Abstract
The present study was conducted for 1 year from March 2010 to February 2011 to identify gastro-intestinal parasites of backyard chickens and to estimate its prevalence in and around Shimoga, a malnad region of Karnataka. A total of 250 gastro-intestinal tracts were collected from backyard chickens for the detection of gastrointestinal parasites. Among the 250 birds screened, 183 (73.2 %) were found positive for gastrointestinal parasites by gross examination of gastrointestinal tract. Out of 183 positive cases, 94 (51.36 %) were found positive for cestodes, includes 73 (77.6 %) Raillietina tetragona, 12 (12.8 %) Raillietina echinobothrida and 9 (9.6 %) Raillietina cesticillus. Whereas, 53 (28.96 %) were found harbouring nematode parasites includes 33 (62.3 %) had Ascaridia galli, 12 (22.6 %) had Heterakis gallinarum and 8 (15.1 %) had both A. galli and H. gallinarum infection. The remaining 36 (19.67 %) had mixed infections of both cestode and nematode parasites. The microscopic examination of the gut contents and faecal samples showed presence of coccidian oocysts and eggs of A. galli, H. gallinarum and Capillaria spp. respectively.
Keywords: Prevalence, Gastrointestinal parasites, Backyard chickens, Shimoga
Introduction
Poultry farming is one of the most important form of animal husbandry activities and a popular form of enterprise in recent years. Even though the impact of parasitic diseases has decreased in farm birds reared on cage systems due to modernization, scientific management and adoption of effective bio-security measures, the birds maintained on deep litter system and backyard free range birds are still remain susceptible to parasitic infection because of litter contamination, scavenging habits and environmental contaminants.
The helminthic infection is considered to be one of the most significant constraints in poultry production especially in humid tropical climatic conditions of India which favour faster propagation and development of larval stages of helminth parasites (Matta and Ahluwalia 1981; Malhotra 1983; Kulkarni et al. 2001). The helminthic infections in backyard chickens adversely affect the successful poultry farming by causing unthriftiness, emaciation, weight loss and lowered egg production. The nematodes viz., Ascaridia galli and Heterakis gallinarum are more frequently encountered parasites and causes heavy economic loss to the poultry industry due to decreased feed conversion ratio, weight loss, lowered egg production and mortality in young birds. Both exotic and desi birds are equally susceptible even after adopting strict managemental practices.
The scientific poultry managemental practices are responsible for the reduction in the incidence of parasitic infections. However, due to abundance of intermediate hosts like beetles, ants and houseflies which are commonly seen on poultry litter, droppings, feeds etc. are responsible for transmission of various helminth parasites among the flock. Hence, an attempt was made in the present study to record the actual status of gastrointestinal parasites in backyard chickens in and around Shimoga, a malnad region of Karnataka state.
Materials and methods
Study period
The study was conducted for 1 year between March 2010 to February 2011 to identify gastro-intestinal parasites affecting backyard chickens and to estimate its prevalence in and around Shimoga, a malnad region of Karnataka.
Sample collection and identification of parasites
In the present study, a total of 250 intestines of backyard chickens were collected from the chicken outlets of local poultry slaughter houses located in and around Shimoga and brought to the laboratory for processing. The intestines were dissected longitudinally and screened for the presence of parasites. The recovered parasites were washed thoroughly for three times to remove the debris. Then the parasites were processed and mounted as per the standard protocol (Bowman 2009). The species identification was done based on the morphological characters and micrometry as per (Soulsby 1982).
Intestinal contents were also examined by sedimentation and flotation methods as per the procedure of Bowman (2009) for the presence of parasitic egg/ova. The faecal samples found positive for coccidian oocysts were kept for sporulation at room temperature using 2.5 % potassium dichromate solution. The sporulation was observed by every 12 h intervals. Then, the sporulated oocysts were used for speciation based on morphology, micrometry and time taken for sporulation as per Soulsby (1982).
Results and discussion
In the present study, out of 250 backyard chickens screened, 183 (73.2 %) were harbored gastrointestinal parasites. This is in accordance with the Katoch et al. 2012; Saad et al. (1989) and Puttalakshmamma et al. (2008), who reported 72.0, 77.3 and 71 % helminthic infections in local chickens, respectively. The higher prevalence of helminthic infections in local chickens was reported from other countries includes Morocco, 89.9 % (Hassouni and Belghyti 2006); Nigeria, 87.7 % (Yorio et al. 2008) and Iran, 96 % (Eslami et al. 2009). The difference might be due to variation in the type of managemental practices adopted, geographical locations and the number of samples included in the study. The lower prevalence of 10.5 % helminth infection was also reported by Baboolal et al. (2012) in broiler chickens of Trinidad. The probable reason for such a low prevalence could be due to regular use of anthelmintics, confinement of commercial broilers and short lifespan.
Among 183 positive, 94 (51.36 %) were found positive for cestodes, 53 (28.96 %) were harboured nematodes and the remaining 36 (19.67 %) had mixed infection of both cestodes and nematodes indicating the highest prevalence of cestode followed by nematode parasites (Table 1). Similarly Nadakal et al. (1973) and Puttalakshmamma et al. (2008) reported the highest prevalence of cestodes followed by nematodes and trematodes in desi birds. In contrast to the present study, Katoch et al. (2012) and Baboolal et al. (2012) observed the higher prevalence of nematodes followed by cestode parasites in backyard chickens of Jammu and broiler chickens of Trinidad region respectively. This could be due to the scavenging habits of backyard chickens, usually seek their food in the superficial layers of the soil, drains etc. which may contain intermediate hosts for cestode parasites.
Table 1.
Prevalence of gastro-intestinal parasites in backyard chicken of Shimoga region
| Species | No. positive | % Positive | Total positive | |
|---|---|---|---|---|
| Cestodes | Raillietina tetragona | 73.0 | 77.60 | 94 (51.36 %) |
| Raillietina echinobothrida | 12.0 | 12.80 | ||
| Raillietina cesticellus | 09.0 | 09.60 | ||
| Nematodes | Ascaridia galli | 33.0 | 62.30 | 53 (28.96 %) |
| Heterakis gallinarum | 12.0 | 22.60 | ||
| Ascaridia galli & Heterakis gallinarum | 08.0 | 15.10 | ||
| Mixed infection | Cestodes and Nematodes | 36.0 | 19.67 | 36 (19.67) |
| Total screened | 250 | 183.0 (73.20 %) | ||
During the present study, none of the backyard chickens harboured trematode parasites. This might be due to non availability of necessary intermediate host for trematode parasites in and around Shimoga region. This is in accordance with the observations of Puttalakshmamma et al. (2008) in desi birds of Bangalore, Baboolal et al. (2012) in broiler chickens of Trinidad and Katoch et al. (2012) in backyard chickens of Jammu.
Out of 94 (51.36 %) backyard chickens positive for cestodes, 73 (77.6 %) were found positive for Raillietina tetragona (Fig. 1), 12 (12.8 %) had Raillietina echinobothrida (Fig. 2) along with the nodular lesions on the intestinal mucosa (Fig. 5) and 9 (9.6 %) showed Raillietina cesticillus (Fig. 3). Similar observation was made by Puttalakshmamma et al. (2008) in desi birds of Bangalore region but Katoch et al. (2012) reported higher prevalence of R. cesticellus followed by R. echinobothrida and R. tetragona (Fig. 4) in backyard chickens of Jammu. The variation in the species wise prevalence might be due to change in the geographical location (Fig. 5).
Fig. 1.

Scolex of R. tetragona (100×)
Fig. 2.

Scolex of R. echinobothrida (100×)
Fig. 5.
Adult worms of R. echinobothrida causes nodular lesions on the intestinal mucosa (Nodular taeniasis) of backyard chicken (10×)
Fig. 3.

Scolex of R. cesticellus (100×)
Fig. 4.

Adult worms of Raillietina spp. recovered from the intestine of backyard chicken (10×)
Among 53 (28.96 %) backyard chickens positive for nematodes, 33 (62.3 %) had A. galli (Fig. 6), 12 (22.6 %) had H. gallinarum (Fig. 7) and 8 (15.1 %) showed both A. galli and H. gallinarum infection. Among nematode parasites, the highest prevalence of A. galli was observed followed by H. gallinarum. This is in comparison with observations of Katoch et al. (2012); Puttalakshmamma et al. (2008); Baboolal et al. (2012).
Fig. 6.

Adult worms of A. galli recovered from the intestine of backyard chicken (10×)
Fig. 7.
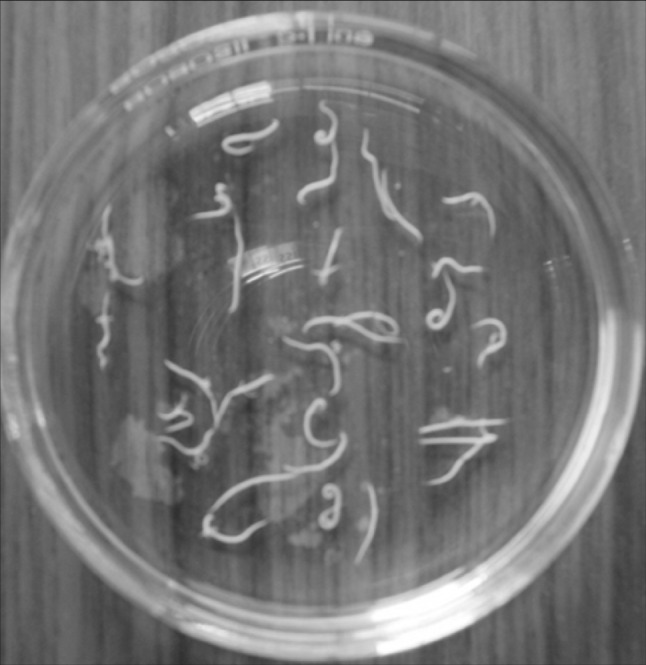
Adult worms of H. gallinarum recovered from the intestine of backyard chicken (10×) from the intestine of backyard chicken (10×)
Out of 183 (73.2 %) positive cases, 18 (9.8 %) backyard chickens showed mixed helminthic infections of A. galli, R. tetragona and R. echinobothrida. Among mixed infection, A. galli and R. tetragona were recorded in 13 (72.2 %) cases, A. galli and R. echinobothrida infection was recorded in 3 (16.7 %) cases and all the three helminths viz. A. galli, R. tetragona and R. echinobothrida was observed in 2 (11.1 %) backyard chickens. However Raote et al. (1991) reported 50.97 % mixed infection in desi birds in Akola whereas; Katoch et al. (2012) reported 32 % mixed infection in backyard chickens of Jammu. The lowest incidence of mixed infection in the present study might be due to regional variation and the number of samples included in the study.
The intestinal contents and the faecal samples of 183 (73.2 %) positive cases were examined by sedimentation and flotation methods for detection of parasitic eggs/ova, 39 (21.3 %) samples found positive for A. galli and H. gallinarum eggs, 9 (4.9 %) samples showed Capillaria spp. eggs whereas, 12 (6.5 %) had coccidian oocysts. During the present study, two types of eimerian oocysts were recorded and identified as Eimeria tenella and Eimeria necatrix based on the morphology, micrometry and time taken for sporulation as per Soulsby (1982). Morphologically, the E. tenella oocysts were elongated whereas, E. necatrix were round. The micrometry of E. tenella and E. necatrix oocysts measured 18.6 × 22.5 um and 13.7 × 15.3 um respectively. The time taken for sporulation by E. tenella and E. necatrix was recorded as 16 and 24 h respectively. The present findings are in agreement with Puttalakshmamma et al. (2008) who reported two predominant coccidian species such as E. tenella and E. necatrix oocysts in desi birds of Bangalore region.
Acknowledgments
The authors are thankful to the Dean, Veterinary College, Shimoga for providing facility to carry out the research work.
References
- Baboolal V, Vijaya S, Gyan L, Brown G, Offiah NV, Adesiyun AA, Basu AK. The prevalence of intestinal helminths in broiler chickens in Trinidad. Veterinarski Arhiv. 2012;82(6):591–597. [Google Scholar]
- Bowman DD. Georgis’ Parasitology for Veterinarians. 9. St. Louis: Saunders Elsevier; 2009. [Google Scholar]
- Eslami A, Ghaemi P, Rahbari S. Parasitic infections of free-range chickens from Golestan Provinces, Iran. Iran J Parasitol. 2009;4:10–14. [Google Scholar]
- Hassouni T, Belghyti D. Distribution of gastrointestinal helminths in chicken farms in the Gharb region-Morocco. Parasitol Res. 2006;99:181–183. doi: 10.1007/s00436-006-0145-8. [DOI] [PubMed] [Google Scholar]
- Katoch R, Yadav A, Godara R, Khajuria JK, Borkataki S, Sodhi SS. Prevalence and impact of gastrointestinal helminths on body weight gain in backyard chickens in subtropical and humid zone of Jammu, India. J Parasit Dis. 2012;36(1):49–52. doi: 10.1007/s12639-011-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni GM, Narladkar BW, Deshpande PD. Helminthic infections in desi fowl (Gallus gallus domesticus) in Marathwada region. J Vet Parasitol. 2001;15:137–139. [Google Scholar]
- Malhotra KS. Population distribution of Heterakis pusilla in Gallusgallus from India. J Helminthol. 1983;57:117–126. doi: 10.1017/S0022149X00009378. [DOI] [PubMed] [Google Scholar]
- Matta SC, Ahluwalia SS. Note on the survey of gastrointestinal helminths of domestic fowls in UP. Indian J Anim Sci. 1981;51:1013–1015. [Google Scholar]
- Nadakal AM, Mohandas A, John KO, Muraleedharan K. Contribution to the biology of the fowl cestode Raillietina echinobothrida with a note on its pathogenicity. Trans Am Microsc Soc. 1973;92(2):273–276. doi: 10.2307/3224924. [DOI] [PubMed] [Google Scholar]
- Puttalakshmamma GC, Ananda KJ, Prathiush PR, Mamatha GS, Suguna Rao Prevalence of gastrointestinal parasites of poultry in an around Bangalore. Vet World. 2008;1:201–202. doi: 10.5455/vetworld.2008.201-202. [DOI] [Google Scholar]
- Raote YV, Joshi MV, Bhandarkar AG, Bhawat SS. Prevalence of helminth parasites in desi birds of Akola region of Maharashtra. Indian J Poult Sci. 1991;26(2):128–129. [Google Scholar]
- Saad MB, El Sadig AA, Shammat AM. Helminth parasites of the local breed of poultry in Kordofan region, Sudanese. J Vet Sci Anim Husb. 1989;28:54–55. [Google Scholar]
- Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated animals. 7. London: ELBS and Baillere Tindal; 1982. [Google Scholar]
- Yorio KP, Adang KL, Fabiyi JP, Adamu SU. Helminthes parasites of local chickens in Bauchi State, Nigeria. Sci World J. 2008;3:35–37. [Google Scholar]



