Abstract
Cystic Echinococcosis is a parasitic disease with cosmopolitan distribution caused by the tape worm Echinococcus granulosus. Fibrous layer is developed around the cyst as a host immune response reaction. The aim of this study was to evaluate the rate of IL-4 gene expression in fibrous layer of bovine and ovine hepatic hydatid cysts using quantitative technique of Real-Time PCR. In this descriptive study the samples of hydatid cyst fibrous layer were taken from 6 bovine and 6 ovine hepatic hydatid cysts. Samples of normal liver tissue close to the cyst were also taken as controls. Total RNA from each sample was extracted and then converted to cDNA. Afterward, the rate of IL-4 gene expression for each sample was evaluated using real-time PCR technique. Data were analyzed by REST software (version 2.0.13, 2009). In sheep the rate of IL-4 gene expression in the fibrous layer of hepatic hydatid cysts was 1.98 times more than the rate of IL4 gene expression in control samples, but the difference was not significant (P = 0.561). In cattle the rate of IL-4 gene expression in the fibrous layer of hepatic hydatid cysts was 9.84 times more than that of control samples which was statistically significant (P < 0.001). With high rate of IL4 expression especially in fibrous layer of bovine hydatid cyst, it can be concluded that this interleukin may play an important role in host parasite relationship.
Keywords: Hydatid cyst, IL-4, Gene expression, Real-Time PCR, Fibrous layer
Introduction
Cystic Echinococcosis (CE) or hydatidosis is a parasitic disease with cosmopolitan distribution, which is caused by the tape worm Echinococcus granulosus. This zoonotic infection is a major public health and economic problem in many regions of the world (Fauser and Kern 1997; McManus et al. 2003; Rigano et al. 1999).
Hydatid cyst, which is the larval stage of E. granulosus, develops in intermediate hosts viscera, consist of two parasite-derived layers, an inner nucleated germinal layer and an outer acellular laminated layer surrounded by a host-produced fibrous capsule as the consequence of the host immune response (Zhang et al. 2003; Zhang and McManus 2006; Zhang et al. 2011). In fertile hydatid cyst brood capsules and protoscoleces bud off from the germinal membrane (Zhang et al. 2011).
Both humoral and T cell mediated responses seem to play an important role in immunology of hydatid cyst. These responses are considered to be regulated by cytokines (Arai et al. 1990). As to the production of distinct cytokine patterns, immune responses to this parasite can be divided into a Th1-type reaction and a Th2-type reaction (Fauser and Kern 1997; Mosmann et al. 1986). It has been shown that, like other helminthic infections (Allen and Maizels 1996; Finkelman et al. 1991; Lange et al. 1994; Mosmann and Sad 1996; Pearce et al. 1991), hydatid cyst induces two very distinct Th1 and Th2 cytokine secretion patterns (Zhang et al. 2003). Existence of complex mixture of antigens in hydatid cyst fluid (McManus and Bryant 1995), which probably contain distinct epitopes for each T-cell subset (Zhang et al. 2003) that may explain why Th1 and Th2 interleukins secret simultaneously in hydatid cyst infected hosts. So early Th1 cytokines, which can kill the parasite (Vuitton 2003) shifts to Th2 cytokine response in the chronic stage (Zhang et al. 2012).
IL-4 can affect a variety of target cells in different ways. It has an important role in regulating antibody production, inflammation and the development of effector T-cell responses (Jeong et al. 2004). Production of interleukin-4 (IL-4) is directing the immune system toward Th2-dependent allergic reactivity (Maggi 1998; Mueller et al. 2002; Nelms et al. 1999). It is made in response to immunologic recognition, principally by CD4 T lymphocytes (Ben-Sasson et al. 1990; Zhang et al. 2003). IL-4 is responsible for the production of IgE in mice (Finkelman et al. 1990; Zhang et al. 2003).
In patients with chronic hydatidosis a high level of IL4 has been reported (Rigano et al. 1995a, b, 1996; Zhang et al. 2003) and measurement of serum IL-4 may be a useful marker for the follow up of patients with CE (Zhang and McManus 2006). Mice injected with a vector expressing IL-4 showed higher cyst load than the load in control mice (Al-Qaoud and Abdel-Hafez 2008). So IL-4 may play an important role in hydatid cyst development in the mammalian host (Zhang et al. 2011).
Although many studies have been performed about the IL4 levels in hydatid cyst patients or in animal model, but no study has so far been performed about the IL-4 level in fibrous layer of hydatid cysts where cellular response to the parasite take place. So regarding the role of fibrous layer that controlling the hydatid cyst growth, in this work IL-4 gene expression in fibrous layer of hydatid cyst in comparison with its expression in normal surrounding tissue of the cyst in two well-known intermediate hosts, sheep and cattle, has been investigated using real-time PCR technique.
Materials and methods
Sampling
In this study, hydatid cyst samples were collected from Khomeinshahr slaughterhouse located in Isfahan province, central Iran. Hydatid cysts from 6 sheep and 6 cattle were collected. For every cyst about 2 cm2 of fibrous layer of ovine and bovine hepatic hydatid cysts was cut, and then each fibrous layer put into a tube containing RNA stabilizer (RNAlater®; Qiagen Inc.). Also for each cyst 2 cm of host normal tissue close to the cyst was also cut and put in RNA later. All samples were then stored at −70 °C until use.
RNA extraction
Total RNA was extracted using RNA extraction kit (Jena Bioscience Inc.) according to the manufacturer instructions. Genomic DNA was eliminated with RNase-Free DNase kit (Qiagen Inc.). Total isolated RNA was quantified using Nano Drop® ND-1000 spectrophotometer. Only samples with A260/A280 ratios between 1.80 and 2.00 were analyzed further. Samples were stored at −70 °C until real-time PCR was performed.
cDNA synthesis
cDNA synthesis was performed using cDNA synthesis kit (Thermo Scientific Inc.) according to the manufacturer instructions. For this purpose oligo dT primers were used. Samples were reverse transcribed using 100 μg/ml RNA under conditions of 65 °C for 5 min, 42 °C for 60 min and 70 °C for 10 min. The synthesized cDNA were stored at −70 °C.
Real-time PCR
PCR amplifications were performed using the Quanti Fast SYBR Green PCR Kit (Fermentas) in a total volume of 20 μl. Primers were designed by Gene Runner software. Real-time reverse transcription PCR (RT-PCR) was run on the ABI Step one Plus (Applied Biosystems, ABI, USA). The following cycling conditions were used: initial denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Melting curve analysis was performed to identify all amplified PCR products. Polymerase chain reactions were performed in duplicate. Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ) was used as reference gene in both bovine and ovine samples. Relative gene expression normalized to YWHAZ and calculation was performed using 2−∆∆Ct method. PCR primers that were used for real-time PCR are shown in Table 1. All samples were tested in duplicate.
Table 1.
The primers used for real-time PCR
| Gene name | Primer sequences (forward/reverse) (5′–3′) | Product size (bp) |
|---|---|---|
| IL-4 Bovine | TGGCAAGCAAGACCTGTTC TCCTTCATAATCGTCTTTAGCC |
91 |
| IL-4 Ovine | GTATGTACCAGCCACTTCGTC TCCATGCATGAATTCTTTCTC |
104 |
| YWHAZ Bovine | ACCTACTCCGGACACAGAACA TTGCTCAGTTACAGACTTCATGC |
124 |
| YWHAZ Ovine | TGAACTCCCCTGAGAAAGCC CCGATGTCCACAATGTCAAGT |
147 |
Data analysis
Data were analyzed by REST software (version 2.0.13, 2009).
Results
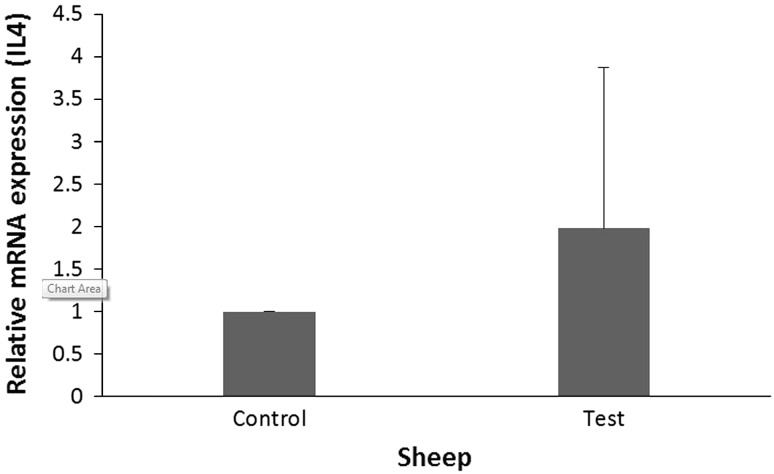
In sheep the rate of IL-4 gene expression in the fibrous layer of hepatic hydatid cysts was 1.98 times more than that of control group (normal liver), but the difference was not significant (P = 0.561) (Table 2). The results of IL4 gene expression in fibrous layer of sheep has been shown in Fig. 1.
Table 2.
The expression rate of IL 4 in ovine and bovine hydatid cyst fibrous layer
| Isolated cyst | No | IL 4 expression rate | Std. error | 95 % CI | P |
|---|---|---|---|---|---|
| Sheep | |||||
| Control | 6 | 1 | 0.047–56.698 | 0.003–1, 116.511 | 0.561 |
| Test | 6 | 1.980 | |||
| Cattle | |||||
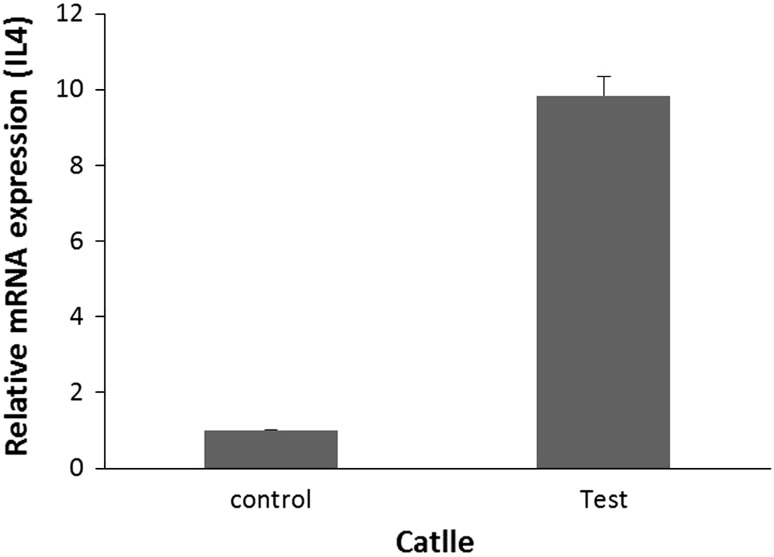
| Control | 6 | 1 | 3.451–31.740 | 2.028, 95.533 | <0.001 |
| Test | 6 | 9.842 | |||
Fig. 1.
IL4 gene expression in fibrous layer of ovine hydatid cyst (test group) in comparison with the rate of IL4 gene expression in normal liver tissue close to hydatid cyst location (control group)
Bovine IL-4 gene expression in test group samples (fibrous layer of hepatic hydatid cysts) was 9.84 times more than that of control group (normal liver) which was statistically significant (P < 0.001). The results of IL4 gene expression in fibrous layer of bovine hydatid cyst has been shown in Fig. 2.
Fig. 2.
IL4 gene expression in fibrous layer of bovine hydatid cyst (test group) in comparison with the rate of IL4 gene expression in normal liver tissue close to hydatid cyst location (control group)
Discussion
Results of this investigation revealed that IL4 gene expression in fibrous layer of hydatid cyst was significantly higher than the rate of expression of gene of this interleukin in normal liver close to the hydatid cyst. Also the rate of IL4 gene expression in fibrous layer of hydatid cyst in cattle was much higher than that of sheep. Regarding the role of IL4 in hydatid cyst infection, it has been shown that in peripheral blood mononuclear from hydatid cyst patients stimulated with this parasite antigens, IL4 increased significantly (Rigano et al. 1995a). Also a high level of IL4 has been reported in patients with chronic hydatid cyst (Rigano et al. 1995b; Zhang et al. 2003; Rigano et al. 1996) Fibrous layer develops by host as a protective response to hydatid cyst. Expression of high level of IL4 in fibrous layer shows that this cytokine may play a key function in regulation of immune response in favor or against the parasite.
In hydatid cyst infection early Th1-polarized cytokine production, which can kill the parasite at the initial stages of development (Vuitton 2003; Zhang et al. 2008), shifts to a predominant Th2 cytokine response in the later chronic stage. It has been shown that Patients with chronic hydatid cyst generate both Th1 and Th2 responses (Baz et al. 2006; Zhang et al. 2008). As Th1 and Th2 cytokines usually down-regulate each other (Zhang et al. 2008; Pearce and MacDonald 2002), this is likely due to echinococcal antigens containing distinct epitopes for each T cell subset (Fraize et al. 2005; Ortona et al. 2003; Zhang et al. 2008). In this study we used fertile cyst containing protoscoleces, so all of the cyst used in this investigation were in chronic stage with Th2 cytokine response.
It seems that the size and developmental stage of hydatid cyst is correlated with cell response in the fibrous layer. In this context it has been shown that in most cattle having larger mature hydatid cysts, CD8 cells were predominant in the pericystic adventitial layer, which also had fewer CD4 cells and a few B cells. However, in cattle having intensive regressive and complicated hydatid cysts, lymphocytes infiltrating the adventitial layer were mostly composed of CD4 cells. Also it has been shown that the smallest unilocular hydatid cysts were surrounded by an epithelioid cell layer composed of mononuclear cells bound to a laminated layer. These cells have usually been considered to be derived from macrophages (Sakamoto and Cabrera 2003). So variation in rate of IL4 expression in fibrous layer of hydatid cyst observed in our study may be related to the developmental stages of the cysts.
Severe infiltration and accumulation of numerous eosinophils and infiltration of lymphocytes and macrophages were seen in the fibrous layer surrounding hydatid cysts. So eosinophils infiltrating and their products such as IL4 in the fibrous layer may have an important role in formation of hydatid lesions in cattle (Sakamoto and Cabrera 2003).
IL4 is secreted by eosinophils, so the presence of these cells in fibrous layer may be an indication of liver tissue damage (Goh et al. 2013) or may be related to cellular immune response to the hydatid cyst. In this context different studies have shown a potential role for eosinophils in tissue repair (Goh et al. 2013; Heredia et al. 2013; Rosenberg et al. 2012). Also it has been shown that IL-4 secreted by eosinophils has been implicated in both wound repair and liver regeneration (Anthony et al. 2007; Chen et al. 2012; Gallagher et al. 2007; Goh et al. 2013; Murray and Wynn 2011; Palm et al. 2012). So high level of IL4 gene expression in fibrous layer of hydatid cyst observed in this work may also related to tissue repair.
Hydatid cysts have a different behavior in sheep and cattle. In this context Himonas et al. showed that 64 % of hydatid cyst in sheep were fertile while only 16 % of hydatid cyst in cattle were fertile (Himonas et al. 1987). So this difference may be considered as an explanation for difference in the rate of IL4 expression in cattle and sheep observed in this study.
In conclusion, this work is the first report about the level of IL4 gene expression in the fibrous layer surrounding hydatid cysts. With high rate of IL4 expression especially in fibrous layer of bovine hydatid cyst, it can be concluded that this interleukin may play an important role in host parasite relationship. For better understanding the function of IL4 gene expression in fibrous layer of hydatid cyst, it is recommended that the profile of other interleukin genes expression in this layer is determined.
Acknowledgments
The authors would like to acknowledge Vice-chancellor of Research and Technology, Isfahan University of Medical Sciences for financial support of this work.
Conflict of interest
The authors have no conflict of interests.
References
- Allen J, Maizels R. Immunology of human helminth infection. Int Arch Allergy Immunol. 1996;109:3–10. doi: 10.1159/000237225. [DOI] [PubMed] [Google Scholar]
- Al-Qaoud KM, Abdel-Hafez SK. The induction of T helper type 1 response by cytokine gene transfection protects mice against secondary hydatidosis. Parasitol Res. 2008;102:1151–1155. doi: 10.1007/s00436-008-0883-x. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K-i, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Baz A, Ettlin GM, Dematteis S. Complexity and function of cytokine responses in experimental infection by Echinococcus granulosus. Immunobiology. 2006;211:3–9. doi: 10.1016/j.imbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson S, Le Gros G, Conrad D, Finkelman F, Paul W. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990;145:1127–1136. [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser S, Kern P. T-lymphocyte cytokine mRNA expression in cystic echinococcosis. Acta Trop. 1997;64:35–51. doi: 10.1016/S0001-706X(96)00638-9. [DOI] [PubMed] [Google Scholar]
- Finkelman Fred D., Holmes Joanne, Katona Ildy M, Urban Joseph F., Beckmann M. Patricia, Park Linda S., Schooley Kenneth A., Coffman Robert L., Mosmann Timothy R., Paul William E. Lymphokine Control of In Vivo Immunoglobulin Isotype Selection. Annual Review of Immunology. 1990;8(1):303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Pearce EJ, Urban JF, Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Parasitol Today. 1991;7:62–66. doi: 10.1016/0169-4758(91)90035-M. [DOI] [PubMed] [Google Scholar]
- Fraize M, Sarciron M, Azzouz S, Issaadi N, Bosquet G, Petavy A. Immunogenicity of two Echinococcus granulosus antigens EgA31 and EgTrp in mice. Parasitol Res. 2005;96:113–120. doi: 10.1007/s00436-005-1322-x. [DOI] [PubMed] [Google Scholar]
- Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- Goh YS, Henderson NC, Heredia JE, Eagle AR, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himonas C, Frydas S, Antoniadol-Sotiriadou K (1987) The fertility of hydatid cysts in food animals in Greece. In: Helminth Zoonoses. Springer, pp 12–21
- Jeong H-J, Jeong HJ, Chung HS, Kim YH, Moon BS, Sung KK, Bai SJ, Cho KH, Kim YK, Hong SH, Shin T, et al. Differential regulation by Seogak Jihwang-Tang on cytokines production in peripheral blood mononuclear cells from the cerebral infarction patients presenting with altered consciousness. J Ethnopharmacol. 2004;94:289–294. doi: 10.1016/j.jep.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Lange AM, Yutanawiboonchai W, Scott P, Abraham D. IL-4-and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunology. 1994;153:205–211. [PubMed] [Google Scholar]
- Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology. 1998;3:233–244. doi: 10.1016/S1380-2933(97)10005-7. [DOI] [PubMed] [Google Scholar]
- McManus D, Bryant C. Biochemistry, physiology and molecular biology of Echinococcus Echinococcus and hydatid disease. Wallingford: CAB International; 1995. pp. 135–181. [Google Scholar]
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mueller TD, Zhang J-L, Sebald W, Duschl A. Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim Biophys Acta. 2002;1592:237–250. doi: 10.1016/S0167-4889(02)00318-X. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Ortona E, et al. Molecular and immunological characterization of the C-terminal region of a new Echinococcus granulosus Heat Shock Protein 70. Parasite Immunol. 2003;25:119–126. doi: 10.1046/j.1365-3024.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, Caspar P, Grzych J-M, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano R, Profumo E, Felice O, Ortona E, Teggi A, Siracusano A. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol. 1995;99:433–439. doi: 10.1111/j.1365-2249.1995.tb05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–285. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano R, Profumo E, Teggi A, Siracusano A. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clin Exp Immunol. 1996;105:456–459. doi: 10.1046/j.1365-2249.1996.d01-796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano R, Profumo E, Ioppolo S, Notargiacomo S, Teggi A, Siracusano A. Serum cytokine detection in the clinical follow up of patients with cystic echinococcosis. Clin Exp Immunol. 1999;115:503–507. doi: 10.1046/j.1365-2249.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2012;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Cabrera PA. Immunohistochemical observations on cellular response in unilocular hydatid lesions and lymph nodes of cattle. Acta Trop. 2003;85:271–279. doi: 10.1016/S0001-706X(02)00226-7. [DOI] [PubMed] [Google Scholar]
- Vuitton DA. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop. 2003;85:119–132. doi: 10.1016/S0001-706X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol Med Microbiol. 2006;47:24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ross AG, McManus DP. Mechanisms of immunity in hydatid disease: implications for vaccine development. J Immunol. 2008;181:6679–6685. doi: 10.4049/jimmunol.181.10.6679. [DOI] [PubMed] [Google Scholar]
- Zhang Wenbao, Wen Hao, Li Jun, Lin Renyong, McManus Donald P. Immunology and Immunodiagnosis of Cystic Echinococcosis: An Update. Clinical and Developmental Immunology. 2012;2012:1–10. doi: 10.1155/2012/101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Li J, Lin R, Wen H, McManus DP. recent advances in the immunology and serological diagnosis of echinococcosis. In: Al-Moslih M, editor. Serological diagnosis of certain human, animal and plant diseases. Rijeka: InTech; 2012. [Google Scholar]