglutinin lectin in sheep infected with Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet Parasitol. 2012;187(1–2):267–274. doi: 10.1016/j.vetpar.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Rosa IA, Rochal DA, Souza W, Urbina JA, Benchimol M. Ultrastructural alterations induced by D 24 (25)-sterol methyltransferase inhibitors on Trichomonas vaginalis. FEMS Microbiol Lett. 2011;315:72–78. doi: 10.1111/j.1574-6968.2010.02178.x. [DOI] [PubMed] [Google Scholar]
- Roussel F, De Carli G, Brasseur P. A cytopathic effect of Trichomonas vaginalis probably mediated by a mannose/N-acetyl-glucosamine binding lectin. Int J Parasitol. 1991;21(8):941–944. doi: 10.1016/0020-7519(91)90170-c. [DOI] [PubMed] [Google Scholar]
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby HA, El-Moghazy FM. In vitro effect of Nigella sativa oil on adult Toxocara vitulorum. Pak J Biol Sci. 2013;16(22):1557–1562. doi: 10.3923/pjbs.2013.1557.1562. [DOI] [PubMed] [Google Scholar]
- Sharma Arishya, Ng Tzi Bun, Wong Jack Ho, Lin Peng. Purification and Characterization of a Lectin fromPhaseolus vulgaris cv.(Anasazi Beans) Journal of Biomedicine and Biotechnology. 2009;2009:1–9. doi: 10.1155/2009/929568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Carbohydrate–lectin interactions in infectious diseases. Adv Exp Med Biol. 1996;408:1–8. [PubMed] [Google Scholar]
- Sutcliffe S, Alderete JF, Till C, Goodman PJ, Hsing AW, Zenilman JM, De Marzo AM, Platz EA. Trichomonosis and subsequent risk of prostate cancer in the prostate cancer prevention trial. Int J Cancer. 2009;124:2082–2087. doi: 10.1002/ijc.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagboto S, Townson S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol. 2001;50:199–295. doi: 10.1016/s0065-308x(01)50032-9. [DOI] [PubMed] [Google Scholar]
- Warton A, Honnigberg B. Structure of Trichomonads as revealed by scanning electron microscopy. J Protozool. 1979;26:56–62. doi: 10.1111/j.1550-7408.1979.tb02732.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1995) Metronidazole. In: Drugs used in parasitic diseases. WHO, Geneva
- World Health Organization (2001) Global prevalence and incidence of selected curable sexually transmitted infections: Overview and estimates, Geneva, Switzerland. Document:WHO/HIV_AIDS/2001.02,WHO/CDS/CSR/EDC/2001.10
- Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9:69–74. doi: 10.1078/0944-7113-00084. [DOI] [PubMed] [Google Scholar]


