Abstract
Leishmaniasis is one of the most neglected human diseases with an estimated global burden ranking second in mortality and fourth in morbidity among the tropical infections. Chemotherapy involving the use of drugs like glucantime is the mainstay treatment in endemic areas of Iran. Drug resistance is increasingly prevalent, so search for alternative therapy is gathering pace. Medicinal herbs, like wormwood Artemisia, have chemical compounds effective against a number of pathogens. In this study, the efficacy of ethanol extract from Artemisia absinthium (Asteraceae) against Leishmania major L. was investigated in vitro. The outcome of different effective doses (1–40 mg/ml) of ethanol extracts from this medicinal herb, A. absinthium, on a standard Iranian parasite strain of L. major was examined. The L. major promastigote cell sensitivity and mortality or viability effects due to the addition of herbal extract were measured using the MTT assay and the flow cytometry technique, respectively. There was complete agreement between the two assays. The lethal concentration (LC50) was measured as 101 mg/ml. Some contrasting relationships between the medicinal herb concentrations and the viability of parasites were observed; so that there was an increased multiplication of the parasite at low concentrations of the drug, but an anti-parasitic apoptotic effect was seen at high concentrations of A. absinthium. It was concluded that there might be one or more chemical constituents within the herbal extract of wormwood which at high concentration controlled cell division and affected the relevant activity within the only one giant mitochondrion in this flagellate parasite. At low doses, however, it showed the opposite effect of leading to mitotic cell divisions.
Keywords: Wormwood, Artemisia, Viability assay, MTT, Leishmania, IC50, LC50
Introduction
Species of Leishmania are vector-borne flagellate protozoan parasites of humans and other vertebrate hosts that lead to the conditions known as leishmaniases (Azizi et al. 2012a, b). These are classified as one of the most neglected tropical diseases with an estimated global burden of disease ranking second in mortality and fourth in morbidity among the tropical infections (Kedzierski 2011). These are a range of diseases that affect the skin, mucosa, and/or internal organs. They are manifested in severity from skin scars to serious disfigurement and fatal systemic infection (WHO 2010). Transmission to the vertebrate host is by Phlebotomus sand flies from a reservoir host to humans (Moemenbellah-Fard et al. 2003; Azizi et al. 2011).
Current treatments for leishmaniasis rely on chemotherapy, such as pentavalent antimonial drugs like sodium stibogluconate and meglumine antimonate which have been prescribed for over 70 years, to ameliorate disease and on vector control to reduce pathogen transmission. Many side effects are associated with the use of these drugs. Resistance to these drugs is also increasing and alternative search strategies should be looked for (Croft et al. 2006; Hadighi et al. 2006; Manzano et al. 2013). About 1.5 million new cases of leishmaniasis are reported every year and there is a critical need to develop better therapies (St. George et al. 2006).
Medicinal plants are indispensible sources of diverse types of bioactive organic compounds. One of these shrubs, Artemisia absinthium L. (Asteraceae/Compositae), commonly known as wormwood, is an erect, medium-sized herb with greenish silvery leaves and white twigs with strong aroma. About 400 species are described globally of which some 30 species are present in Iran only two of which are native to this country. They are known to have diverse antiparasitic (Rocha et al. 2005; Sen et al. 2007; Abdel-Sattar et al. 2010), antibacterial (Ramezani et al. 2004; Valdes et al. 2008; Ahameethunisa and Hopper 2010), antifungal (Kordali et al. 2005), antioxidant and antidepressant (Mahmoudi et al. 2009; Ali et al. 2013) and cytotoxic (Tariku et al. 2011) effects. Their efficacy could be due to the presence of an endoperoxide bridge at the heart of artemisinin, a bitter substance derived from extracts of wormwood (Krishna et al. 2008).
To the best of authors’ knowledge, there is no corroborative report on the effect of wormwood extracts on Leishmania major within the scope of this investigation so far. The aim of this study was to find the effective dose of hydro-alcoholic extract from A. absinthium on the standard Iranian strain of L. major and to determine the efficacy of different doses of this medicinal herb on the parasite.
Materials and methods
Plant identification and preparation
In May 2010, aerial parts (stem, leaves and flowers) of wormwood, A. absinthium, or Afsanthin as it is known in Iran, during blossoming stage were collected from the foothills of Darab, 260 km to the east of the capital city of Shiraz in the southern Iranian Fars province (30°45′N, 50°45′E, at an altitude of about 780 m a.s.l.). A voucher specimen is deposited in the herbarium of Eram botanical garden at Shiraz University, Shiraz, Iran. The plants were air dried in the shade at ambient temperature. Morphological features of A. absinthium were determined using Flora Iranica key (Rechinger 1965–2008) and a Stereomicroscope. The height of this plant measures about 30–60 cm with tubular corolla of flowers on the shoot extremities.
Isolation of extracts
The wormwood plants were ground into very fine powder with an electric stainless steel grinder. Air-dried plant material (200 g) from the aerial parts of A. absinthium was then percolated three times with 80 % ethanol solvent and subjected to hydro-distillation for 3 h in a Clevenger type glass apparatus model Soxhlet. After extraction, the resultant samples were dried over a rotary evaporator to delete water and kept in amber vial at 4 °C prior to the viability assays. The sample yielded 23.7 g of solvent extract on a dry weight basis of 200 g.
Parasite culture
Promastigotes of L. major (MRHO/IR/75/ER) were cultured in the dark in heat-sterilized brain heart infusion (BHI) medium with 10 % fetal calf serum (FCS), 200 mg streptomycin and 200 K unit of penicillin antibiotics at a temperature of 25 ± 2 °C in an atmosphere of 5 % CO2 in an incubator. Once promastigote culture reached its log or exponential phase, subculture samples were used for further studies.
Promastigote viability using the MTT assay
Leishmania major viability was assessed using the colorimetric 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay based on tetrazolium salt reduction to formazan crystals by mitochondrial dehydrogenases (Meerloo et al. 2011). Inoculums of 2 × 106 ml−1 promastigote cells were seeded into each of the 96-well ELISA plates. Subcultures to be assayed were incubated with the MTT reagent. Viable promastigotes can metabolize the MTT reagent into purple-color formazan. An increase in the absorbance at 600 nm, due to the formation of the formazan, indicates active mitochondria and thus viable promastigote cells. Absorbance data were acquired at 600 nm using a microplate enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad®).
Using dilution method, the solvent extract from A. absinthium was added to the fourth well onward with concentrations of 100, 200, 400, 600, 800 and 1,000 µg/ml into two plates (for measurements at 24 and 72 h). Two other plates received concentrations of 1, 2, 5, 10, 20 and 40 mg/ml. About 15 µl of A. absinthium without cell was poured into well number I (negative control). In well number II (positive control), 40 µl cells and 110 µl BHI without A. absinthium was inserted. In well number III (negative control), only 15 µl BHI without cell and plant extract was poured in. The remaining volumes of these wells were filled with 20 µl cell and BHI so that their final volumes reached 150 µl.
The plates were then incubated at 25 °C for 48 and 72 h. The number of live promastigotes of Leishmania in each well was counted after 24, 48 and 72 h with a Neobar slide on an inverted compound microscope. Then 0.3 g MTT powder with 600 λ was dissolved in PBS for one hour, filtered through 0.22 mesh filter and after 48 and 72 h 15 µl/mg of MTT reagent was added to each parasite-containing well. Four hours after incubation at 26 °C, 150 µl (equivalent to the volume in original culture medium) of acidic isopropanol (prepared by a mixture of 50 ml of two molar HCl acid in a 2.5 l isopropanol solution) was added to each well in order to dissolve the formazan crystals. The plates were incubated in the dark for 15 min. The photosensitive absorbance of plates were obtained by subtraction of optical densities at 540 and 720 nm wavelengths using an ELISA microplate reader (Bio-Rad®) since the relative number of viable cells was directly related to the light absorption rate of samples (Meerloo et al. 2011). The percentage cell viability was calculated for control and A. absinthium-exposed cells using the following formula:
where Ac is the absorbance of control well, At the absorbance of A. absinthium-treated well, and Ab the absorbance of blank well. Eventually, the data were expressed in terms of IC50 (50 % inhibition concentration of promastigote cell growth) with a concentration of A. absinthium which inhibited the growth of 50 % of promastigote cells.
Flow cytometry procedure
This was based on Ferreira-da-Silva et al. (2010) with slight modifications. The parasites were washed at 200 g centrifugal force. Parasites (1 × 107 cells) were exposed for two hours to different concentrations (1, 2, 4, 6, 8, 10, 20, 50, 100, 200 and 400 λ) of herbal extract. A test tube with parasite without propidium iodide (PI) as control for calibration, a tube with parasite as negative control stained later with 15 λ PI, and a tube with parasite exposed to 0.2 % saponin as positive control stained later with 15 λ PI were also considered. Furthermore, two test tubes with 4 and 8 % dimethyl sulphoxide (DMSO) with parasites were used. The samples were transferred to dark chamber in flow cytometry apparatus (4-colored BD FACS Calibur model, BD Biosciences Co, USA) for data acquisition and analysis with 10 000 cells. The Cell Quest PRO software was used to analyze the data. In this study, the LC50 was measured as 101 mg/ml.
Data analysis
Statistical analyses of the differences in mean values between different experimental groups were done with Student’s t test. The significance of difference was measured by analysis of variance (ANOVA) and with a confidence interval of 95 %, P-values of 0.05 or less were considered significant.
Results
Inhibition of parasite growth by A. absinthium
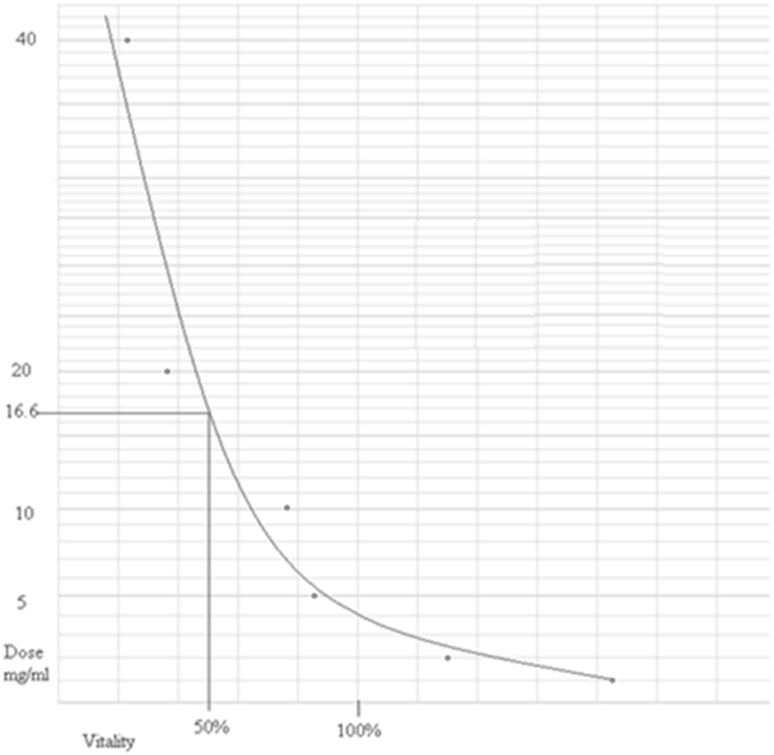
In this study, the L. major promastigote cell sensitivity and mortality or viability effects due to the addition of the herbal extracts were measured using the MTT assay and the flow cytometry technique, respectively. There was complete agreement between the two assays. The cytotoxic efficacy of A. absinthium based on the LC50 value using the viability rate of L. major promastigotes was investigated with the MTT method. The result of MTT method showed the inhibitory effects on parasite growth in vitro. The lethal effects of all tested extracts were measured. It was found that there was an inverse relationship between the medicinal herb concentrations and the viability of parasites, so that there was an increased replication of the parasite at lower concentrations of the drug, but an anti-parasitic effect was seen at higher concentrations of A. absinthium. The data obtained from MTT absorbance showed that after 72 h all L. major promastigotes were killed due to lack of food. Low doses (1–2 mg) of this herbal extract caused replication of cells, but at high (16.6–40 mg) doses 50–100 % of parasite cells were dead (Fig. 1).
Fig. 1.
Vitality curve of Leishmania major promastigotes exposed to different concentrations of Artemisia absinthium ethanol extracts after 48 h post-MTT assay (LC50 = 16.6 mg/ml)
Leishmanicidal activity using flow cytometry
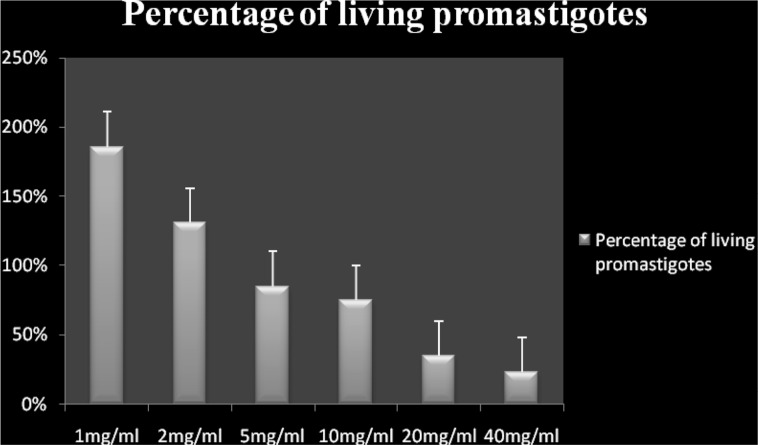
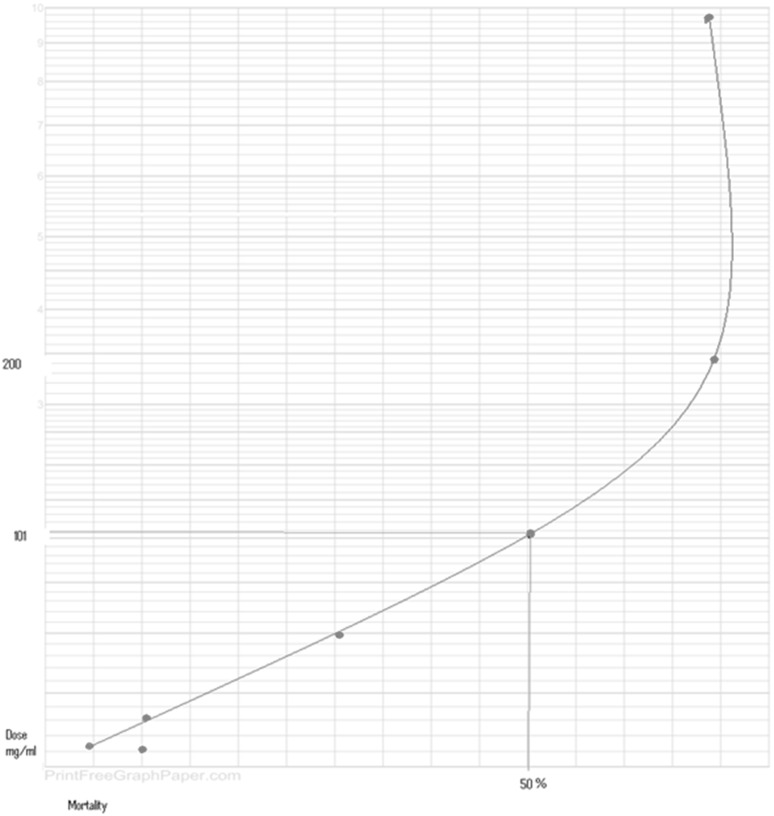
The exposed promastigotes to different extract concentrations were treated with PI stain and the results were noted (Figs. 2, 3). The percentages of apoptotic dead cells as well as necrotic deaths were examined after two hours using the flow cytometry. It was found that the extract concentration at which 50 % of cells were dead (LD50), was 101 mg/ml (Fig. 4).
Fig. 2.
Histogram exhibiting the percentage growth of living promastigotes of Leishmania major 48 h after exposure to different concentrations of Artemisia absinthium extracts
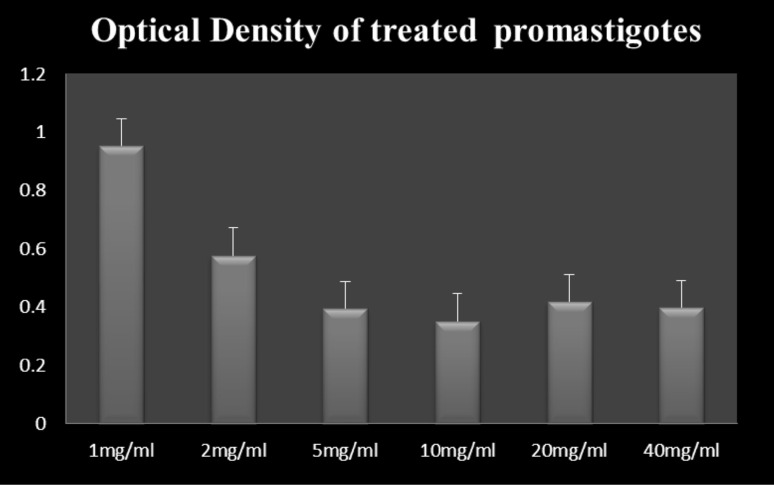
Fig. 3.
Histogram of average optical density of treated promastigotes of Leishmania major 72 h after exposure to different concentrations of Artemisia absinthium extracts
Fig. 4.
Mortality curve of Leishmania major promastigotes exposed to different concentrations of Artemisia absinthium ethanol extracts after two hours using flow cytometry method (LC50 = 101 mg/ml)
Discussion
Leishmaniasis, after malaria, is the second most important vector-borne disease in Iran (Fakoorziba et al. 2011; Azizi et al. 2012a, b; Moemenbellah-Fard et al. 2012), though occasionally other malignant infections are also superimposed (Moemenbellah-Fard et al. 2009; 2014). There is a shortage of cheap and effective chemotherapeutic agents for the treatment of leishmaniasis. Drug resistance as well as multiple side effects is associated with the use of these chemical agents. The efficacy of different concentrations of herbal extracts of wormwood, A. absinthium, on L. major promastigotes in the logarithmic phase of growth was investigated using MTT colorimetric assay at 48 and 72 h. Using the PI stain, the mortality rates on exposure to different doses of these extracts after 2 h were monitored using a flow cytometry apparatus. So flow cytometry technique was used to measure mortality or viability effects of the extracts, but the result of MTT method exhibited inhibitory effects on parasite growth. It was shown here that there was complete agreement between the two methods.
Several studies have attempted to demonstrate the potential anti-Leishmania effects of various herbal extracts in the past. It has previously been stated that various extracts from the leaves of different wormwood plants had anti-Leishmania activity in vitro (Hatimi et al. 2001; Ganguly et al. 2006). The latter report found IC50 values ranged from 0.21 to 0.58 mg/ml for Absinthium indica, while another report on A. absinthium against L. major demonstrated an IC50 value of 0.28 mg/ml (Kheiri Manjili et al. 2012). Essential oils from A. absinthium demonstrated growth inhibitory effects at certain concentrations against promastigotes of two different Leishmania species (Tariku et al. 2011). Similarly, the growth inhibitory activities of 11 different Artemisia species were shown against the promastigotes of L. major (Emami et al. 2012). Anti-Leishmania effects due to certain essential oils derived from cultivated A. absinthium were further exhibited in a recent paper (Bailen et al. 2013). The IC50 of L. major promastigote cells was also determined to be 25 and 50 µg/ml for Artemisia sieberi and artemisinin, respectively (Heydari et al. 2013). The outcome from MTT colorimetric assay in the present study indicated that this medicinal herb (A. absinthium) was mitogenic at low doses of 1-2 mg and increased the mitotic divisions of parasites. At high concentrations, however, an inverse or inhibitory effect on the parasite was noted. The IC50 was calculated to be 16.6 mg which was in line with that of others (Emami et al. 2012; Heydari et al. 2013).
The flow cytometry studies were in perfect conformity to these findings and exhibited that after 2 h exposure with the herbal medicine of wormwood, 50 % of parasitized cells underwent apoptosis with a dose of 101 mg/ml. It was thus concluded that there was one or more chemical compound within the herbal extract of wormwood which at high concentration control cell division and affect the relevant biochemical activity within mitochondria. At low concentrations, however, it showed the opposite effect. It led to mitotic cell divisions.
Acknowledgments
The authors appreciate the improvements to this article that were meticulously proposed by the anonymous peer reviewers. The present paper was extracted from the results of an approved MSc student thesis (No: 90-01-42-3213 Dated 17 March 2011) conducted by the second author, Ms. Fatemeh Shahidi-Hakak. It was financially supported by Shiraz University of Medical Sciences (SUMS). We are also indebted to Ms. Shahrbanu Naderi for assistance in the cell culture. Thanks are due to the Vice-chancellor for Research and Technology at SUMS, for permitting the use of facilities at the university. No competing financial interests exist. No other conflict of interest is also declared.
Contributor Information
Kourosh Azizi, Email: azizik@sums.ac.ir, Email: azizi_ko@yahoo.com.
Fatemeh Shahidi-Hakak, Email: shahidi_fateme78@yahoo.com.
Qasem Asgari, Email: asgarig@yahoo.com, Email: asgarig@sums.ac.ir.
Gholam Reza Hatam, Email: hatamghr@sums.ac.ir, Email: hatam908@yahoo.com.
Mohammad Reza Fakoorziba, Email: mrfakoor@yahoo.com, Email: fakoorziba@sums.ac.ir.
Ramin Miri, Email: rmiri@sums.ac.ir.
Mohammad Djaefar Moemenbellah-Fard, Phone: +98 711 7251001-8, Email: momenbf@sums.ac.ir, Email: momenbf@yahoo.com.
References
- Abdel-Sattar E, Maes L, Salama MM. In vitro activities of plant extracts from Saudi Arabia against malaria, leishmaniasis, sleeping sickness and Chagas disease. Phytother Res. 2010;24:1322–1328. doi: 10.1002/ptr.3108. [DOI] [PubMed] [Google Scholar]
- Ahameethunisa AR, Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Compl Alt Med. 2010;10:6. doi: 10.1186/1472-6882-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Shah I, Shah SWA, Ahmed G, Shoaib M, Junaid M, Ali W, Ahmed Z. Antioxidant and relaxant activity of fractions of crude methanol extract and essential oil of Artemisia macrocephala Jacquem. BMC Compl Alt Med. 2013;13:96. doi: 10.1186/1472-6882-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi K, Moemenbellah-Fard MD, Fakoorziba MR, Fekri S. Gerbillus nanus (Rodentia: muridae): A new reservoir host of Leishmania major. Ann Trop Med Parasitol. 2011;105:431–437. doi: 10.1179/1364859411Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi K, Abedi F, Moemenbellah-Fard MD. Identification and frequency distribution of Leishmania (L.) major infections in sand flies from a new endemic ZCL focus in southern Iran. Parasitol Res. 2012;111:1821–1826. doi: 10.1007/s00436-012-3029-0. [DOI] [PubMed] [Google Scholar]
- Azizi K, Moemenbellah-Fard MD, Kalantari M, Fakoorziba MR. Molecular detection of Leishmania major kDNA from wild rodents in a new focus of zoonotic cutaneous leishmaniasis in an oriental region of Iran. Vector-Borne Zoonotic Dis. 2012;12:844–850. doi: 10.1089/vbz.2011.0872. [DOI] [PubMed] [Google Scholar]
- Bailen M, Julio LF, Diaz CE, Sanz J, Martinez-Diaz RA, Cabrera R, Burillo J, Gonzalez-Coloma A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind Crop Prod. 2013;49:102–107. doi: 10.1016/j.indcrop.2013.04.055. [DOI] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in Leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami SA, Taghizadeh SZ, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran J Basic Med Sci. 2012;15:807–811. [PMC free article] [PubMed] [Google Scholar]
- Fakoorziba MR, Baseri A, Eghbal F, Rezaee S, Azizi K, Moemenbellah-Fard MD. Post-earthquake outbreak of cutaneous leishmaniasis in a rural region of southern Iran. Ann Trop Med Parasitol. 2011;105:217–224. doi: 10.1179/136485911X12899838683449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-da-Silva MF, Pons AH, Tedesco RC, Barbosa HS. Viability and infectivity analysis of Toxoplasma gondii under axenic conditions. Sci Med. 2010;20:93–98. [Google Scholar]
- Ganguly S, Bandyopadhyay S, Bera A, Chatterjee M. Antipromastigote activity of an ethanolic extract of leaves of Artemisia indica. Ind J Pharmacol. 2006;38:64–65. doi: 10.4103/0253-7613.19859. [DOI] [Google Scholar]
- George S, Bishop JV, Titus RG, Selitrennikoff CP. Novel compounds active against Leishmania major. Antimicrob Agents Chemother. 2006;50(2):474–479. doi: 10.1128/AAC.50.2.474-479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmaniatropica parasites. PLoS Med. 2006;3(5):e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatimi S, Boudouma M, Bichichi M, Chaib N, Idrissi NG. In vitro evaluation of antileishmania activity of Artemisia herba-alba. Ass Bull Soc Pathol Exot. 2001;94:29–31. [PubMed] [Google Scholar]
- Heydari FE, Ghaffarifar F, Soflaei S, Dalimi A. Comparison between in vitro effects of aqueous extract of Artemisia seiberi and artemisinin on Leishmania major. Jundishapur J Nat Pharmaceut Prod. 2013;8(2):70–75. doi: 10.17795/jjnpp-9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski L. Leishmaniasis. Human Vaccines. 2011;7(11):1204–1214. doi: 10.4161/hv.7.11.17752. [DOI] [PubMed] [Google Scholar]
- Kheiri Manjili H, Jafari H, Ramazani A, Davoudi N. Anti-leishmanial and toxicity activities of some selected Iranian medicinal plants. Parasitol Res. 2012;111:2115–2121. doi: 10.1007/s00436-012-3059-7. [DOI] [PubMed] [Google Scholar]
- Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem. 2005;53:1408–1416. doi: 10.1021/jf048429n. [DOI] [PubMed] [Google Scholar]
- Krishna S, Bustamante L, Haynes RK, Staines HM. Artemisinins: their growing importance in medicine. Trends Pharmacol Sci. 2008;29(10):520–527. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Ebrahimzadeh MA, Ansaroudi F, Nabavi SF, Nabavi SM. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr J Biotech. 2009;8:7170–7175. [Google Scholar]
- Manzano JI, García-Hernández R, Castanys S, Gamarro F. A new ABC half-transporter in Leishmania major is involved in resistance to antimony. Antimicrob Agents Chemother. 2013;57:3719–3730. doi: 10.1128/AAC.00211-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerloo JV, Kaspers GJL, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731(10):237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Moemenbellah-Fard MD, Kalantari M, Rassi Y, Javadian E. The PCR-based detection of Leishmania major infections in Meriones libycus (Rodentia: muridae) from southern Iran. Ann Trop Med Parasitol. 2003;97:811–816. doi: 10.1179/000349803225002651. [DOI] [PubMed] [Google Scholar]
- Moemenbellah-Fard MD, Benafshi O, Rafinejad J, Ashraf H. Tick-borne relapsing fever in a new highland endemic focus of western Iran. Ann Trop Med Parasitol. 2009;103:529–537. doi: 10.1179/136485909X451852. [DOI] [PubMed] [Google Scholar]
- Moemenbellah-Fard MD, Saleh V, Banafshi O, Dabaghmanesh T. Malaria elimination trend from a hypo-endemic unstable active focus in southern Iran: predisposing climatic factors. Pathog Glob Health. 2012;106:358–365. doi: 10.1179/2047773212Y.0000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moemenbellah-Fard MD, Shahriari B, Azizi K, Fakoorziba MR, Mohammadi J, Amin M (2014) Faunal distribution of fleas and their blood-feeding preferences using enzyme-linked immunosorbent assays from farm animals and human shelters in a new rural region of Southern Iran. J Parasit Dis (In Press) [DOI] [PMC free article] [PubMed]
- Ramezani M, Fazli-Bazzaz BS, Saghafi-Khadem F, Dabaghian A. Antimicrobial activity of four Artemisia species of Iran. Fitoterapia. 2004;75(2):201–203. doi: 10.1016/j.fitote.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Rechinger KH. (Ed.) 1965–2008. Flora Iranica, No. 1–164, Akademische Druch-u, Verlagsanstat, Graz-Austria
- Rocha LG, Almeida JRGS, Macêdo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol. 2007;56:1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- Tariku Y, Hymete A, Hailu A, Rohloff J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem Biodiv. 2011;8:614–623. doi: 10.1002/cbdv.201000331. [DOI] [PubMed] [Google Scholar]
- Valdes AFC, Martinez JM, Lizama RS, Vermeersch M, Cos P, Maes L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L and Artemisia absinthium L. Mem Inst Oswaldo Cruz. 2008;103(6):615–618. doi: 10.1590/S0074-02762008000600019. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2010) Control of the leishmaniases. WHO Tech Rep Ser 949 Geneva, Switzerland [PubMed]