Abstract
The present study aims to determine the age- wise, sex- wise and month-wise prevalence along with seasonal fluctuations of Trichuris spp. in ovines and caprines slaughtered during a 12 month period in local abattoirs in Srinagar region from August 2011 to July 2012. Adult parasites were identified on the basis of morphological characters (Soulsby Helminths, arthropods and protozoa of domes- 229 ticated animals, CLBS and Bailliere Tinda, London,1982). The highest prevalence (66.6 %) was in the month of Jan, 2012 whereas prevalence was lowest in the month of August 2011. Trichuris count in ovines increased in autumn (42.02 %), reached maximum levels in winter (59.37), and then tended to decline until spring (53.22 %) and reached minimum levels in summer (30.6 %), before increasing again in mid-autumn. Thus with respect to climatic conditions of area from which exotic ovines were imported, Trichuris prevalence was more prevalent in dry season(55.5 %) than in wet season (36.36 %). Moreover, an association was observed between sex and age of the host with prevalence of Trichuris infection. Of the representative examined samples, Trichuris infection was 44.07 % in female host comparative to 38.07 % infection in males (p > 0.05). Likewise young animals were more infected (53.8 %) than the adult ones (32.9 %) and kids (37.5 %). Moreover, Trichuris spp. were more prevalent in goats than in sheep(p < 0.05). Hence, it was concluded that prevalence of Trichuris spp. infecting ovines varied with respect to season, age and sex.
Keywords: Trichuris, Ovines, Caprine, Prevalence, Age and sex
Introduction
Gastrointestinal parasite infections are a world-wide problem for both small- and large-scale farmers due to the availability of a wide range of agro-ecological factors suitable for diversified hosts and parasite species. Economical losses are caused by gastrointestinal parasites in a variety of ways; they cause losses through lowered fertility, reduced work capacity, a reduction in food intake and lower weight gains, treatment cost and mortality in heavy parasitized animals. The host-parasites relationships in case of Nematode parasites result into large scale damage at the site of attachment consequently economical loss (Padwal et al. 2011). Small ruminant helminthiasis is found to be an important problem and Trichuriasis is among one such problem caused by Trichuris spp. Trichuris spp. prefer the caecum and the first section of the colon up to the beginning of the disk-like section (Rehbein et al. 1997). The present paper deals with studying the seasonal and age wise prevalence of Trichuris spp. Seasonal prevalence of spp. is done to find the time at which infections with infective larvae begin, rise to a peak and decline, so that the treatment can be timed to prevent development of serious infection. Age wise prevalence has been carried out so as to determine which age group is more prone to Trichuris spp. and the one with least susceptibility.
Materials and methods
Study area
The study was conducted at local abattoirs in Srinagar district situated in the centre of Kashmir Valley–state of India, from September 2011 to August 2012.
Study animals
The study was done on various breeds of sheep imported to Jammu and Kashmir State from outside state. The various breeds examined were Marwadi, Jaiselmeri, Desi, Bakerwali and Bawalpuri. The animals were 1 to above 4 years age from both sexes. A total of 270 caeca were examined for adult parasites.
Postmortem worm recovery
The intestines were examined carefully from the parietal surface to detect the gross pathological changes caused by Trichuris spp., if any. Then the intestine was cut along the log axis with the help of scissors and the internal mucus membranes were also thoroughly examined. Parasites were collected according to the procedures by Urquhart et al. The recovered worms were washed several times in normal saline, and then preserved in 70 % alcohol. The nematodes were then cleared in lactophenol, identified under the microscope with reference to the literature (Soulsby 1986) and then kept in nematode storing solution.
Report recording
Age of animals was recorded prior to slaughtering of animal. Age of small ruminants was categorized into: 2–12 months (kids), 12–24 month (young) and above 2 years (Adults). Seasonal prevalence was studied throughout the year dividing into four seasons: winter (Dec–Feb.), spring (March–May), summer (June–July) and autumn (Sep–Oct).
Parasitological data
The data recorded during this study included the following:
Month–wise prevalence of Trichuris spp.
Season–wise prevalence of Trichuris spp.
Age–wise prevalence of Trichuris spp.
Sex–wise prevalence of Trichuris spp.
Data analysis
Prevalence of Trichuris spp. was calculated as number of individuals of a host species infected with Trichuris species/number of host examined. Chi square test was employed to examine the effect of season, age and sex of the host on the level of parasitism.
Results and discussion
Monthwise prevalence of Trichuris spp.
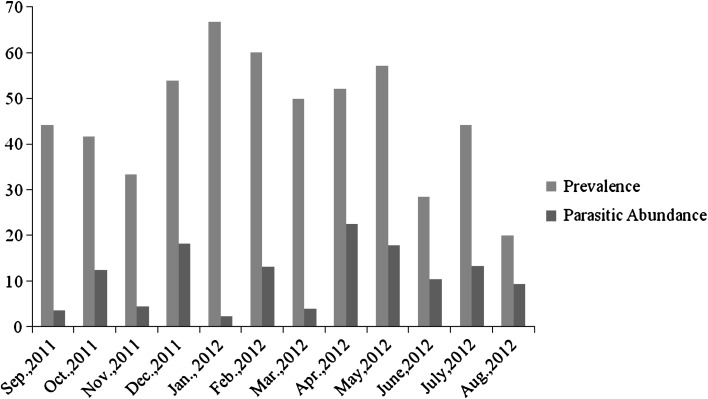
Out of representative 270 caeca, 116 were infected with Trichuris spp. accounting to overall prevalence of 42.96 % in exotic breeds of Ovines. The current findings are in close association to reports of Lone et al. (2011). Highest prevalence was recorded during the month of January 2012 (66.6 %) whereas lowest prevalence was recorded in the month of Aug 2012 (20 %).However, parasitic abundance showed inverse association to prevalence (Fig. 1).
Fig. 1.
Monthwise prevalence and parasitic abundance
Seasonal prevalence of Trichuris spp.
The seasonal dynamics of nematode infection are the consequence of complex inter-relationships between the sheep, their husbandry and the prevailing climate (Viassof et al. 2001). In the present study, highest prevalence was during winter month i.e., 59.37 % and least in summer i.e., 30.6 % (p = 0.009) which was in accordance to findings of Saha et al. (1996). High incidence of parasitic infection during winter could be attributed to suitable climatic conditions and the availability of food i.e. pastures during their development (Padwal et al. 2011). Umur (2005) reported that the late high wave of infection, occurring in winter, may have derived from eggs deposited by young and mature sheep grazing on pasture in late October and September months (Table 1).
Table 1.
Seasonal prevalence Trichuris Spp
| No. examined | No. positive | Prevalence | Chi square | |
|---|---|---|---|---|
| Spring | 62 | 33 | 53.22 | 11.629 (p = 0.009) |
| Summer | 88 | 27 | 30.6 | |
| Autumn | 88 | 37 | 42.04 | |
| Winter | 32 | 19 | 59.375 |
Sexwise prevalence of Trichuris spp.
In present investigations, females show high prevalence (44.07 %) in comparison to males (38.9 %), however statistically insignificant (p > 0.05) which could be probably because of sampling error. Our findings are in accordance to (Asanji and Williams 1987; Pal and Qayyum 1992; Patel et al. 2001; Raza et al. 2007; Saiful Islam KBM and Taimur MJFA 2008). High prevalence in females could be due to the physiological peculiarities. Above factors constitute stress factors thus, reducing their immunity to infections, and for being lactating mothers, females happen to be weak, as a result of which they are more susceptible to the infections besides some other reasons (Kuchai et al. 2011; Blood and Radostits 2000).
Agewise prevalence of Trichuris spp.
Moreover, young animals showed high parasitic prevalence (53.8 %) as compared to adults (32.9 %). Our results were in close association to report of Islam (1989) that a high occurrence of Trichuris spp. was observed in the middle aged animals. These findings of the effect of host age on the prevalence of nematode infection is also confirmed by earlier observations carried out in Kashmir on sheep (Tariq et al. 2008). The low level of parasitism reported in adult animals was due to the immunity of the host. Previous infection and age of the host provide effective protection against re-infection (Soulsby 1986). However, the low level of immunity in adults is initially low but increases with the intensity and duration of exposure of infection.
Breedwise prevalence of Trichuris spp.
During the study, goats were found more prone to Trichuris infection than sheep. It could be assumed to fact that sheep do have a considerably higher immunological response to gastrointestinal parasites compared with that of goats (Urquhart et al. 1996) (Table 2).
Table 2.
Proportion of each category investigated and the associated prevalence
| Sex | Examined | Infected | Prevalence | Chi square value |
|---|---|---|---|---|
| Female | 211 | 93 | 44.07 | p > 0.05 |
| Male | 59 | 23 | 38.9 |
| Age | Examined | Infected | Prevalence | |
|---|---|---|---|---|
| 2–12 months (kids) | 56 | 21 | 37.5 | 11.629 (p = 0.009) |
| 12–24 month (young) | 117 | 63 | 53.8 | |
| Above 2 years (Adults) | 97 | 32 | 32.9 |
| Host | Examined | Infected | Prevalence | |
|---|---|---|---|---|
| Sheep | 173 | 70 | 40.46 % | 4.075 (p = 0.04) |
| Goat | 97 | 49 | 50.51 % |
Conclusion
Findings in current paper indicate the need of hour to study other epidemiological factors to better understand the knowledge regarding Trichuris spp. Moreover, abattoir study should be properly studied so that veterinarians understand importance of checking health status of slaughtered sheep for further meat consumption by human population. Strict policies should be adopted by Govt to have regular checks on slaughtered animals at local abattoirs. To decrease the harmful effects of the parasite, young and adult stock should be pastured separately. Moreover, to get clear epidemiological picture of Trichuris, comprehensive study should be launched in the Kashmir where sheep and goats are abundant and practically participating in cash incomes particularly on mandatory eve of Eid-ul-Azha, sources of food to farming communities and play significant role in these sector.
Acknowledgments
The authors would like to extend their gratitude to Parasitology laboratory, Department of Zoology, University of Kashmir for its technical and material support in the realization of this study.
References
- Asanji MF, Williams MO. Variables affecting population dynamics of gastrointestinal helminth parasites of small farm ruminants in Sierra Leone. Bull Anim Health Prod Afr. 1987;35:308–313. [Google Scholar]
- Blood DC, Radostits OM. Veterinary Medicine. 7. London: Bailliere Tindall; 2000. [Google Scholar]
- Islam MK (1989) Studies on some epidemiological and pathological aspects of Trichuris spp. infection in Black Bengal goats in Mymensingh, Bangladesh, Thesis. M. Sc. (Vet. Science) in Parasitology, Bangladesh Agricultural University, Mymensingh
- Kuchai JA, Chishti MZ, Zaki MM, Javid A, Dar SA, Muzaffar R, Tak H. Epidemiology of helminth parasites in small ruminants of Ladakh, India. Online J Anim Feed Res. 2011;1(5):239–242. [Google Scholar]
- Lone BA, Chishti MZ, Ahmad F. Prevalence of coccidian and Gastrointestinal nematode infections in goats of Baramulla district of Kashmir valley. Glob Vet. 2011;7(1):27–30. [Google Scholar]
- Padwal N, Humbe A, Jadhav S, Borde SN. Seasonal variation of intestinal Trichuris sp. in sheep and goats from Maharashtra State. Int Multidiscip Res J. 2011;1(12):17–18. [Google Scholar]
- Pal RA, Qayyum M. Breed, age and sex-wise distribution of gastro-Intestinal helminths of sheep and goats in and around Rawalpindi region Pakistan. Vet J. 1992;12:60–63. [Google Scholar]
- Patel MD, Nauriyal DS, Hasnani JJ, Gupta RS. Prevalence of gastrointestinal parasitism in goats maintained under semi-intensive and field management systems. Indian J Vet Med. 2001;21:99–101. [Google Scholar]
- Raza MA, Iqbal Z, Jabbar A, Yaseen M. Point prevalence of gastrointestinal helminthiasis in ruminants in southern Punjab. Pakistan J Helminthol. 2007;81:323–328. doi: 10.1017/S0022149X07818554. [DOI] [PubMed] [Google Scholar]
- Rehbein S, Lindner T, Kollmannsberger M, Winter R, Visser M. Helminth infection of slaughtered sheep in Upper Bavaria. 3. Distribution of colonization of nematodes in the large intestine of sheep. Berl Munch Tierarztl Wochenschr. 1997;110(6):223–228. [PubMed] [Google Scholar]
- Saha SB, Pramanik S, Mukherjee GS. Prevalence of gastrointestinal nematodes of goats in West Bengal. Indian J Anim Sci. 1996;11(1):51–52. [Google Scholar]
- Saiful Islam KBM and Taimur MJFA Helminthic and protozoan internal parasitic infections in free ranging small ruminants of Bangladesh. Slov Vet Res. 2008;45(2):67–72. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 6. London: CLBS and Bailliere Tindal; 1982. p. 788. [Google Scholar]
- Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7. Ed. London: Bailliere Tindall; 1986. pp. 212–342. [Google Scholar]
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS. Epidemiology of gastrointestinal nematodes of sheep managed under traditional husbandry system in Kashmir valley. Vet Parasitol. 2008;158:138–143. doi: 10.1016/j.vetpar.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Umur S. An abattoir Survey of Gastro-Intestinal Nematodes in Sheep in the Burdur Region Turkey. Turk J Vet Anim Sci. 2005;29:1195–1201. [Google Scholar]
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Veterinary Parasitology. 2. London: Blackwell Science Ltd; 1996. pp. 102–103. [Google Scholar]
- Viassof A, Leathwick DM, Heath AC. The epidemiology of nematode infections of sheep. N Z Vet J. 2001;49:213–221. doi: 10.1080/00480169.2001.36235. [DOI] [PubMed] [Google Scholar]