Abstract
The study was conducted to ascertain the actual status of gastro-intestinal parasites in pigs maintained under different rearing systems in Shimoga region, Karnataka state. A total of 150 Pigs fecal samples were examined, which includes 50 from organized piggery farm, Veterinary College Shimoga, 50 from private piggery farm of Shimoga and 50 from free range desi pigs of Shimoga city. The fecal samples were processed and examined by direct and sedimentation method. Out of 50 fecal samples examined from organized piggery farm, 19 were found positive for different parasitic eggs, Out of 50 fecal samples screened form private farm, 28 harbored different parasites, whereas from 50 free range desi pigs fecal samples examined, all showed one and other parasitic eggs/ova. The percent prevalence of parasitic infection is more in free range desi pigs compared to Yorkshire breeds maintained under stall fed condition.
Keywords: Yorkshire pigs, Free range desi pigs, Gastrointestinal parasites
Introduction
The swine husbandry practices are less throughout the Karnataka state, but conventional desi pig rearing is very common in coastal Karnataka and southwestern region, due to the religious constraints and misconceptions of pork meat. In recent years, the swine industry is improving with introduction of cross breed pigs and utility of the pork in the urban areas.
In swine industry proper management and preventive measures against diseases can increase the reproductive performance, feed utilization and decreases mortality and morbidity of diseases. Among parasitic diseases, the gastrointestinal parasites are responsible for substantial loss of productivity in pigs in terms of inefficient feed conversion, poor growth rate, reduced weight gain, decreased litter size and the condemnation of affected organs after slaughter (Sowemimo et al. 2012). The poor environmental hygiene and improper management is reported as risk factors for gastrointestinal parasitic infection in pigs. The range and intensity of gastrointestinal parasitism depends on the type of swine production system (Nansen and Roepstorff 1999).
The prevalence of gastrointestinal parasites in pigs are widely reported from all over the world by many workers viz., Ascaris suum, Oesophagostomum spp., Strongyloides ransomi, Hyostrongylus rubidus Trichostrongylus axei, and Trichuris suis (Manuel et al. 1989; Dutta et al. 2005). In addition to helminth parasites, the pigs also harbor many intestinal protozoan parasites viz., Cryptosporidium spp., Giardia lamblia, Balantidium coli and Eimeria spp. in developing countries (Bauri et al. 2012).
Since there is no report on actual status of gastrointestinal parasites of pigs in Shimoga––a malnad region of Karnataka, the present study was undertaken to ascertain the occurrence of gastro-intestinal parasites in pigs maintained under three different managemental conditions includes, extensive management/organized farm, semi extensive/private farms and backyard/free range conditions Fig. 1.
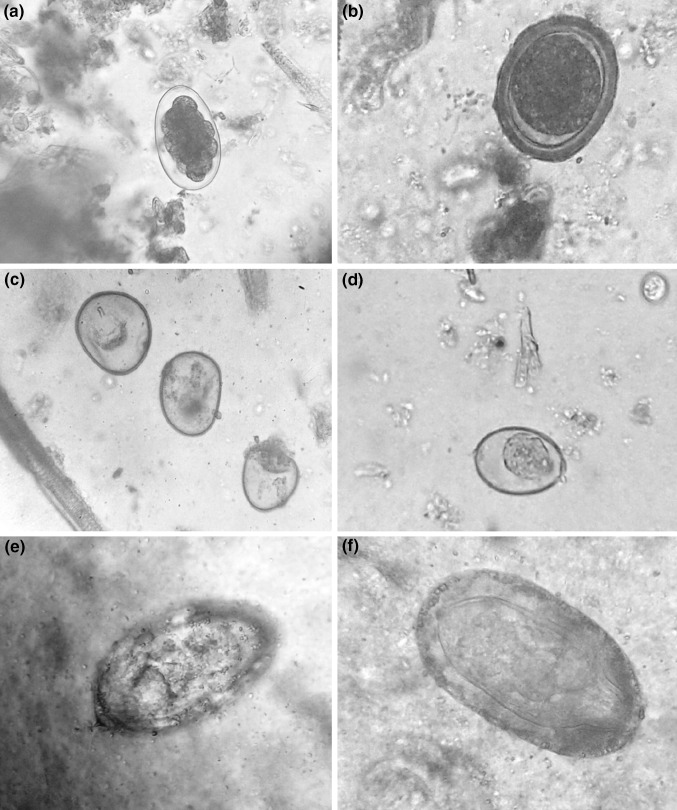
Fig. 1.
a Strongyle egg, b Ascaris suum egg, c Cyst of Balantidium coli, d Coccidian oocysts, e Schistosoma spp. egg, f Macrocanthorhynchus hirudinaceus egg
Materials and methods
In the present study, a total of 150 faecal samples of pigs reared under three different managemental conditions includes, 50 samples from organized piggery farm (Fig. 2a) Veterinary College, Shimoga, 50 samples from private piggery farms (Fig. 2b) of Shimoga and 50 samples from free range local desi pigs (Fig. 2c) in and around Shimoga respectively were used to find the prevalence of gastrointestinal parasites. Both organized and private farms were maintained Yorkshire and crossbred pigs whereas, the free range desi pigs were of indigenous local breeds.
Fig. 2.
a Organized piggery farm, b Private piggery farm, c Free range local desi pigs
The fresh faecal samples were randomly collected per rectally from individual pigs in a dry, clean polythene zipped bag and immediately brought to the laboratory after affixing a proper identification label. The faecal samples were examined grossly for the presence of mucus, blood or any parasitic segments and then processed by direct and sedimentation method as per standard procedure (Bowmann and Dwite 2009) for the detection of parasitic eggs/ova.
Results
In the present study, out of 150 faecal samples examined, 97 (64.6 %) samples found positive for various gastrointestinal parasitic eggs/ova. Among parasite species 26 (17.3 %) samples found positive for Strongyle eggs, 11 (7.3 %) showed A. suum eggs 17 (11.3 %) harbored B. coli cyst, 10 (6.6 %) found positive for Trichuris eggs and 08 (5.0 %) harbored coccidian oocysts, 6 (4.0 %) samples showed Schistosoma spp. eggs and the remaining 19 (12.0 %) had mixed infection with Strongyle eggs, B. coli cyst, and Trichuris spp. eggs respectively (Table 1). Among positive cases, 47.0 % of exotic pigs reared under organized and private farms found positive for parasitic eggs/ova, whereas all (100 %) indigenous local breeds on free range harbored gastrointestinal parasites (Table 1).
Table 1.
Prevalence of Gastrointestinal parasites of pigs in Shimoga, Karnataka
| Parasite species | Exotic (Yorkshire) pigs | Indigenous pigs | Total positive | Percent prevalence | |
|---|---|---|---|---|---|
| Organized piggery farm | Private piggery farm | Desi free range pigs | |||
| Ascaris suum | 00 | 03 | 08 | 11 | 7.3 |
| Strongyle | 04 | 11 | 11 | 26 | 17.3 |
| Balantidium | 07 | 04 | 06 | 17 | 11.3 |
| Trichuris | 02 | 03 | 05 | 10 | 6.6 |
| Coccidia spp. | 03 | 02 | 03 | 08 | 5.0 |
| Schistosoma spp. | 00 | 00 | 06 | 06 | 4.0 |
| Mixed infectiona | 03 | 05 | 11 | 19 | 12.0 |
| Total positive | 19 | 28 | 50 | 97 | 64.6 |
| Total examined | 50 | 50 | 50 | 150 | |
| Percent positive | 38.0 | 56.0 | 100 | 64.6 | |
| % Prevalence in breeds | 47.0 | 100 | |||
aMixed infection = Strongyle eggs, Balantidium coli cysts, Trichuris eggs and Macrocanthorhynchus hirudinaceus egg
Out of 50 faecal samples examined from organized piggery farm, 19 (38.0 %) samples were found positive for parasitic eggs/ova. Among 19 positive cases, 4 samples showed Strongyle eggs, 7 harbored B. coli cyst, 2 found positive for Trichuris eggs and 3 harbored coccidian oocysts and the remaining 3 had mixed infection with Strongyle eggs, B. coli cyst, and Trichuris spp. eggs respectively (Table 1).
However, in private farm, out of 50 faecal samples screened, 28 (56.0 %) samples showed positive for parasitic eggs/ova. Among positive samples, 11 found positive for Strongyle eggs, 3 showed A. suum eggs, 4 harbored B. coli cyst, 3 samples found positive for Trichuris eggs, 2 harbored Coccidian oocysts and 5 samples had mixed infection with Strongyle eggs, B. coli cyst, Macrocanthorynchus hirudinaceus eggs and Trichuris spp. eggs respectively (Table 1).
Whereas, out of 50 faecal samples of free range desi pigs examined, 100 % prevalence was observed with one and the other parasitic eggs/ova. Among positive cases, 8 samples showed A. suum eggs (Fig. 1b), 11 harbored Strongyle eggs, (Fig. 1a) 6 harbored B. coli cyst (Fig. 1c), 5 found positive for Trichuris spp. eggs, 3 harbored coccidian oocysts (Fig. 1d), 6 samples showed Schistosoma spp. eggs (Fig. 1e) and 11 samples had mixed infection with B. coli cyst Strongyle eggs and Macrocanthorhynchus hirudinaceus eggs (Fig. 1f, Table 1).
The statistical analysis was done by Chi square test and found significant difference between different managemental conditions of pig and the occurrence of gastrointestinal parasites.
Discussion
In the present study, the overall prevalence of 47.0 and 100 % parasitic infections was observed in exotic and desi pigs respectively. This is in agreement with the findings of Muraleedharan et al. (1994) who reported the 100 % prevalence of parasitic infections in desi pigs of Mysore and Mandya districts of Karnataka. Similarly Rajeshwari and Chauhan (2006) reported the prevalence of 42.4 % in adult exotic (Yorkshire) pigs along with a low prevalence of 42.1 % in adult desi pigs of Bangalore district, Karnataka. The reason for variation in the prevalence rate corresponds to desi pigs with the present study might be due to managemental practices adopted and the number of samples included in the study whereas, the low prevalence in desi pigs of Bangalore urban area in particular may be due to less exposure of pigs to parasitic infection compare to free range desi pigs of Shimoga region.
The present study recorded the prevalence of 38.0 % in organized farm, 56.0 % in private farms and 100 % in free range desi pigs. This finding is in agreement with Dutta et al. (2005) who reports almost similar prevalence of 38.7 % in intensive farm and 53.2 % in semi intensive farm, but low prevalence of 55.3 % in free range pigs of West Bengal. In contrast to the present study, Deka et al. (2005) reported the low prevalence of 34.6 % gastrointestinal parasites in pigs from private and unorganized abattoirs of Mizoram state. Whereas, Borthakur et al. (2007) reported low prevalence of 37.5 % parasitic infections in pigs of indigenous managemental systems of Aizawl district of Mizoram. Similarly Godara and Sharma (2010) also reported the low prevalence of 26.6 % parasitic infections in pigs of organized farms of Jaipur. The variations in the above findings could be due to differences in the managemental practices adopted for rearing pig and change in the geographical locations.
Among gastrointestinal parasites recorded during the present study, the nematode infection was found more predominant followed by the protozoan infections. Of the parasitic infections, the high prevalence of Strongyle spp. was observed followed by A. suum, B. coli and Coccidian parasites respectively. This finding is in agreement with Deka et al. ( 2005) who reported the higher prevalence of nematode infection in pigs of Mizoram state and Dutta et al. ( 2005) reported the higher prevalence of Strongyle spp. (26.2, 33.3 and 30.1 %), followed by A. suum (13.7, 23.8 and 30.8 %) in intensive, semi intensive and free range pigs respectively.
It is concluded that, the prevalence of parasitic infection is more in free range desi pigs compared to intensive farm of rearing. The present study also recorded the higher prevalence of gastrointestinal nematode parasites among helminths followed by intestinal protozoan parasites.
Acknowledgments
The authors are thankful to Dean, Veterinary College, Shimoga, Karnataka-577204 for providing the necessary facility and support to conduct the present research work.
References
- Bauri RK, Ranjan Rajeev, Deb AR, Ranjan R. Prevalence and sustainable control of Balantidium coli infection in pigs of Ranchi, Jahrkahnd, India. Vet World. 2012;5(2):94–95. doi: 10.5455/vetworld.2012.94-99. [DOI] [Google Scholar]
- Borthakur SK, Rahmani S, Sarma K. Prevalence of gastrointestinal helminths in pigs in Aizawl. J Vet Parasitol. 2007;21(2):173–174. [Google Scholar]
- Bowmann D Dwite. Georgi’s parasitology for veterinarians. 9. St, Louis Missouri: Saunders Elsevier; 2009. [Google Scholar]
- Deka DK, Borthakur SK, Patra G. Parasitosis in domestic animals and birds of Aizawl, Mizoram. J Vet Parasitol. 2005;19(1):51–53. [Google Scholar]
- Dutta S, Ghosh JD, Sasmal NK, Mukherjee GS. Prevalence of gastrointestinal helminths affecting pig farms of West Bengal, India. J Vet Parasitol. 2005;19(1):23–26. [Google Scholar]
- Godara R, Sharma RL. Parasitic infections in livestock at Jaipur. J Vet Parasitol. 2010;24(2):193–195. [Google Scholar]
- Manuel MF, Santos AV, Lucas S. Prevalence of gastrointestinal helminths affecting backyard piggery farms Philippine. J Vet Anim Sci. 1989;15:47–53. [Google Scholar]
- Muraleedharan K, Syed Ziauddin K, Margoob Hussain P, Puttabyatappa B, Seshadri SJ. Prevalence of parasitic infections among small domestic animals. Karnataka J Agric. Sci. 1994;7(1):64–68. [Google Scholar]
- Nansen P, Roepstorff A. Parasitic helminths of the pig: factors influencing transmission and infection levels. Int J Parasitol. 1999;29:877–891. doi: 10.1016/S0020-7519(99)00048-X. [DOI] [PubMed] [Google Scholar]
- Rajeshwari YB, Chauhan AK. Influence of age on occurrence of gastrointestinal parasites in pigs. Mysore Agric Sci. 2006;40(1):123–124. [Google Scholar]
- Sowemimo OA, Asaolu SO, Adegoke FO, Ayanniyi OO. Epidemiological survey of gastrointestinal parasites of pigs in Ibadan, Southwest Nigeria. J Public Health Epidemiol. 2012;4(10):294–298. doi: 10.5897/JPHE12.042. [DOI] [Google Scholar]