Abstract
Fish infections can be readily detected at autopsy, with the recovery of entire tapeworms followed by microscopic examination of the scolex. The examination of faecal material may reveal detached segments from adult tapeworms or eggs, which possess an operculum at the apex. The presence of Bothriocephalus acheilognathi may also be achieved by a squash plate method. Glass slides or plates are used to flatten the intestinal tract and the worms are detected by reflected light and low-power microscopy. Although mature parasites may be conspicuous within the intestine of infected fish, the detection of light infections or presence of juvenile parasites and plerocercoids requires microscopic examination. While these infections may hold little importance to the disease status of individuals, detection may be critical for effective disease control and limiting the spread of infected fish. The pathology may be divided generally into: (i) damage caused by scolex attachment; and (ii) damage caused by the presence of strobila within the intestine lumen. Other organs may exhibit signs of pathological change. For example, infected fish can show signs of nutritional deficiency, with atrophy of hepatocytes within the liver. In severe cases, these changes are consistent with starvation. B. acheilognathi causes a number of physiological changes in juvenile fish. These include: (i) protein depletion; (ii) altered digestive enzyme activity; (iii) elevated muscle fatigue in heavily infected hosts; and (iv) mortality of young fishes. B. acheilognathi can have pronounced detrimental effects on fish. These include severe damage to the intestinal tract, physiological disturbance, reduced growth, condition loss and death. Records of 100 % mortality in hatchery reared common carp (Cyprinus carpio) highlight the pathogenic potential of this parasite.
Keywords: Fish, Cyprinus carpio, Bothriocephalus acheilognathi, Scolex, Strobila, Kashmir
Introduction
The Asian Tapeworm Bothriocephalus acheilognathi Yamaguti 1934 (Cestoda: Bothriocephalidea), is the most important pathogenic cestode of cyprinid fish, which causes bothriocephaliasis and one of the most dangerous helminth parasites of cultured carp (Bauer et al. 1977; Nie and Hoole 2000). The parasite has also been recorded in a range of other freshwater teleost fishes, prompting concern of the disease in wild fish populations (Clarkson et al. 1997; Heckmann 2000). It is listed as a ‘Pathogen of Regional Concern’ by the US Fish and Wildlife Service (2010). B. acheilognathi has been reported under more than 20 different specific names and the most frequently used are Bothriocephalus gowkongensis Yeh 1955 and Bothriocephalus opsariichthydis Yamaguti 1934 (for a list of synonyms—see Kuchta and Scholz 2007). According to Pool and Chubb (1985) and Pool (1987), all descriptions of Bothriocephalus tapeworms from cyprinid hosts represent the same parasite, differing only in length and the shape of the scolex because different methods were used to fix the worm. B. acheilognathi is indigenous to East Asia, but has spread rapidly throughout the world with the trade of fish. The parasite has now been recorded from every continent excluding Antarctica. The ability to successfully colonize new geographical regions has been facilitated by its simple, two-host life cycle (involving common copepod species as an intermediate host) and euryxenous host specificity (very wide range of suitable fish hosts). This has led to the transmission and establishment of B. acheilognathi to many new host species in areas where it has been introduced (Scholz 1999; Salgado-Maldonado and Pineda-Lopez 2003). Once established it may endanger native fish populations, including ecologically sensitive species and fishes that are phylogenetically unrelated to those in which it was introduced (Font and Tate 1994; Dove and Fletcher 2000). B. acheilognathi can have pronounced detrimental effects on fish. These include severe damage to the intestinal tract, physiological disturbance, reduced growth, condition loss and death. Records of 100 % mortality in hatchery reared common carp (Cyprinus carpio) highlight the pathogenic potential of this parasite.
Description
Bothriocephalus acheilognathi typically measures between 3.5 and 8 cm in length and up to 4 mm in width (Yeh 1955), although specimens of 60 cm and even 1 m (Table 1) have been observed (Baer and Fain 1958; Granath and Esch 1983a) Size is a highly variable morphological parameter as it depends on: (i) Ecological conditions (Nedeva and Mutafova 1988); (ii) Host size (Davydov 1978); (iii) Host species (Molnar and Murai 1973a; Granath and Esch 1983a); (iv) Host age; and (v) Intensity of infection (Davydov 1978). Upon relaxation, parasites can also increase in length by a factor of 1.5–2 (Pool 1987). Consequently, parasite size is not a valid feature on which to base identification, despite earlier beliefs (Molnar and Murai 1973a). An important morphological characteristic of B. acheilognathi is its heart-shaped scolex, with a weakly developed apical disc and a pair of deep, slit-like grooves (bothria) positioned dorsoventrally along the scolex (Figs. 1, 2). The scolex is much wider than the first body segments (proglottides). The strobila (body) of the tapeworm consists of numerous proglottides each containing one set of reproductive organs. The shape of these segments differs with maturity. Immature segments lacking fully developed genital organs are always wider than they are long; whereas more developed gravid segments bearing eggs are rectangular and longer than they are wide. However, contraction and relaxation of the segments also causes extreme variation in the length and width ratio of the strobila (Brandt et al. 1981). The male reproductive system is formed by numerous spherical testes situated in the medulla (the region internal to the inner longitudinal musculature). A muscular cirrus sac localized anterior to the ovary opens on the dorsal side of segments into a common genital atrium, which is situated alongside the median line of the body. The female reproductive system is composed by a bilobed ovary situated near the posterior margin of each segment. The vagina, which is short and slightly sinuous, opens into the common genital atrium posterior to the male genital pore. Vitelline follicles are very numerous, circumcortical and confluent between segments. The uterus is saccular, spherical to oval, and opens on the ventral side in the anterior third of the segment. The eggs are thick-walled, operculate (i.e. with a cap—the operculum) on a narrower pole, and usually unembryonated (without a formed embryo) when released into the water.
Table 1.
Characteristics of Bothriocephalus spp. from freshwater Fishes (Measurements in micrometers)
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Species | Bothriocephalus pearsei | B. claviceps | B. formosus | B. acheilognathi |
| Host | Cichlasoma cichlasoma | Anguilla rostrata | Percopsis omiscomaycus | Eleotris Pimephales, |
| Locality | Mexico | Canada | USA | Hawaii, Canada |
| Feature/Authority | Scholz et al. (1996a) | Scholz (1997) | Scholz (1997) | Scholz (1997) |
| Total length (µm) | 26–32 | 54–137 | 21–33 | 22–35 |
| Maximum width (µm) | 0.91 | 2.19 | 0.89–1.06 | 0.71–1.43 |
| Mature segments (L) | 288–496 | 232–448 | 325–530 | 200–508 |
| Mature segments (W) | 528–873 | 934–1,300 | 853–1,025 | 576–1,320 |
| Length/width ratio | 1:1.0–3.1 | 1:2.3–5.6 | 1:1.9–2.9 | 1:1.6–5.0 |
| Gravid segments (L) | 345–1,060 | 240–487 | 280–812 | 280–1,015 |
| Gravid segments (W) | 496–914 | 893–2,110 | 812–1,060 | 480–1,430 |
| Length/width ratio | 1:0.83–2.4 | 1:2.2–7.4 | 1:1.0–3.0 | 1:0.45–4.5 |
| Scolex (L) | 720–880 | 1,180–1,360 | 384–536 | 569–731 |
| Scolex (W) | 224–344 | 232–344 | 134–202 | 660–1120 |
| Terminal disc (W) | 173–224 | 200–344 | 99–131 | 232 |
| Neck (W) | 154–173 | 152–200 | 118–147 | 160–504 |
| Testes number | 28–51 | 39–80 | 24–60 | 33–56 |
| Testes (L) | 28–65 | 56–125 | 28–49 | 34–68 |
| Testes (W) | 21–46 | 40–86 | 26–49 | 26–57 |
| Cirrus-sac (L) | 55–80 | 74–154 | 59–86 | 35–81 |
| Cirrus-sac (W) | 55–80 | 68–93 | 65–81 | 39–84 |
| Ovary (L) | 50–84 | 234–751 | 179–288 | 336–576 |
| Ovary (W) | Absent medially | 77–131 | 58–122 | 70–144 |
| Distribution of vitelline follicles | 14–45 | Absent medially | Confluent | Confluent |
| Vitelline follicles (L) | 13–43 | 17–66 | 18–52 | 19–53 |
| Vitelline follicles (W) | 54–67 | 12–48 | 14–40 | 15–43 |
| Eggs (L) | 32–39 | 54–63 | 49–54 | 45–59 |
| Eggs (W) | 31–37 | 32–35 | 27–41 |
| 5 | 6 | 7 | 8 | |
|---|---|---|---|---|
| Species | Bothriocephalus acheilognathi | B. claviceps | B. claviceps | B. acheilognathi |
| Host | Schizothorax and Cyprinus | Anguilla japonica | Anguilla rostrata | Schizothorax and Cyprinus |
| Locality | Srinagar (Jehlum) | Japan (Lake Biwa) | North America | Srinagar (Jehlum and Dal Lake) |
| Feature/Authority | Ara et al. (2000) | Scholz et al. (2004) | Scholz et al. (2004) | Akhter et al. (2008) |
| Body length (µm) | 175 | 139–161 | 155 | 170 |
| Maximum width (µm) | 3.0 | 2.21–2.37 | 2.9 | 2–3 |
| Scolex (L) | 1.44–1.49 | 1610–1640 | 460 | 0.16 |
| Scolex (W) | 1.15–1.17 | 432–568 | 300 | 0.18 |
| Bothria (L) | 0.58–0.60 | 1,180–1,340 | – | 0.2–0.05 |
| Neck (W) | 1.0–1.03 | 220–316 | – | 0.06–0.05 |
| Testes (L) | 0.02–0.04 | – | – | 0.02–0.06 |
| Testes (W) | 0.01–0.02 | 0.02–0.03 | ||
| Ovary (L) | 0.25–0.27 | – | – | 0.26–0.28 |
| Ovary (W) | 0.04–0.07 | – | – | 0.04–0.07 |
| Eggs (L) | 0.049–0.051 | 52–70 | 58–63 | 0.058 |
| Eggs (W) | 0.021–0.024 | 34–43 | 37–40 | 0.062 |
L Length; W Width
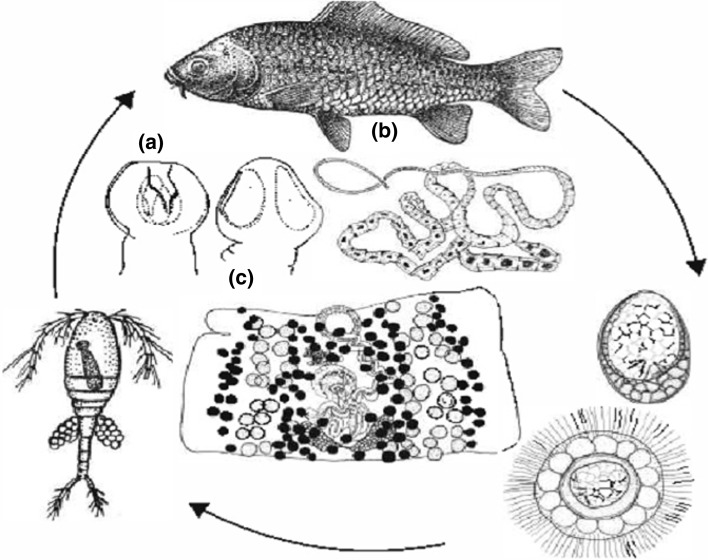
Fig. 1.
Life cycle and Morphology of Bothriocephalus acheilognathi. a Scolex b Total view (segmented body) c Mature segments (proglottis)
Fig. 2.
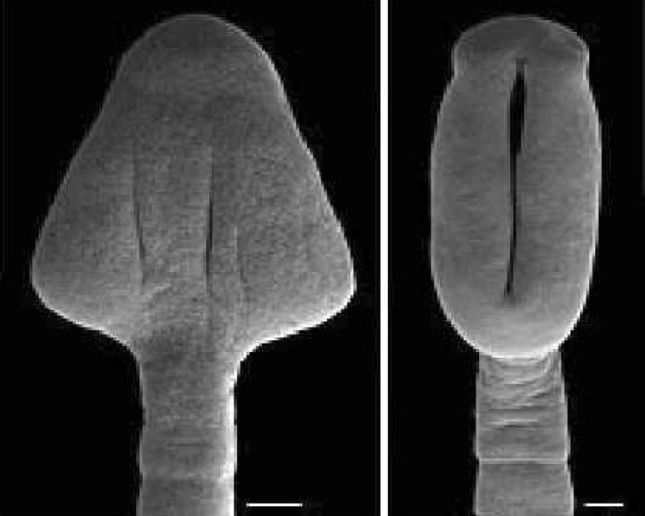
Scanning electron micrographs of the scolex of Bothriocephalus acheilognathi (Bar = 100 µm)
Life cycle and transmission
B. acheilognathi has a simple two host life cycle, involving a planktonic copepod (Copepoda: Cyclopidae) as an intermediate host (Fig. 1). In favorable conditions, the life cycle may be completed in about 1 month. Eggs shed by adult parasites into the gut lumen are released into the water with faeces. Depending on water temperature, an embryo (six-hooked oncosphere or hexacanth) is formed within the egg in a few days. The larva (coracidium) is surrounded by ciliated cells which enable its active movement in the water after hatching from the egg. A number of copepods are suitable intermediate hosts in both experimental and natural conditions. These include species of Acanthocyclops, Cyclops, Macrocyclops, Megacyclops, Mesocyclops, Thermocyclops and Tropocyclops (Marcogliese and Esch, 1989) which are considered common in most fresh waters throughout the world (Pool 1984). Other planktonic crustaceans, such as diaptomids and cladocerans, are not suitable intermediate hosts (Molnar, 1977). After ingestion, the coracidium loses its ciliature and penetrates the gut into the body cavity where is develops from an oncosphere into a plerocercoid (previously also called procercoid Chervy 2002 for terminology of cestode larvae). Larval development is completed in a few weeks depending on the water temperature: within 21–23 days at 28–29 °C (Liao and Shih 1956), but 1.5–2 months at 15–22 °C (Davydov 1978). The life cycle is completed when fish ingest infected copepods. Once established within the intestine of a suitable fish, egg production may begin in as little as 20 days (Liao and Shih 1956). It has been shown that transmission of adult parasites can occur from fish to fish via predation by piscivorous fish on infected prey, a phenomenon known as postcyclic transmission (Odening 1976; Hansen et al. 2007). Local spread caused by aquatic birds, such as Anas platyrhynchos and Chlidonias niger, was assumed to take place based on experiments conducted by Prigli (1975) and field observations (finding of B. acheilognathi in Ixobrychus minutus) by Borgarenko (1981), but this mode of parasite dissemination needs verification. There are also records of the tapeworm in an amphibian (axolotl Ambystoma dumerilii—Garcia-Prieto and Osorio Sarabia 1991) and a snake Thamnophis melanogaster (Perez-Ponce de Leon et al. 2001) although these records may represent only accidental infection.
Definitive (fish) hosts
The most suitable hosts of B. acheilognathi are cyprinids, especially the common carp (C. carpio) and grass carp (Ctenopharyngodon idella). However, the parasite has been reported from approximately 200 species of freshwater fishes, representing ten orders and19 families (Salgado-Maldonado and Pineda-LOpez 2003; R. Kuchta—unpublished data). Nevertheless, maturity of the worm may be reached in only a proportion of these fish species (Dove and Fletcher 2000). Holmes (1979) identified three classes of host, in terms of their ability to allow the maturation of parasites: (i) ‘required hosts’; (ii) ‘suitable hosts’; and (iii) ‘unsuitable hosts’. In ‘required hosts’ parasites usually obtain full maturity. In ‘suitable hosts’ parasites may gain sexual maturity, but are only found in small numbers, while in ‘unsuitable hosts’ parasites may establish but do not reach maturity. Consequently, although a parasite may infect many fish species, the maintenance of the parasite population may rely on a much narrower range in which reproduction takes place (Riggs et al. 1987). Small fish are more commonly and intensively infected with B. acheilognathi than large hosts (Leong 1986). Brouder (1999) detailed a strong negative correlation between size of host and infection intensity of B. acheilognathi.
Geographical distribution
B. acheilognathi was originally described from a small cyprinid fish, Acheilognathus rhombeus (Temminck & Schlegel) (Cypriniformes: Cyprinidae), from Lake Ogura, Kyoto Prefec-ture, Honshu, in Japan (Yamaguti 1934). The parasite is endemic in China, Japan and the Amur River, Asia (Yamaguti 1934; Yeh 1955; Dubinina 1971). Initial movements of B. acheilognathi were linked closely with the spread of carp westwards from Japan and China to Europe during the 1960s and 1970s (Minervini et al. 1985). The spread of B. acheilognathi throughout most parts of the world has been well documented (Bauer and Hoffman 1976) and represents one of the best examples of parasite translocation through man-assisted activities (Bauer and Hoffman 1976; Dove and Fletcher 2000). The rapid spread of this parasite has been assisted by the trade in many cyprinid species for: (i) aquaculture (Minervini et al. 1985); (ii) the ornamental fish industry (Edwards and Hine 1974; Evans and Lester 2001); (iii) aquatic weed control (Maitland and Campbell 1992); (iv) mosquito control (Dove and Fletcher 2000); and (v) the fishing bait industry (Heckmann 2009). The tapeworm was introduced between 1954 and 1962 into the European part of the former USSR as a result of uncontrolled imports of grass carp and common carp from the River Amur and China. Infections of B. acheilognathi became widespread in farmed carp as well as in a variety of wild fishes (Malevitskaya 1958; Radulescu and Georgescu 1962; Bauer and Hoffman 1976). The increased importance of carp for food and weed control led to the rapid spread of B. acheilognathi throughout Europe. By 1970–1975, the tapeworm had colonized several countries of Central and Eastern Europe (Austria, Bulgaria, former Czechoslovakia, Germany, Hungary, Poland and former Yugoslavia—Buza et al. 1970; Petkov 1972; Bauer and Hoffman 1976; Minervini et al. 1985). The worms, originally described in Japan and China, were apparently already introduced populations, while the natural host and geographical origin of this tape worm is the grass carp (Ctenopharyngodon idella) of the Amur River. By 1954–1962, infections had become widespread in farmed fish (in European and Chinese carp) as well as in a variety of wild fish in both the Asian and European USSR (Bauer and Hoffman, 1976). By 1970–1975 records of infections came from Hungary, Yugoslavia, East and West Germany (Molnar and Murai 1973a; Korting 1974; Bauer and Hoffman 1976), and by 1980, worms had become prevalent in France (Denis et al. 1983), Britain (Andrews et al. 1981), South Africa (Van As et al. 1981), USA, and Mexico. Worms were introduced into Mauritius via South Africa (Van As, pers. comm.). New records also came from Asia; Israel, Iraq (Khalifa 1986), Malaysia (Fernando and Furtado 1964), Sri Lanka (Fernando and Furtado 1963) and Korea (Kim et al. 1985). Allied species: B. aegypticus, known only from Egypt (Amin 1978), and B. kivuensis, which occurs in Central and South Africa (Baer and Fain 1960; Mashego 1982). The origin of African populations of B. acheilognathi, first described as Bothriocephalus (Clesthobothrium) kivuensis from barbells (Barbus spp.) by Baer and Fain (1958) in Zaire (now the Democratic Republic of the Congo), is not clear. The tapeworm has since been reported from Egypt and South Africa (Rysavy and Moravec 1975; Brandt et al. 1981). The tapeworm was introduced to the New World in 1965 with grass carp imported from China to Mexico (Lopez Jimenez 1981), then to the USA (Texas) in 1975 and Canada (British Columbia) in 1983 (Hoffman 1999; Choudhury et al. 2006). It was suspected that bait minnows (Plagopterus argentissimus) represented the main source of the tapeworm infecting fish populations in the western USA (Heckmann 2000, 2009). Bothriocephalus acheilognathi has been reported in 13 states of the USA (Hoffman 1999; Choudhury et al. 2006), and from the Great Lakes area (Marcogliese 2008). There is only one published record of B. acheilognathi from South America (Rego et al. 1999), most probably as a result of the import of carp from Europe to Brazil (Cornelio Procopio, Parana). Records of B. acheilognathi from India, Iraq, Israel, Korea, Malaysia, the Philippines, Sri Lanka and Turkey confirm its wide distribution in Asia (Paperna 1996; Hoffman 1999). In Australia, B. acheilognathi was detected in goldfish (Carrasius auratus (L.)) and koi carp (Cyprinus carpio (L.)) (Langdon 1992). The tapeworm is now widely distributed in many native finfish species in eastern Australia (Dove and Fletcher 2000). The parasite was imported to New Zealand with grass carp but was then eradicated during quarantine (Edwards and Hine 1974). The ability of B. acheilognathi to colonize isolated geographical localities has been confirmed by records of the parasite in Puerto Rico, Hawaii and Mauritius and remote subterranean sink- holes (cenotes) in Yucatan (Bunkley-Williams and Williams 1994; Font and Tate 1994; Scholz et al. 1996a). B. acheilognathi has so far been recorded in six continents as a result of the introduction of cyprinids (grass carp, carp), and guppies (mosquito-fish - Gambusia and Poecilia) by man to control mosquito larvae (Hoffman and Schubert 1984; Salgado-Maldonado and Pineda-LOpez 2003). However, the distribution area is limited between 60°N and 40°S. The spread of B. acheilognathi further north or further south in the southern hemisphere is unlikely, because the cestode is thermophilic with an optimum temperature between 22 and 25 °C (Bauer et al. 1981; Granath and Esch 1983a; Hanzelova and Itrian 1986).
Importance of the disease
B. acheilognathi is an important pathogen in aquaculture in Asia and Europe (Bauer et al. 1981; Heckmann 2009). Losses of juvenile fish, with up to 100 % mortality, occur in hatchery ponds. In commercial carp farms, fry (length 38–42 mm) can be infected 28-29 days after hatching (Hanzelova and Itrian 1986). The susceptibility of fry is probably because copepods make up a large proportion of the diet of these fish, and the limited space within the intestinal tract to accommodate these large parasites. Heavy tapeworm burdens cause blockage of the intestine and severe pathological changes, leading to reduced growth, condition and survival (Scott and Grizzle 1979; Granath and Esch 1983b; Hoole and Nisan 1994; Hansen et al. 2006). The tapeworm has also been the cause of disease problems in ornamental fish farms in Australia and Central Europe, involving Poecilia reticulata and Xiphophorus maculates (Evans and Lester 2001; R. Kuchta, unpublished data) and mortality of koi carp (Han et al. 2010). Far less information exists on the impact of B. acheilognathi in wild fish populations. The introduction of this alien tapeworm to new localities can endanger native fish species (Dove and Fletcher 2000; Heckmann 2000, 2009; Salgado-Maldonado and Pineda-LOpez 2003). This may be particularly serious in fish that attain only a small size at maturity, with potential for reduced recruitment, growth, fitness and survival. However, equilibrium between host and parasite can develop in a relatively short period, limiting disease impacts (Hoffman 1999). It is recognized that identification and evaluation of the effects of parasites in wild fish populations is problematic, as sick fish are rapidly removed by predators, water flow and necrophages (Blanc 1977).
Materials and methods
The methodology was almost same in all helminths, but it was slightly modified for different taxonomic group in order to get best results as per international standards. SEM of cestodes, were fixed in laboratory and were then taken to USIC (University of Kashmir) for Scanning Electron Microscopy study. Specimens were washed using distilled water, then fixed for about 1/2 h at 4 °C in 1 % osmium tetroxide in 0.1 M sodium cacodylate–HCl. They were washed for about 1 h in several changes of cold buffer (0.1 M sodium cacodylate containing 3 % sucrose and 0.1 M CaCl2). Post-fixation was carried out for about 2 h using 2.5 % glutaraldehyde buffered to pH 7.3. The specimens were left in washing buffer overnight and were then dehydrated using an ascending series of alcohol solutions. Critical point drying was carried out using a Hitachi Critical Point Dryer (HCP-2, Hitachi Koki Co., Ltd. Tokyo Japan) with carbon dioxide as the transition fluid. The specimens were then coated with gold and examined with a Hitachi Toyota Japan (Model-S3000H) at 80 kV.
For histopathological sections, standard methods (Galigher and Kozloff 1971) were employed for the examination of the infected host intestinal tissue. Specimens that have been stored in 70 % ethanol were transferred to 10 % buffered formalin (v/v). The infected host tissue was dehydrated and blocked in paraffin. The blocks were sectioned at 4–6 micrometers (µm), placed on glass slides and stained with Harris hematoxylin and eosin (H & E) and then viewed with an LSM laser (Carl Zeiss, Thornwoood, New York) equipped compound light microscope. Representative pictures were taken with an attached digital camera at various magnifications and stored in a memory disk for future reference. H and E is a standard stain for viewing pathological tissue while Mallory’s trichrome is used for viewing specific cells characteristic of tissue inflammation.
Results and discussion
Diagnosis of infection and clinical signs
Infections can be readily detected at autopsy, with the recovery of entire tapeworms followed by microscopic examination of the scolex (Figs. 2, 3). The examination of faecal material may reveal detached segments from adult tapeworms or eggs, which possess an operculum at the apex. The presence of B. acheilognathi may also be achieved by a squash plate method. Glass slides or plates are used to flatten the intestinal tract and the worms are detected by reflected light and low-power microscopy. Although mature parasites may be conspicuous within the intestine of infected fish (Fig. 3), the detection of light infections or presence of juvenile parasites and plerocercoids requires microscopic examination. While these infections may hold little importance to the disease status of individuals, detection may be critical for effective disease control and limiting the spread of infected fish. Infected fish may become sluggish and swim close to the water surface (Hoole 1994). This may be accompanied by in appetence, slow growth, condition loss, emaciation and signs of anaemia (Liao and Shih 1956; Edwards and Hine 1974; Scott and Grizzle 1979; Hoole and Nisan 1994; Sopinska and Guz 1997). Infected fish may be more susceptible to secondary bacterial infections due to the debilitating effects of the parasite (Clarkson et al. 1997) and destruction of the epithelial layer of the intestine (Bauer et al. 1981). Heavy tapeworm burdens can cause the body of infected carp fry to become noticeably distended and swollen (Scott and Grizzle 1979; Brandt et al. 1981). In very small fish, the movement of tapeworms may be seen through the body wall prior to dissection. The intestinal tract of infected fish is often grossly enlarged, very thin-walled and obviously occluded. In such cases the gut wall can be stretched to the point of transparency, revealing the white-coloured worms within (Fig. 3) and even allowing individual body segments of parasites to be distinguished. Internal organs of infected hosts may become enlarged and the gall bladder swollen and turgid. Parasites usually accumulate at the posterior part of the first loop of the intestine, posterior to the common bile duct opening. Small focal haemorrhages may be detected at the point of scolex attachment, extending in severity to full haemorrhagic enteritis (Hoole and Nisan 1994). In extreme cases, intestinal perforation may result with rupture of the intestinal tract (Scott and Grizzle 1979; Heckmann 2000). Intensity of infection may range from 30 to 156 mature, gravid worms per pond-reared carp (90–160 mm in length), and up to 20, although usually less than ten, in fully-grown grass carp (Scott and Grizzle 1979). The highest number of parasites recorded in a single fish was 467 worms (Liao and Shih 1956), but no data on their size were provided. However, small numbers of very large tapeworms can also cause pronounced pathological changes. These records suggest that disease results from the overall mass of parasites, rather than a defined intensity of infection (Davydov 1977). The pathogenicity of B. acheilognathi may also vary at different times of year due to changes in the number of parasites present, water temperature, metabolic rates, and nutritional status of the host and duration of infection (Scott and Grizzle 1979).
Fig. 3.

Juvenile common carp (Cyprinus carpio) with infection of Bothriocephalus acheilognathi
Macroscopic and microscopic lesions
Histopathological studies have been conducted on a wide range of fish species including the common carp (Sekretaryuk 1983; Hoole and Nisan 1994), grass carp (C. idella—Liao and Shih 1956; Scott and Grizzle 1979), spottail shiner (Notropis hudsonius), fat- head minnow (Pimephales promelas), wound- fin (Plagopterus argentissimus) and round tail Chub (Gila robusta—Heckmann 2000). The pathology may be divided generally into: (i) damage caused by scolex attachment; and (ii) damage caused by the presence of strobila within the intestine lumen (Scott and Grizzle 1979; Hoole and Nisan 1994). Other organs may exhibit signs of pathological change. For example, infected fish can show signs of nutritional deficiency, with atrophy of hepatocytes within the liver. In severe cases, these changes are consistent with starvation (C. Williams, unpublished data).
Pathological changes caused by attachment of B. acheilognathi
Bothriocephalus acheilognathi attaches to the gut wall by its bothria, which engulf the intestinal folds. The parasite attaches near the anterior portion of the intestine, just posterior to the bile duct. An accumulation of tapeworms in this area leads to digestive tract blockage that distends the intestinal wall leading to perforation. When attached, B. acheilognathi envelopes parts of the intestines and induces an inflammatory response. The inflammation can lead to hemorrhage and necrosis. Symptoms can also include, weight loss, anemia, and mortality especially in young fishes, Paperna 1996. Infections can be detected by the presence of eggs or body parts in feces, and by the presence of the tapeworm in the gut of the fish (Fig. 4). Scolex attachment may also be associated with increased mucus production (Scott and Grizzle 1979). Tapeworm attachment also provokes a localized inflammatory response, consisting mainly of lymphocytes. In heavy infections, increased numbers of lymphocytes may occur throughout the lamina propria. Lesions associated with the scolex depend upon the force exerted by the bothria to maintain attachment. The scolex is often pushed firmly against the gut wall causing compression and the formation of localized pits, extending as far as the muscularis (Figs. 4, 5). These attachment sites lead to pronounced thinning of the intestine at these points. Scolex attachment can also result in a loss of brush border and an overall reduction in thickness of the terminal web. In advanced cases of infection, scolex attachment can cause localized ulceration. Desquamative catarrhal enteritis and proliferation of connective tissue around the point of scolex attachment have also been recorded (Bauer et al. 1973). Heckmann (2000) provided unique accounts of advanced scolex penetration. Studies on wound fin and round tail chub revealed severe pathology associated with penetration of the gut wall up to the muscularis, resulting in a prominent inflammatory response, extensive haemorrhaging an necrosis. The scolex of some parasites even continued to penetrate the intestine wall into the body cavity, extending as far as the liver and gonads (Heckmann 2000). This represents an unusual and rarely reported consequence of B. acheilognathi infection. Scolex attachment causes considerable disruption to the intestine, including: (i) destruction of the desmosomal junctions; (ii) loss of the gut microvillous border; (iii) separation and loss of enterocytes; (iv) release of host-cell debris into the gut lumen; and (v) infiltration of leukocytes into the infected area. In hosts less than 4 cm in length, damage through attachment can be extensive and is consistent with disruption of gut enzymes (Hoole and Nisan 1994). In many places, the plasmalemma between the microtriches and that of the epithelial cells of the host intestine are lacking, so that the matrix of the tegument is in direct contact with the cytoplasm of the host cells. Lysozomes have been demonstrated surrounding the microtriches embedded in the host cytoplasm (Smyth and McManus 1989).
Fig. 4.

Scolex of Bothriocephalus acheilognathi engulfing the intestine of common carp causing compression of the mucosa (arrow) and localized haemorrhage
Fig. 5.
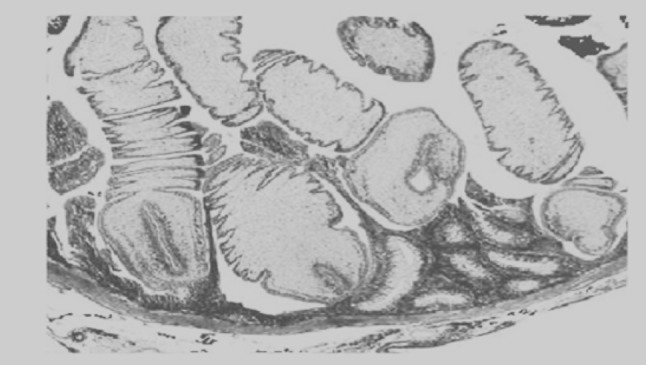
Marked thinning of the intestine, with formation of pits in the intestinal wall caused by the attachment of numerous tapeworms
Pathological changes caused by the strobila of Bothriocephalus acheilognathi
The pathological changes caused by the strobila of B. acheilognathi generally exceed those associated with scolex attachment. The extent and severity of this damage can vary depending on host size, parasite size and intensity of infection (Davydov 1978; Hoole and Nisan 1994). In small cyprinid fish, pathological changes are characterized by distension of the intestine, compression of the intestinal folds and pronounced thinning of the gut wall (Fig. 6). In very heavy infections severe distension may be accompanied by a complete loss of normal gut architecture, with occlusion of the intestine, congestion, compression, pressure necrosis, thinning and atrophy of the mucosa (Nakajima and Egusa 1974a) (Fig. 6). Separation and degeneration of the epithelium, with regions of complete epithelial loss can occur. Lysis of large areas of the mucosa with necrotic changes has also been observed in heavily infected carp fry (Davydov 1977; Sekretaryuk 1983). The presence of parasite eggs caught between the parasites and gut wall can lead to epithelial abrasion, exfoliation of host cells and indentation of the mucosa. This damage, combined with an already compressed gut wall, can lead to ulceration. Inflammatory changes may be pronounced during heavy tapeworm burdens, with increased numbers of lymphocytes and eosinophilic granular cells occurring throughout infected regions. Paradoxically, heavy parasite infections and marked pathological changes have been observed in apparently healthy fish (C. Williams, unpublished data). Nakajima and Egusa (1974a) described no signs of mortality, despite serious histopathological changes in common carp fry. What is seldom clear in these cases are the metabolic and physiological costs of these infections and the energetic or behavioral demands upon infected fish to maintain condition.
Fig. 6.
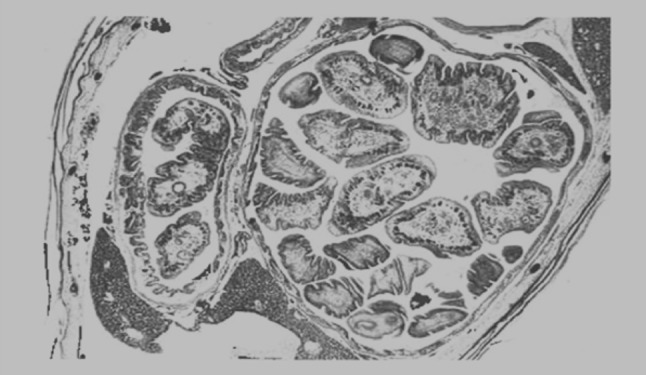
Transverse section of common carp showing attenuation of the gut and partial occlusion from tapeworms within
Disease mechanism
Bothriocephalus acheilognathi causes a number of physiological changes in juvenile fish. These include: (i) protein depletion; (ii) altered digestive enzyme activity; (iii) elevated muscle fatigue in heavily infected hosts; and (iv) mortality of young fishes (Liao and Shih 1956; Davydov 1978; Scott and Grizzle 1979; Granath and Esch 1983b; Brouder 1999; Hansen et al. 2006). Bothriocephaliasis also reduces fat content and causes a decrease in kidney, liver and spleen weight (Balakhnin 1979; Zitnan and Hanzelova 1982). According to Clarkson et al. (1997), B. acheilognathi infection causes a reduction in reproductive capacity, depressed swimming ability and elevated muscle fatigue in heavily infected hosts. The findings of Granath and Esch (1983b) corroborated the reduced ability of mosquito fish to acclimatize with oscillations in water temperature resulting in the mortality of infected fish. Relatively little is known about the relationship between the fish immune response and B. acheilognathi infections, although inflammation occurs in intestines of infected fish and leukocytes were noted on the surface of the parasite (Hoole and Nisan 1994; Nie and Hoole 2000). The interaction between the parasite and pronephric lymphocytes of carp was studied by examining proliferation of lymphocytes isolated from both naive fish and fish injected intraperitoneally with cestode extract (Nie et al. 1996). Parasite extracts increased antibody production and pronephric antibody-producing cells in injected fish (Nie and Hoole 1999), and stimulated proliferation of pronephric lymphocytes in vitro after 5 and 10 days post-injection (Nie et al. 1996). Reduced haemoglobin and total blood volume were reported in infected carp (Kudryashova 1970; Par 1978; Svobodova 1978), but opinions vary as to the intensity of infection necessary to induce these pathogenic effects. Kudryashova (1970) found these effects in fish with more than five worms while Svobodova (1978) did not find any significant effect on various physiological indices in fish harboring between one and 21 worms and attributed the elevated leukocyte count to inflammation of the gut. According to Par (1978), fish with between one and 29 worms had an elevated leukocyte count and those with more than 15 specimens showed marked damage to the gut. Biochemical studies showed reduction in activities of enzymes, such as alanine and aspartate aminotransferase, intestinal trypsin and chymotrypsin, amylase and acid phosphatase (Kudryashova 1970; Matskasi 1984). Reduction in total serum proteins, disrupted carbohydrate and protein metabolism, and elevated oxygen consumption was observed in infected grass carp (Davydov 1978). Morbidity and mortality in winter were attributed to changes in alanine and aspartate aminotransferase activities, which interfered with protein synthesis (Lozinska-Gabska 1981). Inflammation of the gut, severe catarrhal- haemorrhagic enteritis at the parasite attachment point, with proliferation of the peripheral connective tissue, and thinning of the intestinal wall have been attributed to increased acid phosphatase activity during infections (Par 1978; Svobodova 1978; Scott and Grizzle 1979; Sekretaryuk 1983). There is some evidence to suggest B. acheilognathi secretes toxic materials (their composition is not known) leading to intoxication of the host (Degger and Avenant-Oldewage 2009) and damage to the epithelial lining (Hoole and Nisan 1994). According to Bauer et al. (1981) intoxication of the entire host can produce degenerative processes in organs. Hoole and Nisan (1994) revealed through ultra structural studies that electron- dense secretions are released from the surface of B. acheilognathi which may have a protective function for the parasite. Worms adhering to the host’s gut wall injure the lining epithelium, produce and secrete toxic materials, and obstruct the passage of the intestinal contents (Bauer et al. 1977).
Protective/control strategies
Due to the economic importance of Bothriocephalus acheilognathi, its global distribution and expanding host range, considerable efforts have been made to limit disease impacts. These include: (i) the intervention of stringent legislative controls; (ii) extensive prophylaxis; (iii) veterinary examination of fish stocks; (iv) reduction of intensive stock management; and (v) active therapy (Weirowski 1984). Control of the parasite can be directed at either the copepod intermediate hosts (drainage of the ponds in the spring to eliminate planktonic invertebrates) or the fish stage of the life cycle, although these measures are governed by economic and practical considerations. Fish ponds can be allowed to dry and disinfected with unslaked lime (Shcherban 1965). European fish farmers control bothriocephaliasis by drying the ponds annually or treating drained wet ponds with calcium chloride (about 70 kg/ha) or calcium hydroxide (about 2 t/acre) or calcium hypochlorite (HTH) to kill the copepod intermediate host, and treating the fish with anthelmintics. Insecticides employed as ectoparasiticides include Neguvon (Masoten or Dipterex – at 25 ppm, i.e. at 25 g of Masote per ton) or similar compounds (Bromex; Naled), can be used to reduce populations of copepods in ponds (Hoffman 1983). However, these are now banned in many countries as a result of environmental and health concerns. A wide range of Chemotherapeutic agents have been employed with varied success including natural products such as: (i) tobacco dust (Avdosyev 1973); (ii) lupin seeds (Balatski et al. 1976); (iii) conifer needles (Klenov 1969a); and (iv) horse radish leaves (Klenov 1969b). Also a number of compounds and insecticides have been used (Molnar 1970; Edwards and Hine 1974; Fijan et al. 1976; Par et al. 1977; Brandt et al. 1981). A comprehensive review of chemotherapeutic treatments used for the control and eradication of B. acheilognathi is in Bauer et al. (1981) and Williams and Jones (1994). Drugs are usually administered orally. Such preparations are often mixed in oil (corn, soy and fish) and sprayed on to pellets or mixed with feeds. Recent efforts have focused on water- borne chemotherapeutics, which alleviate some of the problems associated with Map- potency and dosage. It is important to distinguish the use of treatment to reduce parasite burden and treatment to achieve complete eradication of the infection. Tapeworms may shed segments during adverse conditions or periods of stress, regenerating when conditions become more favourable. Another important consideration is whether anthelmintics are ovicidal (i.e. kill parasite eggs). This is necessary to avoid the discharge of large numbers of infective eggs to the environment when the worm is evacuated from the fish. The eggs of B. acheilognathi can be killed rapidly by drying, freezing and ultraviolet rays. Among 11 chemicals tested for ovicidal effect, two chlorine-based compounds were found to be effective: (i) 3.1 ppm of sodium dichloroisocyanurate; and (ii) 9 ppm of bleaching-powder (Nakajima and Egusa 1974b). However, there are very few chemical treatments currently licensed for use for tape- worm infections. Furthermore, the use of chemicals for the control of parasites in open water bodies can be very difficult, ineffective, harmful, expensive and illegal.
Conclusions and suggestions for future studies
The Asian tapeworm is pathogenic to fresh- water fishes, especially young carp fry, and may cause great economic loss in hatcheries and fish farms. It has the ability to colonize new regions, and adapt to a wide spectrum of fish hosts. It represents one of the most impressive and deplorable examples of a parasite widely disseminated by man assisted movements of fish. The rate of dissemination and success of colonization has been aided by the cosmopolitan distribution of both intermediate and definitive hosts. However, the spread of Bothriocephalus acheilognathi to many parts of the world has also been the result of inadequate legislative controls, poor preventative measures and lack of appropriate health-checking procedures prior to fish introductions (Scholz and Di Cave 1993; Hoffman 1999; Heckmann 2000). Recent data indicate that the impact of the tapeworm in Europe may have decreased during the last decade. However, surveillance should be maintained to prevent its further expansion to new areas. Efforts are underway to identify the resistance of different strains of common carp used in European aquaculture. Hoole (1994) proposed the development of a vaccine against Bothriocephalus acheilognathi, although practical and economic constraints continue to limit this approach. Exported fish, especially cyprinids and ornamental species (like guppies), should be inspected by veterinarians before their translocation to prevent further dissemination of the tapeworm into new regions. Control measures are generally effective, including treatment of infected fish, but the use of some anthelmintics are no longer allowed because of their negative effect on human health or the environment. Future work must therefore seek to accommodate novel and effective treatments to minimize economic loss. Many aspects of the biology, ecology and pathology of Bothriocephalus acheilognathi are well understood and comprehensively documented. However, many of these observations are restricted to cultured fish populations. Due to the expanding host and geographical ranges of Bothriocephalus acheilognathi, the importance of the parasite to wild fish populations requires further assessment and documentation. This is an important consideration in view of declining global biodiversity and the growing conservation efforts to protect aquatic environments. Comparative studies are needed to understand differences in species susceptibility and disease potential in newly infected hosts and the consequences of the parasite in new environments. Sub lethal effects of the parasite on fish growth, fitness, fecundity, behavior or tolerance to environmental changes may also hold important ecological implications. The physiological and bioenergetics costs of the parasite under natural conditions also require clarification. This information is necessary to provide better understanding of future disease risks and to evaluate the role of this introduced parasite on the health and stability of fish populations.
Acknowledgments
The authors are highly grateful to all the Researchers who have provided valuable information about this fish parasite which have been incorporated in the present publication.
Footnotes
The Editor-in-Chief has retracted this article by Sofi et al.because the article shows significant overlap with a book chapter by Scholz et al.All authors agree to this retraction.
Change history
12/1/2018
The Editor-in-Chief has retracted this article by Sofi et al. (2016) because the article shows significant overlap with a book chapter by Scholz et al. (2012).
Change history
12/1/2018
The Editor-in-Chief has retracted this article by Sofi et al. (2016) because the article shows significant overlap with a book chapter by Scholz et al. (2012).
Contributor Information
Tanveer A. Sofi, Phone: 09797127214, Email: stanveer96@gmail.com
Fayaz Ahmad, Email: rajafayazali@yahoo.co.in.
References
- Akhter S, Ahmad F, Chishti MZ. Infection dynamics of Bothriocephalus acheilognathi in the copepod intermediate host. Oriental Sci. 2008;15:55–59. [Google Scholar]
- Amin OM. Intestinal helminths of some Nile fishes near Cairo, Egypt with redescription of Camallanus kirandensis Baylis 1928 (Nematoda) and Bothriocephalusaegypticus Rysavy and Moravec 1975 (Cestoda) J Parasitol. 1978;64:93–101. doi: 10.2307/3279616. [DOI] [PubMed] [Google Scholar]
- Andrews C, Chubb JC, Coles T, Dearsley A (1981) The occurrence of Bothriocephalusacheilognathi control, revised edn. US Department of the Interior Fish and Wildlife Service
- Ara J, Khan AR, Ahmad F. First record of Pseudophyllidean cestode, Bothriocephalus (Rudolphi, 1808) from fishes of Kashmir. Oriental Sci. 2000;5(1):23–26. [Google Scholar]
- Avdosyev BS. The use of tobacco dust in the control of Bothriocephalusgowkongensis infection in carp. Rybnoe Khozyaistvo. 1973;1973:109–118. [Google Scholar]
- Baer JC, Fain A. Bothriocephalus (Clestobothrium) kivuensis n. sp. cestode parasite d’un barbeau du lac Kivu. Ann Soc Roy Zool Belg. 1958;88:287–302. [Google Scholar]
- Baer JC, Fain A. Observation sur le developpement de Bothriocephalus (Clestobothrium) kivuensis Baer & Fain 1958. Acta Trop. 1960;17:374–377. [PubMed] [Google Scholar]
- Balakhnin IA. Changes in the correlation between the intestinal organs of carp with bothriocephalasis. Gidrobiologicheski Zhurnal. 1979;15:45–49. [Google Scholar]
- Balatski KP, Ivasik VP, Boyarchuk VM, Novosad NL (1976) Bothriocephalus infection in carp. Veterinariya, 1976, 55 (Russia)
- Bauer O. N., Hoffman G. L. Wildlife Diseases. Boston, MA: Springer US; 1976. Helminth Range Extension by Translocation of Fish; pp. 163–172. [Google Scholar]
- Bauer ON, Musselius VA, Strelkov YA (1973) Diseases of Pond Fish. Israel Program for Scientific Translations, Jerusalem, p. 219
- Bauer ON, Musselius VA, Nikolaeva VA, Strelkov YuA. Ichthyopatologiya. Moskow: Izdatelsvo Pishchevaya Promyshlennost; 1977. p. 431. [Google Scholar]
- Bauer ON, Musselius VA, Strelkov YuA. Diseases of Freshwater Fish. Moscow: Legkaya I Pischevaya Promyshenost; 1981. [Google Scholar]
- Blanc Introduced pathogens in European aquatic ecosystems: theoritical aspects and realities. Bulletin Francais de la Peche et de la Pisciculture. 1977;344–345:489–513. [Google Scholar]
- Borgarenko LF (1981) Cestodes of the order Pseudophyllidea in birds in Tadzhikistan. lzvestiya Akademii Nauk Tadzhikskoi SSR Otdelenie Biologicheskikh Nauk, 1979: 99–100
- Brandt FW, Van As JG, Hamilton-Attwell VL. The occurrence and treatment of bothriocephalosis in the common carp, Cyprinus carpio in fish ponds with notes on its presence in the large- mouth yellowfish Barbus kimberleyensis from the Vaal Dam, Transvaal. Water SA. 1981;7:34–42. [Google Scholar]
- Brouder MJ. Relationship between length of roundtail chub and infection intensity of Asian fish tapeworm Bothriocephalus acheilognathi. J Aquat Anim Health. 1999;11:302–304. doi: 10.1577/1548-8667(1999)011<0302:RBLORC>2.0.CO;2. [DOI] [Google Scholar]
- Bunkley-Williams L, Williams E.H (1994) Parasites of Puerto Rican Freshwater Sport Fishes. Puerto Rico Department of Natural and Environmental Resources, San Juan, Puerto Rico, and Department of Marine Sciences, University of Puerto Rico, Mayaguez, Puerto Rico, 168 p
- Buza L, Molnar K, Szakolczai J. Bothriocephalus gowkongensis elofordulasa magyarorszagon. Holaszat. 1970;16:42–43. [Google Scholar]
- Chervy L. The terminology of larval cestodes or metacestodes. Syst Parasitol. 2002;52:1–33. doi: 10.1023/A:1015086301717. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Charipar E, Nelson P, Hodgson JR, Bonar S, Cole RA. Update on the distribution of the invasive Asian fish tapeworm, Bothriocephalus acheilognathi, in the U.S. and Canada. Comparative Parasitol. 2006;73:269–273. doi: 10.1654/4240.1. [DOI] [Google Scholar]
- Clarkson RW, Robinson AT, Hoffnagle TL. Asian tapeworm (Bothriocephalus acheilognathi) in native fishes from the Little Colorado River, Grand Canyon, Arizona. Great Basin Naturalist. 1997;57:66–69. [Google Scholar]
- Davydov VG. Host tissue reaction to different types of cestode attachment. Biologiya VnutrenychVod, Informatsionnyi Bull. 1977;33:45–48. [Google Scholar]
- Davydov ON. Growth, development and fecundity of Bothriocephalus gowkongensis (Yeh, 1955), a parasite of cyprinid fish. Gidrobiologicheskii Zhumal. 1978;14:70–77. [Google Scholar]
- Degger N, Avenant-Oldewage A. Metal accumulation analysis within tissue of Bothriocephalus acheilognathi. J S Afr Vet Assoc. 2009;80:127–128. [Google Scholar]
- Denis A, Gabrion C, Lambert A. The presence in France of two parasites of East Asian origin: diplozoon nipponicum (Monogenea) and Bothriocephalus acheilognathi (Cestoda) in Cyprinus carpio. Bull Francais de Pisciculture. 1983;289:128–134. doi: 10.1051/kmae:1983012. [DOI] [Google Scholar]
- Dove ADM, Fletcher AS. The distribution of the introduced tapeworm Bothriocephalus acheilognathi in Australian freshwater fishes. J Helminthol. 2000;74:121–127. [PubMed] [Google Scholar]
- Dubinina MN. Cestodes from fishes of the River Amur’s basin. Parazitologicheskii Sbo mik. 1971;25:77–119. [Google Scholar]
- Edwards DJ, Hine PM. Introduction, preliminary handling and diseases of grass carp in New Zealand. NZ J Mar Freshwat Res. 1974;8:441–454. doi: 10.1080/00288330.1974.9515518. [DOI] [Google Scholar]
- Evans BB, Lester RJG. Parasites of ornamental fish imported into Australia. Bull Eur Associat Fish Patholog. 2001;21:51–55. [Google Scholar]
- Fernando CH, Furtado JI. A study of some helminth parasites of freshwater fishes in Ceylon. Zeit Parasitenkunde. 1963;23:141–163. doi: 10.1007/BF00260290. [DOI] [Google Scholar]
- Fernando CH, Furtado JI. Helminth parasites of some Malaysian freshwater fishes. Bull Natural Museum Singapore. 1964;32:45–71. [Google Scholar]
- Fijan N, Kezic N, Teskeredzic E, Kajgana L. Treatment of carp bothriocephaliass. Veterinarski Arhiv. 1976;46:245–252. [Google Scholar]
- Font WF, Tate DC. Helminth parasites of native Hawaiian freshwater fishes: an example of extreme ecological isolation. J Parasitol. 1994;80:682–688. doi: 10.2307/3283246. [DOI] [PubMed] [Google Scholar]
- Galigher AE, Kozloff EN. Essentials of practical microtechnique. 2. Philadelphia: Lee and Febiger; 1971. p. 531. [Google Scholar]
- Garcia-Prieto L, Osorio Sarabia D. Distribution of actual de Bothriocephalus acheilognathi en Mexico. Anales del Instituto de Biologia, Universidad Nacional AutOnoma de Mexico, Serie Zoologia. 1991;62:523–526. [Google Scholar]
- Granath WO, Esch GW. Temperature and other factors that regulate the composition and infrapopulation densities of Bothriocephalus acheilognathi (Cestoda) in Gambusia affinis (Pisces) J Parasitol. 1983;69:1116–1124. doi: 10.2307/3280874. [DOI] [Google Scholar]
- Granath WO, Esch GW. Survivorship and parasite-induced host mortality among mosquitofish in a predator free, North Carolina cooling reservoir. Am Midl Nat. 1983;110:314–323. doi: 10.2307/2425272. [DOI] [Google Scholar]
- Han JE, Shin SP, Kim JH, Choresca CH, Jr, Jun JW, Gomez DK, Park SC. Mortality of cultured koi Cyprinus carpio in Korea caused by Bothriocephalus acheilognathi. Afr J Microbiol Res. 2010;4:543–546. [Google Scholar]
- Hansen SP, Choudhury A, Heisey DM, Ahumada JA, Hoffnagle TL, Cole RA. Experimental infection of the endangered bonytail chub (Gila elegans) with the Asian fish tapeworm (Bothriocephalus acheilognathi): impacts on survival, growth, and condition. Can J Zool. 2006;84:1383–1394. doi: 10.1139/z06-126. [DOI] [Google Scholar]
- Hansen SP, Choudhury A, Cole RA. Evidence of experimental postcyclic transmission of Bothriocephalus acheilognathi in bonytail chub (Gila elegans) J Parasitol. 2007;93:202–204. doi: 10.1645/GE-686R.1. [DOI] [PubMed] [Google Scholar]
- Hanzelova V, Ithan R. Embryogenesis and development of Bothriocephalus acheilognathi Yamaguti, 1934 (Cestoda) in the intermediate host under experimental conditions. Helminthologia. 1986;23:145–155. [Google Scholar]
- Heckmann RA. Asian tapeworm, Bothriocephalus acheilognathiYamaguti, 1934, a recent cestode introduction into the western United States of America: control methods and effect on endangered fish populations. Proc Parasitol. 2000;29:1–24. [Google Scholar]
- Heckmann RA. The fate of an endangered fish species (Plagopterus argentissimus) due to an invasive fish introduction (Cyprinella lutrensis) infected with Asian tapeworm (Bothriocephalus argentissimus): recovery methods. Proc Parasitol. 2009;47:43–52. [Google Scholar]
- Hoffman GL. Asian fish tapeworm, Bothriocephalus opsarichthydis, prevention and control Revised Edition. US Dep: Int. Fish & Wildlife Serv; 1983. [Google Scholar]
- Hoffman GL. Parasites of North American freshwater fishes. Berkeley: University of California Press; 1999. [Google Scholar]
- Hoffman GL, Schubert G. Some parasites of exotic fishes. In: Courtenay WR, Stauffer JR, editors. Distribution, Biology, and Management of Exotic Fishes. Baltimore: John Hopkins University Press; 1984. pp. 233–261. [Google Scholar]
- Holmes JC (1979) Parasite populations and host community structure. In: Nickol, BB. (ed) Host-Parasite Interfaces. Proceedings of the Symposium at the University of Nebraska-Lincoln, 5–7 October. Academic Press, London, pp. 27–46
- Hoole D. Tapeworm infections in fish: past and future problems. In: Pike AW, Lewis JW, editors. Parasitic Diseases of Fish. Tresaith: Samara Publishing Limited; 1994. pp. 119–140. [Google Scholar]
- Hoole D, Nisan H. Ultrastructural studies on the intestinal response of carp, Cyprinus carpio L. to the pseudophyllidean tapeworm, Bothriocephalus acheilognathi Yamaguti 1934. J Fish Dis. 1994;17:623–629. doi: 10.1111/j.1365-2761.1994.tb00260.x. [DOI] [Google Scholar]
- Khalifa KA. Cestoda of freshwater farmed fishes in Iraq. J Wildl Dis. 1986;22:278. doi: 10.7589/0090-3558-22.2.278. [DOI] [PubMed] [Google Scholar]
- Kim YG, Kim JY, Chun SK. Life History of Bothriocephalus opsariichthydis Yamaguti (Cestoda; Pseudophyllida) parasitized on Israel carp Cyprinus carpio (Linne). 1. First intermediate host and developing procercoid. Bull Fisheries Science Institute Kunsan Fish Jr coll. 1985;1:1–10. [Google Scholar]
- Klenov AP. Coniferous needles tested against Bothriocephalus in grass carp. Veterinatiya. 1969;46:65–67. [Google Scholar]
- Klenov AP (1969b) Testing phytoncides (onion and leaves of horse-radish) against Bothriocephalus infection in grass carp. Veterinariya 46: 59 (in Russian)
- Korting W. Bothriocephalosis of the carp. Veterinar Med Nachr. 1974;2(74):152–158. [Google Scholar]
- Kuchta R, Scholz T. Diversity and distribution of fish tapeworms of the Bothriocephalidea (Eucestoda) Parassitologia. 2007;49:21–38. [PubMed] [Google Scholar]
- Kudryashova YuV. The effect of Bothriocephalus gowkongensis on the hematological indicators of 2-year-old carp. Doklady Moskovskoi Sel’skokhozyaistvennoi Akademii im. K. A. Timiryazeva. 1970;164:345–349. [Google Scholar]
- Langdon JS (1992) Major protozoan and metazoan parasitic diseases of Australian finfish. In: Munday B (ed) Fin Fish Workshop Refresher Course for Veterinarians, Proceedings 182. Post Graduate Committee in Veterinary Science, University of Sydney, Sydney, Australia (1992), pp. 1–27
- Leong TS. Seasonal occurrence of metazoan parasites of Puntius binotatus in an irrigation canal, Pulau Pinang, Malaysia. J Fish Biol. 1986;28:9–16. doi: 10.1111/j.1095-8649.1986.tb05136.x. [DOI] [Google Scholar]
- Liao HH, Shih LC. Contribution to the biology and control of Bothriocephalus gowkongensis Yeh, a tapeworm parasitic in young grass carp (Ctenopharyngodon idellus C. a. V.) Acta Hydrobiol Sin. 1956;2:129–185. [Google Scholar]
- Lopez Jimenez S. Cestodos de peces I. Bothriocephalus (Clestobothrium) acheilognathi (Cestoda:Bothriocephalidae) Anales del Instituto de Biologia Universidad Nacional AutOnoma de Mexico, Serie Zoologia. 1981;51:69–84. [Google Scholar]
- Lozinska-Gabska M. Activity of aspartate and alanine aminotransferase in the alimentary canal of carp (Cyprinus carpio L.) infected with tapeworms Bothriocephalus gowkongensis Yeh 1955 or Khawia sinensis Hsu 1935. Wiad Parazytol. 1981;27:717–743. [PubMed] [Google Scholar]
- Maitland PS, Campbell RN. Freshwater fishes of the British isles. London: Harper Collins Publishers; 1992. [Google Scholar]
- Malevitskaya MA. O zavoze parazita so slozhnym ciklom razvitija Bothriocephalus gowkongensis pri akklimatizacii amurskich ryb. Dokl. Doklady Akademii Nauk USSR. 1958;123:572–575. [Google Scholar]
- Marcogliese DJ. First report of the Asian fish tapeworm in the Great Lakes. J Great Lakes Res. 2008;34:566–569. doi: 10.3394/0380-1330(2008)34[566:FROTAF]2.0.CO;2. [DOI] [Google Scholar]
- Marcogliese DJ, Esch GW. Experimental and natural infection of planktonic and benthic copepods by the Asian tapeworm, Bothriocephalus acheilognathi. Proc Helmintholog Soc Washington. 1989;56:151–155. [Google Scholar]
- Mashego SN (1982) A seasonal investigation of the helminth parasites of Barbus species in water bodies in Lebowa and Venda, South Africa. Ph.D. thesis, University of the North, Sovenga. p. 191
- Matskasi I (1984) The effect of Bothriocephalus acheilognathi infection on the protease and a-amylase activity in the gut of carp fry. In: Olaha J (ed) Fish, pathogens and environment in European polyculture (Proceedings of an International Seminar, 23–27 June 1981, Szarvas). Symposia Biologica Hungarica 23: 119–125
- Minervini R, Lombardi F, Cave D. Lintroduzione di Bothriocephalus acheilognathi Yamaguti 1934, in Italia: osservazioni su popolazioni naturali e di allevamento di carpa (Cyprinus carpio) Rivista Italiana di Piscicultura e Ittiopatogia. 1985;20:27–32. [Google Scholar]
- Molnar K. An attempt to treat fish bothriocephalosis with devermin. Toxicity for the host and antiparasitic effect. Acta Veterinaria Academiae Scientiarum Hungaricae. 1970;20:325–331. [PubMed] [Google Scholar]
- Molnar K. On the synonyms of Bothriocephalus acheilognathi Yamaguti 1934. Parasitologia Hungarica. 1977;10:61–62. [Google Scholar]
- Molnar K, Murai E. Morphological studies on Bothriocephalus gowkongensis Yeh, 1955 and B. phoxini Molnar 1968 (Cestoda, Pseudophyllidea) Parasitol Hungar. 1973;6:99–110. [Google Scholar]
- Molnar K, Murai E. Morphological studies on Bothriocephalus gowkongensis Yeh 1955 and Bothriocephalus phoxini Molnar 1968 (Cestoda, Pseudophyllidea) Parasitologia Hungarica. 1973;6:99–108. [Google Scholar]
- Nakajima K, Egusa N. Bothriocephalus opsariichthydis Yamaguti (Cestoda: Pseudophyllidea) found in the gut of cultured carp, Cyprinus carpio (Linne)—I morphology and taxonomy. Fish Pathol. 1974;9:31–39. doi: 10.3147/jsfp.9.31. [DOI] [Google Scholar]
- Nakajima K, Egusa N. Bothriocephalus opsariichthydisYamaguti (Cestoda: Pseudophyllidea) found in the gut of cultured carp, Cyprinus carpio (Linne)—II. Anthelmintic effects of some chemicals. Fish Pathol. 1974;9:46–49. doi: 10.3147/jsfp.9.46. [DOI] [Google Scholar]
- Nedeva I, Mutafova T. On the morphology of Bothriocephalus acheilognathi Yamaguti, 1934 (Bothriocephalidae) Khelmintologiya. 1988;26:39–46. [Google Scholar]
- Nie P, Hoole D. Antibody response of carp, Cyprinus carpio to the cestode, Bothriocephalus acheilognathi. Parasitology. 1999;118:635–639. doi: 10.1017/S0031182099004242. [DOI] [PubMed] [Google Scholar]
- Nie P, Hoole D. Effects of Bothriocephalus acheilognathi on the polarization response of pronephric leucocytes of carp, Cyprinus carpio. J Helminthol. 2000;74:253–257. [PubMed] [Google Scholar]
- Nie P, Hoole D, Arme C. Proliferation of pronephric lymphocytes of carp, Cyprinus carpio induced by extracts of Bothriocephalus acheilognathi. J Helminthol. 1996;70:127–131. doi: 10.1017/S0022149X00015273. [DOI] [PubMed] [Google Scholar]
- Odening K. Conception and terminology of hosts in parasitology. Adv Parasitol. 1976;14:1–93. doi: 10.1016/S0065-308X(08)60513-8. [DOI] [PubMed] [Google Scholar]
- Paperna I (1996) Parasites, Infections and Diseases of Fishes in Africa: an Update. CIFA Technical Paper 31, Food and Agriculture Organization of the United Nations, Rome
- Par O. Low-intensity invasion by tapeworm Bothriocephalus gowkongensis, as acting on the physiological and condition parameters of the health state of the carp. Bull VURH Vodriany. 1978;14:26–33. [Google Scholar]
- Par O, Parova J, Prouza A. Mansonil-an effective anthelminthic for the treatment of bothriocephaliasis in the carp. Bull VURH Vodriany. 1977;1:17–25. [Google Scholar]
- Perez-Ponce de Leon G, Jimenez-Ruiz FA, Mendoza-Garfias B, Garcia-Prieto L. Helminth parasites of garter snakes and mud turtles from several localities of the Mesa Central of Mexico. Comp Parasitol. 2001;68:9–20. [Google Scholar]
- Petkov P. Occurrence of Bothriocephalus gowkongensis in carp bred in artificial water reservoirs in the Pleven district. Veterinarnomeditsinski Nauki. 1972;9:75–78. [Google Scholar]
- Pool D. A scanning electron microscope study of the life cycle of Bothriocephalus acheilognathi Yamaguti 1934. J Fish Biol. 1984;25:361–364. doi: 10.1111/j.1095-8649.1984.tb04883.x. [DOI] [Google Scholar]
- Pool DW. A note on the synonymy of Bothriocephalus acheilognathi Yamaguti, 1934, Bothriocephalus aegyptiacus RySavy and Moravec 1975 and Bothriocephalus kivuensis Baer and Fain 1958. Parasitol Res. 1987;73:146–150. doi: 10.1007/BF00536471. [DOI] [PubMed] [Google Scholar]
- Pool DW, Chubb JC. A critical scanning electron microscope study of the scolex of Bothriocephalus acheilognathi Yamaguti 1934, with a review of the taxonomic history of the genus Bothriocephalus parasitizing cyprinid fishes. Syst Parasitol. 1985;7:199–211. doi: 10.1007/BF00011451. [DOI] [Google Scholar]
- Prigli M. The role of aquatic birds in spreading Bothriocephalus gowkongensis Yeh, 1955 (Cestoda) Parasitologia Hungarica. 1975;8:61–62. [Google Scholar]
- Radulescu I, Georgescu R. Contributii la cunoasterea parasitofaunei species Centropharyngodon idella in primuande aclimatizare in RP. Romina. Buletinul Institutului de Cercetari si Proiectari Piscicole. 1962;21:85–91. [Google Scholar]
- Rego AA, Chubb JC, Pavanelli GC. Cestodes in South American freshwater teleost fishes: keys to genera and brief description of species. Revista Brasileira de Zoologia. 1999;16:299–367. doi: 10.1590/S0101-81751999000200003. [DOI] [Google Scholar]
- Riggs MR, Lemly AD, Esch GW. The growth, biomass, and fecundity of Bothriocephalus acheilognathi in a North Carolina cooling reservoir. J Parasitol. 1987;73:893–900. doi: 10.2307/3282507. [DOI] [PubMed] [Google Scholar]
- RySavy B, Moravec F. Bothriocephalus aegyptiacus sp. n. (Cestoda: Pseudophyllidea) from Barbus bynni, and its life cycle. Vestnik Ceskoslovenske Spoleanosti Zoologicke. 1975;39:68–72. [Google Scholar]
- Salgado-Maldonado G, Pineda-LOpez RF. The Asian fish tapeworm Bothriocephalus acheilognathi: a potential threat to native freshwater fish species in Mexico. Biol Invasions. 2003;5:261–268. doi: 10.1023/A:1026189331093. [DOI] [Google Scholar]
- Scholz T. A revision of the species of Bothriocephalus Rudolphi, 1808 (Cestoda: Pseudophyllidea) parasitic in American freshwater fishes. Syst Parasitol. 1997;36:85–107. doi: 10.1023/A:1005744010567. [DOI] [Google Scholar]
- Scholz T. Parasites in cultured and feral fish. Vet Parasitol. 1999;84:317–335. doi: 10.1016/S0304-4017(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Scholz T, Di Cave D. Bothriocephalus acheilognathi (Cestoda: Pseudophyllidea) parasite of freshwater fish in Italy. Parassitologia. 1993;34:155–158. [PubMed] [Google Scholar]
- Scholz T, Spakulova M, Snabel V, Kralova I, Hanzelova V. A multidisciplinary approach to the systematics of Proteocephalus macrocephalus (Creplin, 1825) (Cestoda: Proteocephalidae) Syst Parasitol. 1996;37:1–12. doi: 10.1023/A:1005743413573. [DOI] [Google Scholar]
- Scholz T, Vargas-Vazquez J, Moravec F, Vivas-Rodriguez C, Mendoza-Franco E. Cestoda and Acanthocephala of fish from cenotes (sinkholes) of the Peninsula of Yucatan, Mexico. Folia Parasitol. 1996;43:141–152. [Google Scholar]
- Scholz T, Skerıkova A, Shimazu T, Mark JG. A taxonomic study of species of Bothriocephalus Rudolphi 1808 (Cestoda: Pseudophyllidea) from eels in Japan: morphological and molecular evidence for the occurrence of Bothriocephalus claviceps (Goeze 1782) and confirmation of the validity of B. japonicus Yamaguti 1934. Syst Parasitol. 2004;57:87–96. doi: 10.1023/B:SYPA.0000013835.02539.e0. [DOI] [PubMed] [Google Scholar]
- Scott AL, Grizzle JM. Pathology of cyprinid fishes caused by Bothriocephalus gowkongensis Yeh, 1955 (Cestoda: Pseudophyllidea) J Fish Dis. 1979;2:69–73. doi: 10.1111/j.1365-2761.1979.tb00141.x. [DOI] [Google Scholar]
- Sekretaryuk KV. Morphogistokhimicheskie issledobaniya kishechnika karpa pri botriocephaleze. Parazitologiya. 1983;17:203–208. [PubMed] [Google Scholar]
- Shcherban MP (1965) Cestode Infections of Carp. lzdatelstvo Urozhai, Kiev
- Smyth JD, McManus DP. The physiology and biochemistry of cestodes. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Sopinska A, Guz L. Fenbendazole treatment against Bothriocephalus acheilognathi in carp, Cyprinus carpio. Bull Eur Associat Fish Pathol. 1997;17:86–87. [Google Scholar]
- Svobodova Z. Values of some conformation, condition and physiological parameters of two-year-old carp invaded by the tapeworm Bothriocephalus gowkongensis. Bull VURH Vodnany. 1978;3:21–25. [Google Scholar]
- Van As JG, Schoobee HJ, Brandt FW. Further records of the occurrence of Bothriocephalus (Cestoda: Pseudophyllidea) in the Transvaal S. Afr J Sci. 1981;77:343. [Google Scholar]
- Weirowski F (1984) Occurrence, spread and control of Bothriocephalus acheilognathi in the carp ponds of the German Democratic Republic. In: Olaha J (ed) Fish, pathogens and environment in European polyculture. Proceedings of an International Seminar, June 23–27, 1981, Szarvas. Symposia Biologica Hungarica 23: 149–155
- Williams H, Jones A. Parasitic Worms of Fish. Taylor & Francis, London, UK. Yamaguti S. (1934) Studies on the helminth fauna of Japan. Part 4. Cestodes of fishes. Jpn J Zoology. 1994;6:1–112. [Google Scholar]
- Yamaguti Bothriocephalusgowkongensis (Cestoda: Pseudophyllidea) in the British Isles. J Fish Diseases. 1934;4:89–93. [Google Scholar]
- Yeh IS. On a new tapeworm Bothriocephalus gowkongensis n. sp. (Cestoda: Bothriocephalidae) from freshwater fish in China. Acta Zoologica. 1955;7:69–74. [Google Scholar]
- Zitnan R, Hanzelova V. Negative effects of bothriocephalosis on weight gains in carp. Folia Veterinaria. 1982;26:173–181. [Google Scholar]