Abstract
Of late, toxoplasmosis has gained immense importance as an opportunist parasite in immunocompromised patients. In immunocompromised subjects, the disease is supposed to occur in acute form and causes acute toxoplasmic encephalitis. However, the exact pathogenesis of other vital organs, particularly in acute form of infection, is still a matter of debate. Therefore, an attempt was made to study the pathogenesis of acute form of toxoplasmosis using cryopreserved human RH strain of the parasite in murine models. For this, 100 tachyzoites were given to individual mice and upon the setup of acute form of infection, the mice were euthanized and the organs were processed for histopathology. Histopathology revealed tachyzoites in liver only while severe necrosis due to multiplication of tachyzoites were visible in liver, spleen, lungs and brain. Kidneys and heart appeared more or less normal. Finally, the pathology of disease in these organs is described in detail. The present research has generated some vital information regarding necrotic changes in tissues due to acute toxoplasmosis and will defiantly help the researchers in the better understanding of disease particularly in humans and putting up of suitable treatment regime for human subjects infected with acute toxoplasmosis.
Keywords: Experimental toxoplasmosis, Histopathology, Murine model, RH stain
Introduction
Toxoplasma gondii, an obligate intra cellular coccidian parasite, has got utmost zoonotic relevance in the present scenario. The pathophysiology of disease may either be classified as acute toxoplasmosis that occurs mainly with the direct inoculation of infective form viz. tachyzoites into the host (Parker et al. 1981) or chronic toxoplasmosis that occurs after acute toxoplasmosis when the immune system suppresses the multiplication of tachyzoites leading to the formation of tissue-localized cysts or bradyzoites (Skariah et al. 2010). All the non-feline vertebrates including humans act as intermediate hosts of the parasite showing disseminated tissue infections. It has been estimated that as many as one-third of the world’s population shows serological evidence of infection (Montoya and Liesenfeld 2004). The condition leads to life-threatening consequences both in immunocompromised human patients suffering from acquired immune deficiency syndrome (AIDS) and those with organ transplants (Angel et al. 1997). The latent bradyzoites often reverts to tachyzoites, causing reactivation of acute toxoplasmosis (Skariah et al. 2010) mainly in the brain tissues (Brenier-Pinchart et al. 2004) ultimately leading to acute toxoplasmic encephalitis in immunocompromised patients (Luft and Remington 1992; Porter and Sande 1992) and accounts for around 20 % of AIDS related deaths in the United States (Montoya and Liesenfeld 2004). Presence of brain cysts is often associated with various psychiatric disorders such as schizophrenia (Webster et al. 2006; Daryani et al. 2010) alongside other brain pathologies in both immunocompromised and immunocompetent individuals (Alvarado-Esquivel et al. 2006; Fekadu et al. 2010). The present study was designed to assess the extent of pathogenesis and damage caused by dividing tachyzoites in acute state of infection in Inbred Swiss albino mice using laboratory maintained cryopreserved stock of human RH strain of the parasite.
Materials and methods
Selection of the parent stock and experimental model
Ten Inbred Swiss albino mice of either sex, 6–12 weeks of age and weighing about 25–30 g were obtained from the Laboratory of Animal Resource Section, Indian Veterinary Research Institute (IVRI), India. Animals were kept in polypropylene cages and acclimatized for a period of 15 days prior to experimentation under standard temperature, humidity and light cycle conditions. Animals were fed on a balanced diet ad libitum, consisting of crushed wheat 62 %, maize 30 %, wheat bran 7 %, and common salt 1 %. Fresh potable water was made available ad libitum.
Inoculation of mice with virulent tachyzoites
The mice were infected with virulent 100 tachyzoites of human RH strain of T. gondii, through intraperitoneal route. The strain was being maintained through regular passaging in murine model for over a decade in a cryopreserved stock line in Divisional Protozoology Laboratory, IVRI. Briefly, these tachyzoites were firstly injected into a single mice and the tachyzoites obtained from that mice were further used for the study. The infected mice were routinely observed for the symptoms of acute toxoplasmosis.
Collection of body organs for histopathology
When the general symptoms of acute toxoplasmosis reached their peak extent, the mice were euthanized under chloroform anaesthesia. Briefly, the mice were put into desiccators pre-inoculated with cotton swab dipped in chloroform. The peritoneal fluid was aspirated after inoculation of 5 ml of sterile phosphate buffered saline (PBS, pH 7.2) in the peritoneal cavity with due care of avoiding injury to visceral organs. The process was repeated until the peritoneal contents turn clear. The peritoneal lavage was later stained with Giemsa stain to ascertain the presence of free tachyzoites. There after the peritoneum of the mice was cut open and the gross pathology of all the major organs was recorded. Small tissue pieces of lungs, liver, brain, spleen, kidney and heart were collected and stored in 10 % formal saline solution and were subsequently send to the Department of Pathology, IVRI, for routine histopathology.
Results and discussion
All the experimental mice were routinely examined following challenge for development of clinical signs of toxoplasmosis. The clinical signs started appearing Day 5 post inoculation and reached its peak Day 7 post inoculation. The mice were euthanized on Day 7 post inoculation. The clinical signs included raised hair coat, development of severe ascites with pendulous abdomen, dullness, tachypnoea marked by resting of fore legs either on walls of the cages or on the nozzle of water bottle or on other resting mice.
Staining of peritoneal lavage
A large number of free tachyzoites were seen in the peritoneal fluid (Fig. 1) demonstrating the extent of acute toxoplasmosis. Besides free tachyzoites, a few intact macrophages, filled with the dividing tachyzoites, were also observed.
Fig. 1.
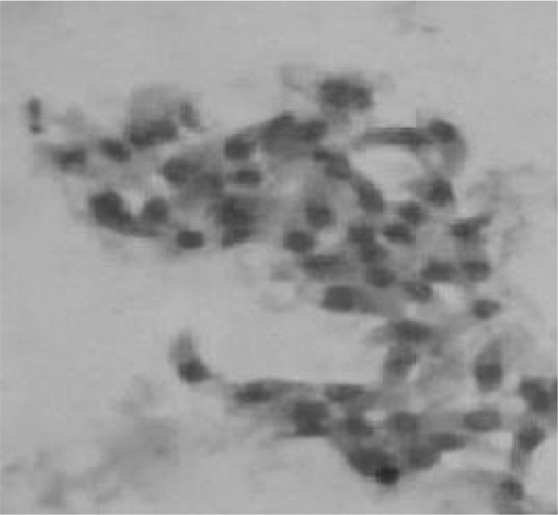
Giemsa stained tachyzoites from the peritoneal lavage of the infected mice
Gross morphology of organs
Upon opening of the peritoneal cavity (Fig. 2), enlargement of liver and spleen was quite remarkable in all the mice. Hepatomegaly was recorded and the liver was pale in colour besides, a few grayish white multiple necrotic foci on surface of liver particularly in the caudal lobes suggesting of hepatopathy (Fig. 3). In spleen, almost similar lesions as described for liver were recorded. Marked splenomegaly (Fig. 4) with spleen almost 2–3 times larger than the normal was seen. Lungs showed clearly marked visible areas of necrosis (Fig. 5). The other major organs viz, heart, brain and kidney appeared more or less normal upon gross examination.
Fig. 2.

In situ organs in the experimentally infected mice upon opening of the peritoneum
Fig. 3.
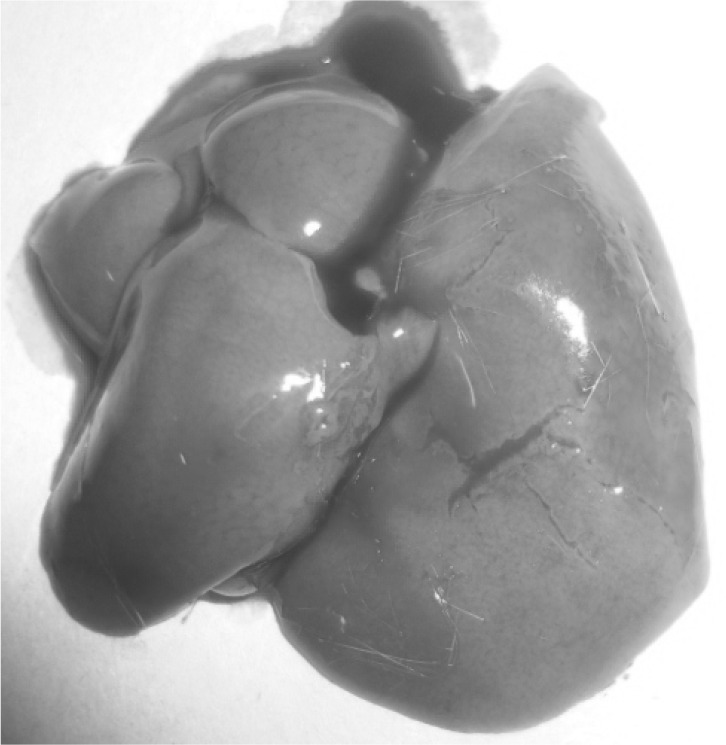
Gross necrotic foci on the liver of the infected mice
Fig. 4.
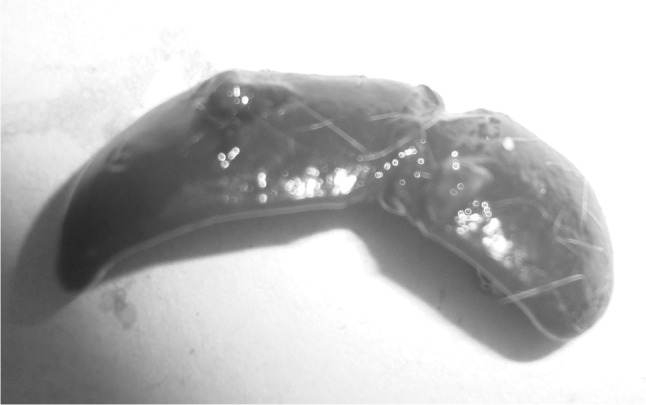
Marked splenomegaly in the infected mice
Fig. 5.
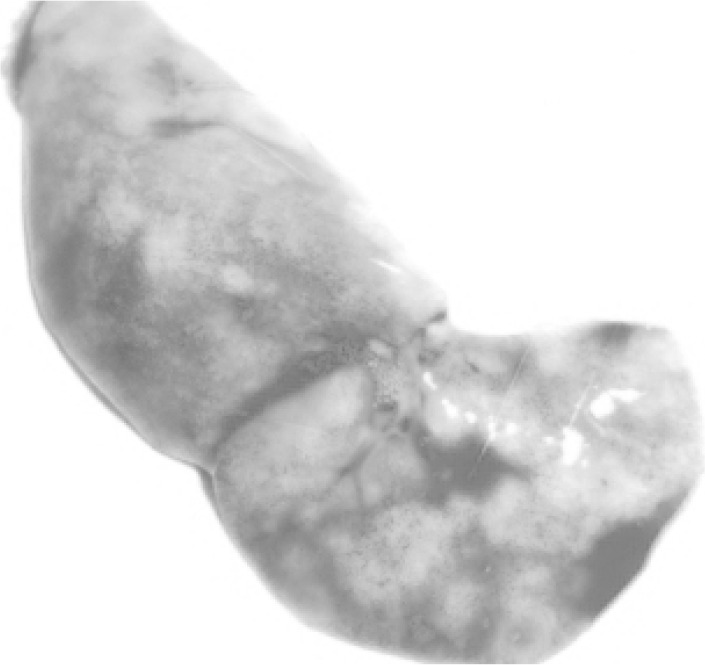
Gross necrotic foci on the lungs of the infected mice
Histopathology
A number of miliary necrotic foci were found scattered throughout the liver suggestive of severe necrosis. The necrotic foci were characterized by pool of necrotic cellular debris along with moderate number of degenerated/intact tachyzoites (Fig. 6). Besides these, there was presence of microgranulomas (4–5 foci/40×). The tachyzoites were present in hepatic parenchymal sinusoidal spaces and along the portal vessels and central vein. Hepatic cells were enlarged and vacuolated. Spleen also showed a number of necrotic foci disseminated in the white and red pulp. Hyperplasia of lymphoid tissue in follicles as well as in white matter was markedly observed (Fig. 7). The number of megakaryocytes was increased. Amyloidosis was seen in spleen of seven out of ten mice suggestive of antigen antibody reaction. In lungs, the capillary plexus in the inter-alveolar septa were mildly thickened with perivascular infiltration of mononuclear cells. There were aggregates of necrotic foci at few places suggestive of acute necrosis (Fig. 8). In brain, few areas of degeneration in the neuropil were observed. Meningitis was also observed in few cases (Fig. 9). The kidneys did not revealed any sign of necrosis or tachyzoites except for the increase in perivascular spaces. The heart did not show any abnormal changes (Fig. 10).
Fig. 6.
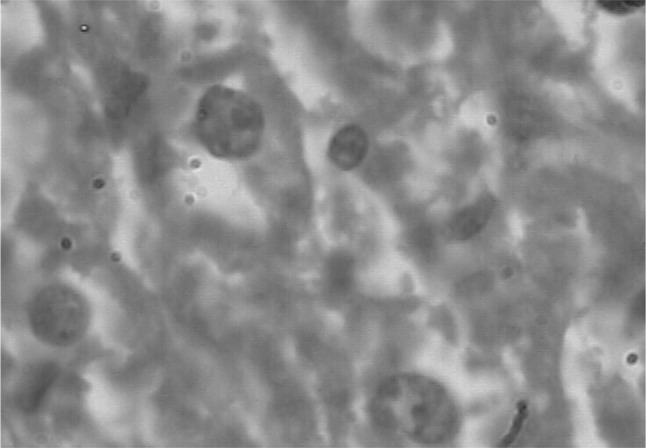
Histopathological section of liver showing tachyzoite and degenerative changes
Fig. 7.
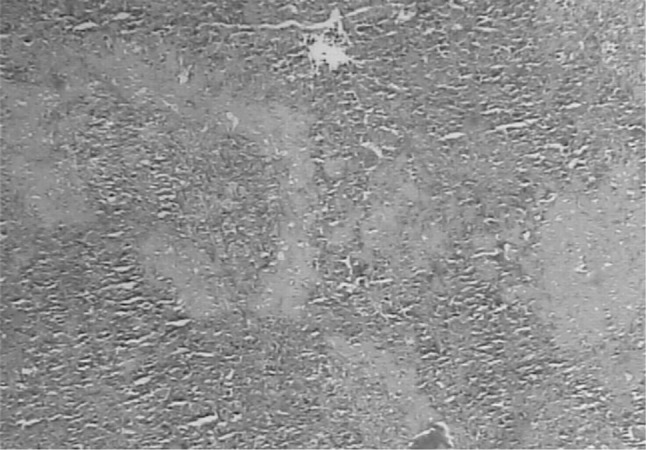
Histopathological section of spleen showing degenerative changes and amyloid deposition
Fig. 8.
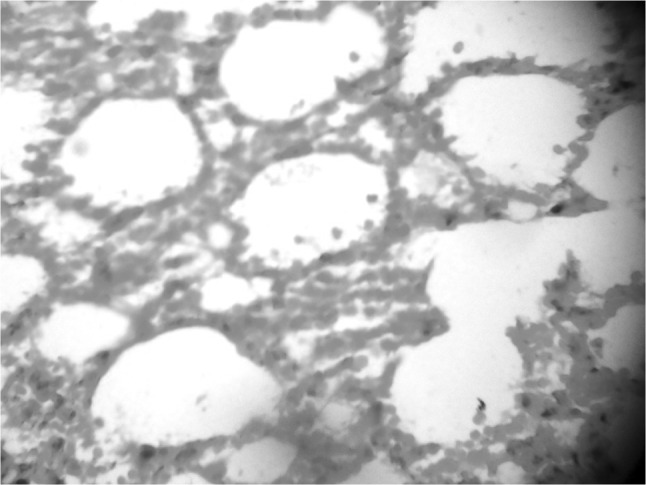
Histopathological section of lung showing degenerative changes
Fig. 9.
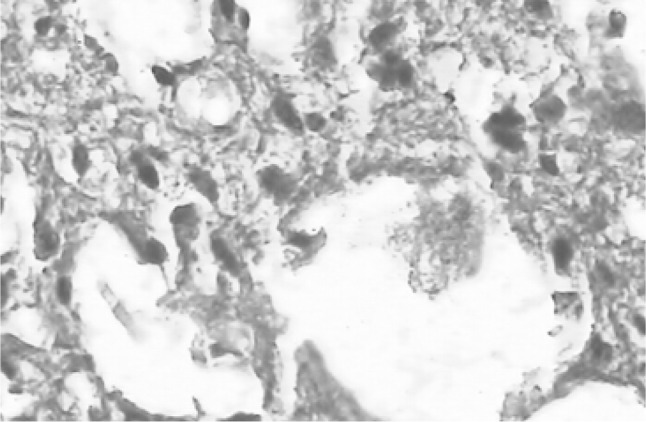
Histopathological section of brain showing degenerative changes
Fig. 10.

Histopathological section of heart
The unique ability of T. gondii to multiply in virtually all the nucleated cells of the host body and its subsequent encystment in the various tissues, coupled with its wide host range consisting of all the warm blooded animals including humans, makes the parasite a matter of serious concern for both animal and human health in the modern times. The reversion of tissue cysts containing bradyzoites back to rapidly dividing tachyzoites resulting into disease reactivation is a common phenomenon in immunocompromised patients (Lyons et al. 2002). 100 tachyzoites are sufficient for the establishment of acute phase of infection in mice (Kumara et al. 2010; Sudan et al. 2014). In the instant case, upon entry into the mice peritoneal cavity the tachyzoites were taken up by the macrophages but they continue to divide inside the macrophages (OIE 2008) leading to their rupture and peritonitis. There after upon entry into the host body vascular system they get disseminated to various parts of the body. The main pathogenesis in toxoplasmosis is due to tissue necrosis caused by rapidly dividing tachyzoites (OIE 2008). In usual instances, the development of the immune response against the dividing tachyzoites suppresses their division and they form tissue cysts containing bradyzoites (OIE 2008; Boothroyd 2009) but in the instant case the RH strain was used and this strain has lost its property to get converted to bradyzoites (Dubey 2008). The mice are considered to be more closer to humans and the pathogenesis in mice is supposed to be somewhat similar to be what could have occurred in humans.
In conclusion, above cited description on the clinical pathogenesis of human strain of toxoplasmosis in mice, that is indiscriminately addressed, has been an eye opener for the academicians as well as researchers to further investigate host parasite interactions with emphasis on differences between experimental and naturally acquired infections, magnitude of the disease, its pathogenesis and pathophysiological impact on health vis-à-vis improved diagnostic for specific diagnosis of the disease exhibiting non specific signs/symptomatology through critically planned in vivo as well as in vitro studies. The advent of molecular biological techniques may improve the efficiency of detecting the hidden pathogen, causing slow progressing disease in canines. The disease which is of prime zoonotic importance needs adequate attention. The present study has given a clearer picture of the damage caused by dividing tachyzoites at the tissue level and will give a broader better vision of the pathology of the disease and will definitely help in the better understanding of the disease course particularly in acute phase of infection.
Acknowledgments
The authors are thankful to the Director, IVRI for providing the facilities and to the ICAR for the fellowship awarded to the first author during the perusal of his master’s programme.
Declaration
The experiments to the laboratory animal were done as per the approval of University Ethical Committee.
References
- Alvarado-Esquivel C, Alanis-Quiñones OP, Arreola-Valenzuela MA, Rodríguez- Briones A, Piedra-Nevarez LJ, Duran-Morales E, Estrada-Martínez S, Martínez-García SA, Liesenfeld O. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. 2006;6:178. doi: 10.1186/1471-2334-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel SO, Matrajt M, Margarit J, Nigro M, Illescas E, Pszenny V, Amendoeiva MRR, Guavnera E, Garberi JC. Screening of active toxoplasma in patients by DNA hybridization with ABGTg7 probe in blood samples. J Clin Microbiol. 1997;35:591–595. doi: 10.1128/jcm.35.3.591-595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd JC. Toxoplasma gondii: 25 years and 25 major advances for the field. Int J Parasitol. 2009;39:935–946. doi: 10.1016/j.ijpara.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenier-Pinchart MP, Blanc-Gonnet E, Marche PN, Berger F, Durand F, Ambroise-Thomas P, Pelloux H. Infection of human astrocytes and glioblastoma cells with Toxoplasma gondii: monocyte chemotactic protein-1 secretion and chemokine expression in vitro. Acta Neuropathol. 2004;107:245–249. doi: 10.1007/s00401-003-0804-0. [DOI] [PubMed] [Google Scholar]
- Daryani A, Sharif M, Hosseini SH, Karimi SA, Gholami S. Serological survey of Toxoplasma gondii in schizophrenia patients referred to psychiatric hospital, Sari city, Iran. Trop Biomed. 2010;27:476–486. [PubMed] [Google Scholar]
- Dubey JP. The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol. 2008;55(6):467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behavior disorders—overview of evidence and mechanisms. Folia Parasitol. 2010;57:105–113. doi: 10.14411/fp.2010.013. [DOI] [PubMed] [Google Scholar]
- Kumara MU, Mishra AK, Rao JR, Tewari AK, Ravindran R. Kinetics of Toxoplasma gondii infection in murine model. J Vet Parasitol. 2010;24(2):133–136. [Google Scholar]
- Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Lyons R, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite–bradyzoite interconversion. Trends Parasitol. 2002;18(5):198–202. doi: 10.1016/S1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- OIE (2008) Toxoplasmosis in OIE Terrestrial Manual 1284–1293
- Parker GA, Langloss JM, Dubey JP, Hoover EA. Pathogenesis of acute toxoplasmosis in specific-pathogen-free cats. Vet Pathol. 1981;18:786–803. doi: 10.1177/030098588101800609. [DOI] [PubMed] [Google Scholar]
- Porter SB, Sande M. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- Skariah S, McIntyre MK, Mordue DG. Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion. Parasitol Res. 2010;107:253–260. doi: 10.1007/s00436-010-1899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudan V, Tewari AK, Singh H. An insight into the behavior, course and kinetics of acute infection of Toxoplasma gondii human RH strain in experimentally infected murine model. Iran J Parasitol. 2014;9(1):114–119. [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Lamberton PHL, Donnelly CA, Torrey EF. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood- stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behavior. Proc R Soc. 2006;273:1023–1030. doi: 10.1098/rspb.2005.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]


