Abstract
The present study aimed to evaluate a polymerase chain reaction (PCR) assay used for detection of Schistosoma mansoni infection in Biomphalaria alexandrina snails in early prepatent period and to compare between it and the ordinary detection methods (shedding and crushing). Biomphalaria alexandrina snails are best known for their role as intermediate hosts of S. mansoni. DNA was extracted from infected snails in addition to non-infected “negative control” (to optimized the efficiency of PCR reaction) and subjected to PCR using primers specific to a partial sequence of S. mansoni fructose-1,6-bus phosphate aldolase (SMALDO). SMALDO gene was detected in the infected laboratory snails with 70, 85, and 100 % positivity at the 1st, 3rd, and 7th day of infection, respectively. In contrast, the ordinary method was not sensitive enough in detection of early prepatent infection even after 7 days of infection which showed only 25 % positivity. By comparing the sensitivity of the three methods, it was found that the average sensitivity of shedding method compared to PCR was 23.8 % and the average sensitivity of crushing method compared to PCR was 46.4 % while the sensitivity of PCR was 100 %. We conclude that PCR is superior to the conventional methods and can detect positive cases that were negative when examined by shedding or crushing methods. This can help in detection of the areas and times of high transmission which in turn will be very beneficial in planning of the exact timing of the proper control strategy.
Keywords: SMALDO, PCR, S. mansoni, Prepatent infection
Introduction
Schistosomiasis remains one of the most common endemic parasitic diseases affecting people in 74 countries, with ~120 millions individuals being symptomatically infected and 20 millions being severely affected. The infection is prevalent in tropical and sub-tropical areas in poor communities without potable water and adequate sanitation (WHO 2013). Males have a higher incidence, most likely because of increased exposure to infected water via bathing, swimming, and agricultural activities (Behrman 2005). Fresh water pulmonate snails of the genus Biomphalaria are best known for their role as intermediate hosts of the widely distributed parasite Schistosoma mansoni (Morgan et al. 2001). Although campaigns for schistosomiasis control based on chemotherapy have reduced the morbidity and prevalence of this disease, transmission usually persists to a greater or lesser extent after delivery of treatment because of continuing local transmission and exposure to reinfection (Fenwick 2006; Satayathum et al. 2006). The transmission of S. mansoni in human populations has been associated with environmental and socioeconomic conditions, but the presence of susceptible Biomphalaria, is essential (Negrao-Correa et al. 2012).
The basic life cycle has an alteration of generation with the sexual generation of adult Schistosoma in the definitive vertebrate host and the asexual one in a molluscan host (Webbe and El Hak 1990). Adult S. mansoni live in the bloodstream. Their eggs pass out of the host with the faeces. In contact with water, free-swimming miracidia emerge from the egg and penetrate the soft tissues of the snail host (Abath et al. 2006). After penetrating the head/foot region of susceptible snails, schistosome miracidia transform into sporocysts. Then, second generation sporocysts known as daughters develop inside the first generation sporocysts through polyembryony. Larvae remain in the head/foot region for approximately 2–3 weeks, after which they can be found in the digestive gland-gonad (DGG) complex where they continue to multiply. When the infection reaches patency at ~4 weeks post infection, cercariae are released from daughter sporocysts and are released from the snail. The specific timing of the above events differs with various host–parasite combinations and maintenance conditions (Humphries 2010).
Snail control is one of the most rapid and accurate means for reducing spread of S. mansoni infection. Patent infection rates in snails are determined by searching for cercariae coming out from snails by two routine examination methods; shedding method, which was induced by exposure of snails to artificial light (Webbe 1965), and crushing method, which was performed by squeezing the snails between two glass slides followed by microscopic examination (Chu and Dawood 1970). These methods are more or less accurate in detection of snail prepatent infection (detection of cercarial shedding is only possible 30 days after infection), time-consuming, unsuitable for routine large-scale screening of snail populations (Caldeira et al. 2004). Subsequently, detailed data on prepatent infection, which can constitute a significant proportion of infected snails populations and correspond to infection prevalence in human populations contacting the sites studied, have not been available (Abbasi et al. 2010; Hamburger et al. 2004). Therefore, development of specific and accurate methods to identify prepatent infection of snails with S. mansoni, should be useful for epidemiological studies to control the prevalence of the infected B. alexandrina (Schmitt et al. 2002).
Several molecular methods were developed depending on detection of specific sequences in genomic DNA by polymerase chain reaction (PCR) and were successfully used for highly sensitive and early detection of infected snails especially in prepatency. For examples; (1) identification of minisatellite repeat mitochondrial DNA was used to detect prepatent S. mansoni infection in B. glabrata snails (Jannotti-Passos et al. 1997), (2) detection of 18S rDNA was used to detect prepatent S. mansoni infection in snails (Hanelt et al. 1997) and (3) amplification of a tandem repeat sequence (121 bp) was used to detect prepatent S. mansoni infection in snails, other than PCR assays, dot hybridization using labeled probes was also used to detect infected snails (Hamburger et al. 1998).
The present study aimed to evaluate a molecular based method, PCR, used for detection of S. mansoni prepatent infection in snails in early prepatent period (in the first few days of infection). This will help to determine exactly the time and areas of high transmission which in turn will be very beneficial in planning of control strategy.
Materials and methods
Study type
Case control experimental study
The study was conducted in the period from August, 2012 to July, 2013 in Parasitology Department, Faculty of Medicine, Zagazig University.
Snails
Two main groups of Biomphalaria alexandrina snails were examined in this study, the laboratory group (L) and the field group (F). The laboratory group includes 80 laboratory bred non infected snails (4–6 weeks age) that were obtained from Schistosoma Biological Supply Center (SBSC), Theodor Bilharz Research Institute (TBRI), Giza, Egypt. They were maintained and exposed to infection in our laboratory in accordance with international, ethical guidelines after approval of the Institutional Committee of the Faculty of Medicine; Zagazig University. This group is used as a reference against the field group.
Viable S. mansoni miracidia were hatched from the eggs obtained from liver of infected mice in our laboratory and used for infection of the laboratory snails. Each snail was exposed for 3 h to eight freshly hatched miracidium in 1 ml dechlorinated water. Exposed snails were maintained under standard laboratory conditions and then examined for infection starting from the 1st day post infection (Prah and James 1977). Some miracidia were taken in small tube containing dechlorinated water and stored at −20 °C to be used as a reference for the positive results. The laboratory snails were equally subdivided into 4 subgroups; uninfected control negative group (L0), snails after 1st, 3rd, and 7th days of infection (L1, L3, L7, respectively). The field group includes 400 snails collected in two seasons; season I (in the autumn: September, October & November) and season II (in the spring: April, May & June), from El-Oreen water channels, Abo Kbeer, Sharqia, Egypt. (The number of each group was the available representative number of samples of each group).
Study design
All laboratory groups were examined by PCR technique except L7 examined firstly by crushing then by PCR. All field snails were identified morphologically as regards: colour, number of whorls, and diameter of the shell, the external examination of the shell revealed that they were dextral, disc shaped, dark brown to black in colour, without striations, the whorls were about 4–5 in number, gradually increasing and generally rounded. The shell is thin, fragile, with concavity on either side. There is a wide open funnel shaped deep umbilicus. As regards the size, it was about 5–12 mm. the snails then examined for infection by shedding method according to Liang et al. (1987) and crushing method according to Chu and Dawood (1970) then both positive and negative snails were examined by PCR technique for detection of infection and the obtained results were tabulated and studied.
DNA extraction and PCR performance
Extraction of DNA from all snails and miracidia was conducted using DNA extraction kit provided from DNeasy® Blood & Tissue Kit (QIAGEN®, Germany) following the manufacturer’s instructions.
A partial sequence (274 bp) of Schistosoma manoni SMALDO DNA was amplified by PCR using the following primers; forward primer: 5′ TCGTCGTCTGTACCGCCAGC 3′ and reverse primer: 5′ AGCGAAGCGGCATCCAAGTCT 3′. The web based tool, Primer 3 (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) was used to design the primers based on the published sequence of SMALDO open reading frame (GenBank accession number L38658) (El-Dabaa et al. 1998). A total PCR reaction volume of 20 μl containing: 10 μl Intron’s Maxime PCR PreMix Kit [containing i-TaqTM DNA Polymerase (5 U/μl), 200 μM dNTPs, Reaction Buffer (10×), Gel loading buffer], 2 μl of the two amplification primers, 2 μl of template DNA (500 ng), and 6 μl DNase free H2O. The amplification was carried out in T Professional Basic Gradient Thermal Cycler (Biometra). The reaction mixtures were started with an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 57 °C for 1 min, and primer extension at 72 °C for 1 min. Finally, an elongation step at 72 °C for 10 min. Amplified PCR products (274 bp) was analyzed by electrophoresis on 1.5 % agarose agar, stained by ethidium bromide (0.5 μg/ml in the running buffer) then, visualized on an ultraviolet transilluminator and photographed.
Statistical analysis
All data were subjected to statistical analysis using SPSS win statistical package version 11. Chi square test and Fisher Exact test were used to compare differences among frequencies in groups. Significance was defined as P < 0.05. The sensitivity is the probability that the assay will be positive when the infection is present. The specificity is the probability that the assay will be negative when the infection is absent. They were calculated using the following formulas: sensitivity (%) = TP/(TP + FN) × 100 and specificity (%) = TN/(TN + FP) × 100 (TP: true positive, FN: false negative, TN: true negative and FP: false positive values).
Results
Among 20 snails tested 1 day post-exposure (L1), 14 (70 %) were found to be PCR positive, while among 20 snails tested 3 days post-exposure (L3), 17 (85 %) were found to be PCR positive. All infected snails tested 7 days post-exposure (L7) were found to be PCR positive (100 %), while all control snails (L0) were negative when tested with PCR. This means that PCR sensitivity and specificity were 100 % (all infected snails appear positive and all non infected snails appear negative) (Tables 1, 2; Fig. 1). The crushing method was not sensitive in detection of early prepatent infection even after 7 days of infection (L7). It detected only (5/20 snails) with 25 % positivity while PCR can detect all infected snails (20/20 snails) with 100 % positivity (Table 1). PCR technique is sensitive in detecting the infection in early prepatency for the laboratory group of snails. L1 (70 %), L3 (85 %) and L7 (95 %). The relation was statistically insignificant (P value >0.05) (Table 2).
Table 1.
Results of PCR for the laboratory group of snails at 1st, 3rd and 7th day post infection and for crushing method at 7th day post infection
| Technique | Groups | Number | Positive | Negative | Positive percentage |
|---|---|---|---|---|---|
| PCR | Control (L0) | 20 | 0 | 20 | 0 |
| 1st day (L1) | 20 | 14 | 6 | 70 | |
| 3 days (L3) | 20 | 17 | 3 | 85 | |
| 7 days (L7) | 20 | 20 | 0 | 100 | |
| Crushing | 7 days (L7) | 20 | 5 | 15 | 25 |
Table 2.
Sensitivity of PCR in detection of infected laboratory snails 1 day, 3 days and 1 weak post infection
| Group | Number | Positive | Negative | Percentage | Chi square χ2 | P |
|---|---|---|---|---|---|---|
| 1st day (L1) | 20 | 14 | 6 | 70 | 2.30 | 0.32 |
| 3 days (L3) | 20 | 17 | 3 | 85 | ||
| 7 days (L7) | 20 | 19 | 1 | 95 |
P > 0.05 was statistically insignificant
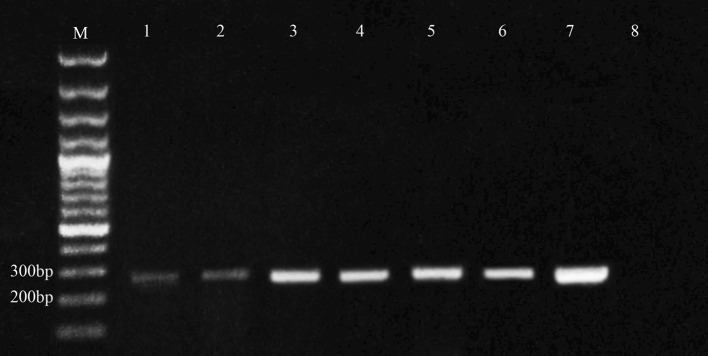
Fig. 1.
Agarose gel electrophoresis for PCR of S. mansoni SMALDO gene (274 bp) in infected laboratory and field snails; lane 1 positive snails after 1 day of infection (L1), lane 2 positive snails after 3 days of infection (L3), lanes 3 positive snails after 7 days of infection (L7), lanes 4 and 5 positive snails for season I, lane 6 positive snails for season II and lane 7 miracidia, control +ve, lane 8 control negative snails (L0). M the marker (bp base pair)
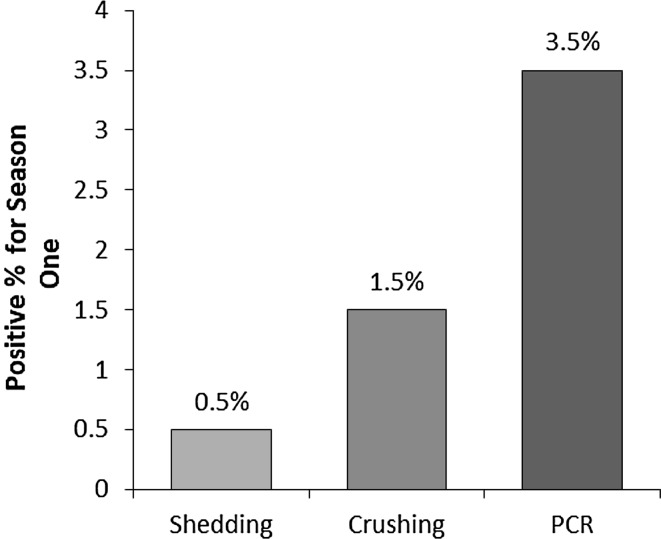
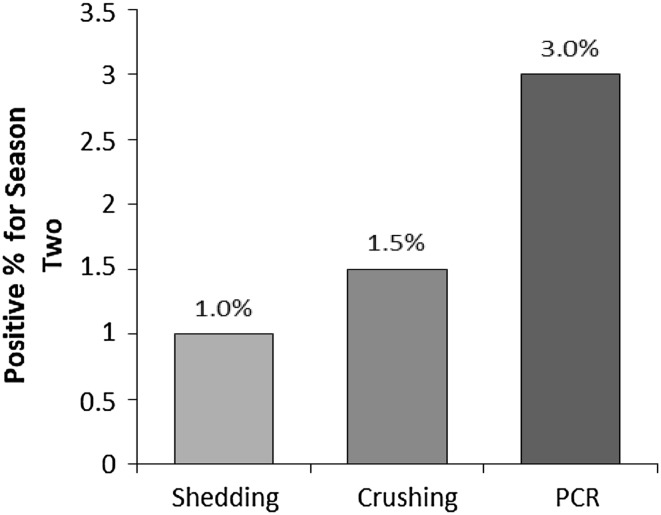
In season I (autumn), only one of the examined field snails was positive (0.5 %) by shedding and three positive snails (1.5 %) detected by crushing, but seven positive snails (3.5 %) detected by PCR. In season II (spring), two snails of the examined field snails were positive (1.0 %) by shedding and three positive snails (1.5 %) detected by crushing, but six positive snails (3 %) detected by PCR (Table 3; Figs. 1, 2, 3). In season I, the sensitivity of shedding method to detect the infected snail was 14 %, however it was negative in all uninfected snails (specificity 100 %). While in season II, the sensitivity of shedding method to detect the infected snail was 33.3 %, however it was negative in all uninfected snails in season II (specificity 100 %) (Table 4). Statistical relationship between shedding method and PCR in season I and II (autumn and spring) was statistically insignificant (P > 0.05) (Table 5).
Table 3.
The results of shedding method, crushing method and PCR technique in field snails in season I and II (the autumn, the spring)
| Season | No. | Shedding | Crushing | PCR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative no. | Positive no. | Positive (%) | Negative no. | Positive no. | Positive (%) | Negative no. | Positive no. | Positive (%) | ||
| Autumn | 200 | 199 | 1 | 0.5 | 197 | 3 | 1.5 | 193 | 7 | 3.5 |
| Spring | 200 | 198 | 2 | 1.0 | 197 | 3 | 1.5 | 194 | 6 | 3 |
Fig. 2.
Comparison between the percentage of positive snails by shedding method, crushing method and PCR technique in season 1 (the autumn)
Fig. 3.
Comparison between the percentage of positive snails by shedding method, crushing method and PCR technique in season 2 (the spring)
Table 4.
Sensitivity and specificity of shedding method in season I and II (autumn and spring)
| PCR | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Shedding (season I) | |||
| Positive | 1 | 0 | 1 |
| Negative | 6 | 193 | 199 |
| Total | 7 | 193 | 200 |
| Shedding (season II) | |||
| Positive | 2 | 0 | 2 |
| Negative | 4 | 194 | 198 |
| Total | 6 | 194 | 200 |
In season I the Sensitivity = 14.3 % and Specificity = 100 %. While in season II the Sensitivity = 33.3 % and Specificity = 100 %
Table 5.
Statistical relationship between shedding method and PCR in season I and II (autumn and spring)
| Season | Total | Shedding positive | PCR positive | P (Fisher Exact test) |
|---|---|---|---|---|
| One | 200 | 1 | 7 | 1.0 |
| Two | 200 | 2 | 6 |
P > 0.05 was statistically insignificant
In season I, the sensitivity of crushing method to detect the infected snail was 42.9 %, however it was negative in all uninfected snails (specificity 100 %). While in season II, the sensitivity of crushing method to detect the infected snail was 50 %, however it was negative in all uninfected snails in season II (specificity 100 %) (Table 6). Statistical relationship between crushing method and PCR in season I and II (autumn and spring) was statistically insignificant (P > 0.05) (Table 7).
Table 6.
Sensitivity and specificity of crushing method in season I and II (autumn and spring)
| PCR | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Crushing (season I) | |||
| Positive | 3 | 0 | 3 |
| Negative | 4 | 193 | 197 |
| Total | 7 | 193 | 200 |
| Crushing (season II) | |||
| Positive | 3 | 0 | 3 |
| Negative | 3 | 194 | 197 |
| Total | 6 | 194 | 200 |
In season I the Sensitivity = 42.9 % and Specificity = 100 %. While in season II the Sensitivity = 50 % and Specificity = 100 %
Table 7.
Statistical relationship between crushing method and PCR in season I (autumn) and II (spring)
| Season | Total | Crushing positive | PCR positive | P (Fisher Exact test) |
|---|---|---|---|---|
| One | 200 | 3 | 7 | 1.0 |
| Two | 200 | 3 | 6 |
P > 0.05 was statistically insignificant
By comparing the sensitivity of the three methods, it was found that the average sensitivity of shedding method compared to PCR was 23.8 % and the average sensitivity of crushing method compared to PCR was 46.4 % (Table 8).
Table 8.
Average sensitivity of shedding, crushing and PCR in season I (autumn) and II (spring)
| Season | Total case | Shedding | Crushing | PCR | |||
|---|---|---|---|---|---|---|---|
| Positive no. | Sensitivity (%) | Positive no. | Sensitivity (%) | Positive no. | Sensitivity (%) | ||
| Autumn | 200 | 1 | 14.3 | 3 | 42.9 | 7 | 100 |
| Spring | 200 | 2 | 33.3 | 3 | 50.0 | 6 | 100 |
| Average | 23.8 | 46.4 | 100.0 | ||||
Discussion
Biomphalaria alexandrina is prevalent in Egypt, during the last decade it becomes the most dominant species in both upper and Lower Egypt forming a main threat for schistosomiasis transmission in the South of Egypt leading to infection of previously non infected population (Hamed 2010). The detection of infected snails in different sites can be considered sensitive and allows more rapid effective control of the intermediate host of schistosomiasis infection. Rapid and accurate detection of the prepatent schistosome infection in B. alexandrina snails is crucial for controlling S. mansoni infection (Abu El Einin et al. 2009). The traditional methods for examination of infected snails (shedding and crushing) are less sensitive, time consuming and less accurate in detection of prepatent schistosomal infections. However, PCR has been successfully and specifically used for monitoring of snail prepatent infection rates which reflect infection prevalence in human populations.
Molecular tools such as conventional PCR and improved DNA amplification methods have been shown capable of detecting schistosome DNA in a variety of samples. A highly repetitive, 121-base pair (bp) sequence has been used to detect DNA from S. mansoni and S. haematobium in stools, serum, urine, and plankton samples (Pontes et al. 2002; Sandoval et al. 2006).
In this work, we evaluate the sensitivity of molecular method for detection of prepatent infection of snails by S. mansoni through amplification of a partial sequence of S. mansoni SMALDO gene using PCR. This molecular method could differentiate between S. mansoni from other morphologically similar parasites that may co-exist in naturally infected snails. This prompts us to carefully check the similarity of primers sequences to other known sequences with BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi). Our results showed that the primers sequences used in this study are unique for the S. mansoni SMALDO sequences and no cross reaction with other schistosomes or molluscan aldolases.
Only one band of S. mansoni SMALDO gene was identified in infected snails as compared to complete negativity of non-infected snails. In contrast, Hamburger et al. (1998) have found more than one band (laddering) of S. mansoni tandem repeat (121 bp) in infected snails. This ladder pattern differs according to concentration of genomic DNA (Hamburger et al. 1998). Therefore, detection of prepatent infection of snails by S. mansoni using SMALDO gene (one specific band pattern) is more practical than tandem repeat (laddering pattern) because the former method allows rapid and easy interpretation. SMALDO gene is superior to tandem repeat because SMALDO is specific only for S. mansoni and not cross reactive with other schistosomes or molluscan aldolases. In addition, the detection of the transcripts of SMALDO could be more sensitive in detection of prepatent infected snails due to RNA redundancy of such gene (Franco et al. 1997). Although PCR assays are highly sensitive and accurate as compared to routine methods, they vary in sensitivity, specificity and detection time. This variation mainly depends on the target sequences selected for PCR amplification. Several research groups have developed PCR methods using various target sequences and these PCR assays showed different sensitivities for detection of Schistosoma DNA. For examples; a PCR assay based on a highly repeated tandemly arranged DNA sequence (121 bp) detects prepatent S. mansoni infection at the 1st day post infection (80 % sensitivity) (Hamburger et al. 1998). A nested PCR targeting the 18S rDNA of S. mansoni detects infection in snails 1 week post infection (100 % sensitivity) (Hanelt et al. 1997). The PCR amplified sequence of the mitochondrial DNA minisatellite repeat from S. mansoni identifies infected snails 1 week post infection and with 100 % sensitivity (Jannotti-Passos et al. 1997).
In the current study, examination of the laboratory infected snails revealed that PCR technique is able to detect early prepatent infection. The SMALDO PCR assay permits detection of the laboratory infected snails up to 1 day after miracidial infection with 70 % actual sensitivity in B. alexandrina infected with 8 miracidia per snail. All snails tested (100 %) 1 week after infection (L7) were positive. However, examination of (L7) group by crushing method reveals that only 5 snails out of 20 are positive (25 %). Hamburger et al. (1998) have reported an early detection of a121 bp tandem repeat region of S. mansoni in infected B. glabratasnails 1 day after infection (80 % sensitivity), 3 days (80 % sensitivity), and 1 week (80 % sensitivity). This difference may be due to gene sequence, where we used a more specific gene (SMALDO gene) while the previous author depend on a repetitive gene. Additionally, it may be attributed to technical errors in infection of the snails. The difference in prevalence of infection among laboratory snails may be attributed to snails´ immune response because the numerous species and strains of Biomphalaria vary in their compatibility as a schistosome host such that some display resistance to infection while others are susceptible. When resistant snails are exposed to S. mansoni miracidia, haemocytes (snail blood cells) migrate towards the recently transformed sporocysts and enclose them in a multilayered cellular encapsulation. Soon after, the sporocysts are killed by a cytotoxic reaction which most likely involves free radicals such as hydrogen peroxide and/or nitric oxide, in contrast, susceptible snails are not able to successfully defend against S. mansoni larvae and an infection will be developed following miracidial exposure (Hahn et al. 2001). Also it may be attributed to the difference in the concentration of schistosomal DNA as regards days of infection (as the infection becomes well established).
All field snails of both seasons were examined by the conventional methods of examination for infection (shedding and crushing) and then subjected to PCR to evaluate the ability for applying PCR method in the field in a trial to overcome the limitation of the conventional methods. In season I (autumn), we found that examination of 200 snails revealed one snail was positive by shedding method (0.5 %), three positive snails by crushing (1.5 %) and seven positive snails by PCR (3.5 %) (4 were previously negative by both methods in addition the three positive snails from shedding and crushing) and in season II (spring) we found that examination of 200 snails revealed two shedding snails (1 %),while there were three positive snails by crushing (1.5 %) and six positive snails by PCR (3 %). Thus the obtained results reflect the sensitivity of PCR in comparison to the other two methods. This was in agreement with Melo et al. (2006) who found that PCR allowed for the identification of infection in pools that were negative by the conventional methods which was able to detect S. mansoni infection in 37.5 % of the snail pools. Hamburger et al. (2004) used the same technique for monitoring infected S. haematobium snails in Kenya. They found that PCR positivity was detected as soon as one day after miracidial exposure, with 4 of the 20 snails tested (20 %) showing PCR positivity, 3 days later 11 of the 20 snails tested (55 %) were PCR positive, and 13 (76.4 %) of 17 exhibited PCR positivity after 5 days exposure to miracidia. The difference in sensitivity of PCR may be attributed to that Hamburger et al. (2004) depend on the hexadecyltrimethyl ammonium bromide (CTAB) method for extraction of genomic DNA, but we used chemical kit which is more simple extraction method and can avoid many technical errors and hazards of the chemical one, at the same time it is more sensitive, rapid, and can yield more pure DNA. Also it may be due to different localities and different Schistosoma species.
New molecular amplification technologies offer a positive step towards community monitoring of local water contamination by Schistosoma eggs and transmission to snails. Use of these sensitive and specific techniques will provide essential data for precise determination of the exact time and prevalence of snail infection. This will greatly help in the programs of Schistosoma control especially mass chemotherapy and snail control. PCR for S. mansoni detection would have the additional advantage of estimating the parasite burden of the infection. Although the cost per reaction is still relatively high, the reagents and equipments for molecular techniques are becoming cheaper, making the discussion concerning high cost less relevant.
In conclusion, PCR is superior to the conventional methods and can detect positive cases that were negative when examined by shedding or crushing methods. However, more extensive validation studies in endemic a1
reas are crucial to fully investigate the suitability of the tools for these purposes, with benefits for the control of schistosomiasis.
References
- Abath FG, Gomes AL, Melo FL, Barbosa CS, Werkhauser RP. Molecular approaches for the detection of Schistosoma mansoni: possible applications in the detection of snail infection, monitoring of transmission sites and diagnosis of human infection. Mem Inst Oswaldo Cruz Rio de Janeiro. 2006;101(Suppl. I):145–148. doi: 10.1590/S0074-02762006000900023. [DOI] [PubMed] [Google Scholar]
- Abbasi I, King CH, Muchiri EM, Hamburger J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency. Am J Trop Med Hyg. 2010;83(2):427–432. doi: 10.4269/ajtmh.2010.09-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu El Einin HM, Mansour WA, El-Dabaa E. Assessment of infected Biomphalaria alexandrina snails by detecting Schistosoma mansoni antigen and specific gene. Aust J Basic Appl Sci. 2009;3(3):2747–2753. [Google Scholar]
- Behrman AJ (2005) Schistosomiasis. EMedicine. Available at: http://www.emedicine.com/emerg/topic857.htm. Accessed 31 March 2005
- Caldeira RL, Jannotti-Passos LK, Lira PM, Carvalho OS. Diagnostic of Biomphalaria Snails and Schistosoma mansoni: DNA obtained from traces of shell organic materials. Mem Inst Oswaldo Cruz Rio de Janeiro. 2004;99(5):499–502. doi: 10.1590/S0074-02762004000500007. [DOI] [PubMed] [Google Scholar]
- Chu KY, Dawood v. Cercarial production from Biomphalaria alexandrina infected with Schistosoma mansoni. Bull World Health Org. 1970;42:574–596. [PMC free article] [PubMed] [Google Scholar]
- El-Dabaa E, Mei H, El-Sayed A, Karim AM, Eldesoky HM, Fahim FA, LoVerde PT, Saber MA. Cloning and characterization of Schistosoma mansoni fructose-1,6-bisphosphate aldolase isozyme. J Parasitol. 1998;84:954–960. doi: 10.2307/3284627. [DOI] [PubMed] [Google Scholar]
- Fenwick A. New initiatives against Africa’s worms. Trans R Soc Trop Med Hyg. 2006;100:200–207. doi: 10.1016/j.trstmh.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Franco GR, Rabelo EML, Azevedo V, Pena HB, Ortega JM, Santos TM, Meira WSF, Rodrigues NA, Dias CMM, Harrop R, Wilson A, Saber MA, Abdel-Hamid H, Faria MSC, Margutti MEB, Parra JC, Pena SDJ. Evaluation of cDNA libraries from different developmental stages of Schistosoma mansoni for production of expressed sequence tags (ESTs) DNA Res. 1997;4:231–240. doi: 10.1093/dnares/4.3.231. [DOI] [PubMed] [Google Scholar]
- Hahn UK, Bender RC, Bayne CJ. Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: role of reactive oxygen species. J Parasitol. 2001;87:292–299. doi: 10.1645/0022-3395(2001)087[0292:KOSMSB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hamburger J, Xin HX, Ramzy RM, Jourdane J, Ruppel A. A polymerase chain reaction assay for detecting snails infected with Bilharzia parasites (Schistosoma mansoni) from very early prepatency. Am J Trop Med Hyg. 1998;59(6):872–876. doi: 10.4269/ajtmh.1998.59.872. [DOI] [PubMed] [Google Scholar]
- Hamburger J, Hoffman O, Kariuki HC, Muchiri EM, Ouma JH, Koech DK, Sturrock RF, King CH. Large scale polymerase chain reaction based surveillance of Schistosoma haematobium DNA in snails from transmission sites in Costal Kenya: a new tool for studying the dynamics of snail infection. Am J Trop Med Hyg. 2004;71(6):765–773. doi: 10.4269/ajtmh.2004.71.765. [DOI] [PubMed] [Google Scholar]
- Hamed MA. Strategic control of Schistosoma intermediate host. Asian J Epidemiol. 2010;3(3):123–140. doi: 10.3923/aje.2010.123.140. [DOI] [Google Scholar]
- Hanelt B, Adema CM, Mansour MH, Loker ES. Detection of Schistosoma mansoni in Biomphalaria using nested PCR. J Paraitol. 1997;83:387–394. doi: 10.2307/3284399. [DOI] [PubMed] [Google Scholar]
- Humphries J. Effects of larval schistosomes on Biomphalaria snails. In: Toledo R, Fried B, editors. Biomphalaria snails and larval trematodes. New York: Springer; 2010. pp. 103–125. [Google Scholar]
- Jannotti-Passos LK, Vidigal T, Dias-Neto E, Pena S, Simpson A, Dutra W, Souza C, Carvalho-Parra J. PCR amplification of the mitochondrial DNA minisatellite region to detect Schistosoma mansoni infection in Biomphalaria glabrata snails. J Parasitol. 1997;83:395–399. doi: 10.2307/3284400. [DOI] [PubMed] [Google Scholar]
- Liang YS, John I, Bruce JI, David AB. Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycle. Proc First Sine Am Symp. 1987;1:34. [Google Scholar]
- Melo FL, Gomes AV, Barbosa CS, Werkhauser RP, Abath FGC. Development of molecular approaches for the identification of transmission sites of schistosomiasis. Trans R Soc Trop Med Hyg. 2006;100:1049–1055. doi: 10.1016/j.trstmh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123(7):211–228. doi: 10.1017/S0031182001007703. [DOI] [PubMed] [Google Scholar]
- Negrao-Correa D, Mattos AC, Pereira CA, Martins-Souza RL, Coelho PM (2012) Interaction of Schistosoma mansoni sporocysts and hemocytes of Biomphalaria. J Parasitol Res. vol 2012 [DOI] [PMC free article] [PubMed]
- Pontes LA, Dias-Neto E, Rabello A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg. 2002;66:157–162. doi: 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- Prah SK, James C. The influence of physical factors on the behavior and infectivity of miracidia of S. mansoni and S. haematobium. II- effect of temperature and ultraviolet light. Helminthology. 1977;51:73–85. doi: 10.1017/S0022149X00007288. [DOI] [PubMed] [Google Scholar]
- Sandoval N, Siles-Lucas M, Lopez AJ, Pérez-Arellano JL, Gárate T, Muro A. Schistosoma mansoni: a diagnostic approach to detect acute schistosomiasis infection in a murine model by PCR. Exp Parasitol. 2006;114:84–88. doi: 10.1016/j.exppara.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection and reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. doi: 10.4269/ajtmh.2006.75.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Wuhrer M, Hamburger J, Jourdane J, Ramzy RM, Gever R. Schistosoma mansoni and Schistosoma haematobium: identification and characterization of glycoconjugate antigens in hemolymph of infected vector snails. J Parasitol. 2002;88:505–513. doi: 10.1645/0022-3395(2002)088[0505:SMASHI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Webbe G. Transmission of bilharziasis 2: production of cercariae. Bull World Health Orag. 1965;33:155–162. [PMC free article] [PubMed] [Google Scholar]
- Webbe G, El Hak S. Progress in the control of schistosomiasis in Egypt. Trans R Soc Trop Med Hyg. 1990;84:394–400. doi: 10.1016/0035-9203(90)90334-B. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2013) Schistosomiasis a major public health Available: http://www.who.int/schistosomiasis/en/(http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi)