Abstract
To identify the larvicidal activities of silver nanoparticles synthesised with Avicennia marina leaf extract against the larvae of Aedes aegypti and Anopheleus stephensi, in vitro larvicidal activities such as LC50 and LC90 were assessed. Further, characterisation such as UV and FTIR analysis were carried out for the synthesised silver nanoparticles. The LC50 value of the synthesised silver nanoparticles was identified as 4.374 and 7.406 mg/L for An. stephensi and Ae. aegypti larvae respectively. Further, the LC90 values are also identified as 4.928 and 9.865 mg/L for An. stephensi and Ae. aegypti species respectively. The synthesised silver nanoparticles have maximum absorption at 420 nm with the average size of 60–95 nm. The FTIR data showed prominent peaks in (3940.57, 3929.00, 3803.63, 3712.97, 2918.30, 2231.64, 1610.50, 1377.17, 1257.59, 1041.59, 1041.56, 775.38, 667.37 and 503.21) different ranges. The biosynthesis of silver nanoparticles with leaf aqueous extract of A. marina provides potential source for the larvicidal activity against mosquito borne diseases. The present study proved the mosquitocidal properties of silver nanoparticles synthesised from mangroves of Vellar estuary. This is an ideal eco-friendly approach for the vector control programs.
Keywords: Biosynthesis, AgNO3, Mangrove, Larvicidal, FTIR
Introduction
Mosquitoes are the most important single group of insects in terms of public health importance, which transmit a number of diseases, such as malaria, filariasis, dengue, Japanese encephalitis, etc. causing millions of deaths every year (Service MV 1983). Repeated use of synthetic insecticides for mosquito control has disrupted natural biological control systems and led to resurgences in mosquito populations (Dhanasekaran et al. 2010). Finding a new safe and efficient drug for treatment of viral respiratory disease, in particular retrovirus infection has been the field of research for many scientist and significant attention has been paid to natural compounds (Zandi et al. 2009). Avicennia marina (Forssk.) Vierh, has been traditionally used for treatment of rheumatism, small pox, ulcers and other ailments (Bandaranayake 2002). Its antiviral activity on poliovirus and herpes simplex virus type-1 has not been reported yet from anywhere. Balakrishnan et al. (2013) analyzed the samples collected from the mangrove forests of Parangipettai, Vellar estuary yielded a mosquitocidal Bacillus, whose extracellular metabolite(s) exhibited mosquito larvicidal and pupicidal activity. Some studies were done about the anti parasitic, antifungal and antibacterial activity of A. marina such as in vitro antimalarial and anticandidal activities as well as cytotoxicity of A. marina (Premanathan et al. 1999; Khafagi et al. 2003; Abeysinghe et al. 2006). The leaves of A. marina have no effect on behavioral changes, morbidity or mortality in rats (Ali and Bashir 1998). Synthesis of nanoparticles by using chemical and physical methods requires high pressure, energy, temperature and toxic chemicals. Plant extracts are suitably scaled up for large scale biosynthesis of silver nanoparticles in a controlled manner according to their size, shape and sensitivity. But the present study is the first attempt for the biosynthesis of nanoparticles by using mangrove plant extract. Among the mangrove plants Rhizophora mucronata is previously proved to have antibacterial, antiplasmodial and antiviral activities (Premanathan et al. 1999; Ravikumar et al. 2009; Ravikumar et al. 2011). Nanosized silver can also be used to treat immunologic and inflammatory diseases (Shin et al. 2007). Currently, silver sulfadiazine is listed as an essential anti-infective topical medicine by the World Health Organization (WHO 2010). In the present study, an attempt has been made to identify the larvicidal activity of silver nanoparticles biosynthesised with A. marina leaf extract.
Materials and methods
Collection of plant material
Fresh matured leaves of A. marina were collected from artificially developed mangrove forest (Lat. 9°38′N and Long. 78°57′E) located on the shores of Vellar estuary, Parangipettai, Southeast coast of India (Tamil Nadu, India). The authentication of the plant species was developed by Prof. K. Kathiresan, Dean & Director, Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai, Tamil Nadu, India.
Biosynthesis of silver nanoparticles
The collected leaf sample was washed with tap water and distilled water to remove the adhering salts and other associated animals. About 10 g of finely cut leaves was placed with 100 mL of sterilized double distilled water and then boiled the mixture for 5 min. The boiled extract was filtered with Whatman No. 1 filter paper. A total of 10 mL of collected filtrate was treated with 90 mL of silver nitrate aqueous solution (21.2 g of AgNO3 powder in 125 mL of Milli Q water) and incubated at room temperature for 10 min, resulting in the formation of brownish yellow solution indicating the synthesis of silver nanoparticles (Parashar et al. 2009).
Characterisation of biosynthesised nanoparticles
About 1 mL of solution (Diluted with 1:20v/v Milli Q water) was monitored in UV–vis spectrophotometer (between 300–700 nm with 5 nm intervals) with different time intervals (15, 30 min, 4, 6 and 8 h). After 8 h of incubation, the solution was centrifuged at 12,000 rpm for 20 min and their pellets were redispersed in sterile distilled water. The centrifugation and redispersion was repeated three times to ensure the complete separation of nanoparticles. The purified pellet was dried and subjected to the FTIR spectroscopy measurement in the diffuse reflectance mode at a resolution of 4 cm–1 in KBr pellets.
Larvicidal bioassay (preliminary screening)
The eggs and egg rafts of Aedes aegypti and Anopheleus stephensi were procured from Vector Control Research Centre (VCRC), Puducherry, India. Filter paper with attached eggs was dipped into a plastic tray containing 500 mL of dechlorinated water for 30–40 min, to hatch out larvae. The reared larvae were maintained for 5 days in standard environment (27 ± 1).
Larval/pupal toxicity test
Laboratory colonies of mosquito larvae/pupae were used for the larvicidal/pupicidal activity. Twenty five numbers of first to fourth instars larvae and pupae were introduced into 500 mL glass beaker containing 249 mL of dechlorinated water and 1 mL of desired concentrations (plant extract and silver nanoparticles). Larval food was given for the test larvae. At each concentration, two to five trials were made and each trial consisted of five replicates. The control was made by mixing 1 mL of acetone with 249 mL of dechlorinated water. The larvae and pupae were exposed to dechlorinated water without acetone served as control. The control mortalities were calculated by using Abbott’s formula (Abbott 1925).
The LC50 and LC90 were calculated from toxicity data by using Probit analysis (Finney 1971).
Statistical analysis
The average larval mortality data were subjected to probit analysis for calculating LC50 and LC90, and other statistics at 95 % confidence limit (LCL-UCL) and Chi square values were calculated using the SPSS 16.0 version (Statistical software package - SPSS Inc., Chicago, IL, USA) to find the regression equation values. Results with p ≤ 0.05 were considered to be statistically significant.
Results
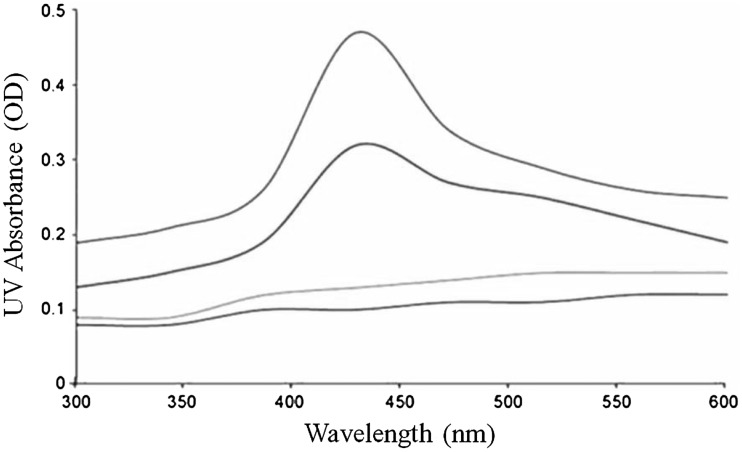
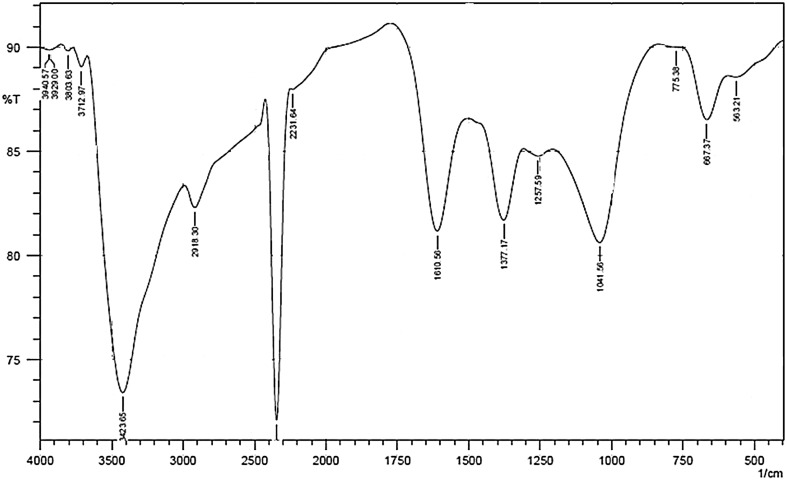
The present study was tested with mangrove leaf extracts from A. marina against the vector borne disease causing mosquito like An. stephensi and Ae. aegypti. It revealed that, mangrove leaf extracts of A. marina showed various ranges of larvicidal activities and the maximum percentage of larvicidal activity. The color change was noted by visual observation in the mangrove leaf extracts when incubated with AgNO3 solution (Fig. 1a, b). Absorption spectrum of leaf extracts at different wavelengths ranging from 200 to 800 nm revealed a peak at 420 nm. Both callus and leaf extracts without AgNO3 did not show any change in colour. The colour intensity of the synthesised silver nanoparticles was increased with increased time duration and the maximum intensity was observed with 420 nm wavelength (Fig. 2). The results of the present study suggest that, the maximum (100 %) percentage of mosquito larvicidal activity was identified with 5 and 10 mg/L An. stephensi and Ae. aegypti fourth instar larvae (Table 1). The results of the dose dependent assay suggested that, the value LC50 was identified as 4.374 and 7.406 mg/L An. stephensi and Ae. aegypti respectively. Further, the LC90 values were identified as 4.928 and 9.865 mg/L for An. stephensi and Ae. aegypti respectively. The results of 95 % fiducial limits (FL), lower confidence limit (LCL), upper confidence limit (UCL) and Chi square (Χ2) values are mentioned in Table 2. The results of the FTIR spectra of the synthesised silver nanoparticles exhibited prominent peaks with (3940.57, 3929.00, 3803.63, 3712.97, 3423.65, 2918.30, 2231.64, 1610.58, 1377.17, 1257.59, 1041.50, 775.38 and 667.37) different values (Fig. 3).
Fig. 1.
Conical flask with silver nitrate (Ag-100 ppm) before (1) and after (2) exposure to A. marina showing AgNO3 a silver nanoparticles after 6 h and b 72 h
Fig. 2.
UV-vis absorption spectra of silver nanoparticles synthesized by A. marina leaf extract with different time intervals
Table 1.
Larvicidal activity of the A. marina synthesised silver nanoparticles against Ae. aegypti and An. stephensi larvae
| Concentrations (mg/L) | % of mortality in Ae. aegypti | % of mortality in An. stephensi |
|---|---|---|
| 20.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 10.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 5.00 | 100.00 ± 0.00 | 98.00 ± 15.88 |
| 2.50 | 90.15 ± 8.79 | 87.58 ± 5.85 |
| 1.25 | 88.68 ± 9.72 | 68.13 ± 3.58 |
Table 2.
Dose dependant larvicidal activity of silver nanoparticles synthesized by A. marina leaf extract against Ae. aegypti and An. stephensi larvae
| Species | Concentrations (mg/L) | % of mortality | LC50 (mg/L) | 95 % FL LCL–UCL |
LC90 (mg/L) | 95 % FL LCL–UCL |
Χ2 |
|---|---|---|---|---|---|---|---|
| Ae. aegypti | 20.00 | 100.00 ± 0.00 | – | – | – | – | – |
| 10.00 | 85.22 ± 2.05 | – | – | – | – | – | |
| 5.00 | 70.49 ± 2.84 | – | – | – | – | – | |
| 2.50 | 65.58 ± 6.27 | 4.374 | 6.674–12.280 | 4.928 | 6.080–10.679 | 0.619* | |
| 1.25 | 50.47 ± 5.23 | – | – | – | – | – | |
| An. stephensi | 20.00 | 100.00 ± 0.00 | – | – | – | – | – |
| 10.00 | 87.83 ± 2.51 | – | – | – | – | – | |
| 5.00 | 69.43 ± 2.13 | – | – | – | – | – | |
| 2.50 | 63.24 ± 5.38 | 7.406 | 7.029–13.626 | 9.865 | 7.982–26.926 | 1.148* | |
| 1.25 | 47.74 ± 5.08 | – | – | – | – | – |
FL fiducial limits, LCL lower confidence limit, UCL upper confidence limit, Χ2 Chi square value
* p ≤ 0.05 level values are represents as mean ± SD values
Fig. 3.
FT-IR spectrum of silver nanoparticles synthesised by A. marina leaf extract
Discussion
Many marine plants synthesized a variety of chemically diverse secondary metabolites in response to selection pressures from herbivores and microorganisms and some of these compounds are recognized as insecticides. The bioactivity of phytochemicals against mosquito larvae can vary significantly depending on plant species, plant parts, age of plant parts, solvent used in extraction and mosquito species (Syed Ali et al. 2012). Most studies on phytochemicals focus on herbs and other medicinal plants. This may due to historical experiment knowledge and some scientific studies have shown that they are particularly active against certain organisms. Several studies have focused specifically on medicinal plants in different geographical regions. Commonly a connection is extrapolated between plant activity against disease agents based on traditional experience and insecticidal activity against mosquitoes. Mosquito control management including periodic larviciding (WHO 1975; Becker et al. 2003) is very helpful in favorable conditions. Larval breeding habitat is a successful and safer way to interrupt larval stages of vectors rather than the infective adult stage (Thangam and Kathiresan 1991). Many plant derived natural compounds was tested for mosquito control (Oladimeji et al. 2007, 2008; Vinayagam et al. 2008; Chakkaravarthy et al. 2011; Almehmadi 2011). Ishibashi et al. (1993) reported the extracts of Aglaia elliptifolia showed insecticidal activity. Early studies envisaged that the Indian marine plant extracts possessed potential larvicidal activity (Thangam et al. 1993; Rao et al. 1995).
The production of the silver nanoparticles synthesized from callus and leaf extracts of A. marina was evaluated through spectrophotometer in a range of wavelength from 300 to 600 nm. This revealed a peak at 420 nm in leaf extracts of A. marina indicating the production of silver nanoparticles. This is similar to the surface plasmon vibrations with characteristic peaks of silver nanoparticles prepared by chemical reduction (Petit et al. 1993; Kong and Jang 2006). Due to the excitation of plasma resonances or inter band transitions, some metallic nanoparticle dispersions exhibit the unique bands/peaks (Kuber and D’Souza 2006). A similar result was obtained by other researchers (Ahmad et al. 2003; Kathiresan et al. 2009). The results of the FTIR used to identify the possible bio molecules responsible for the stabilization of the synthesised silver nanoparticles. The prominent peaks of the FTIR results are showing the correspond values to the amide group (N-Hstretching-3 426.89), aliphatic group (Cyclic CH2-2 925.49), methyl group (CH3-2 869.56), alkane group (CH-2 346.95) alkene (CC-1 631.49 and 669.178) and ether groups (COC-1 031.73). The observed peaks are considered as major functional groups in different chemical classes such as flavonoids, triterpenoids and polyphenols (Asmathunisha et al. 2010). Hence, the terpenoids are proved to have good potential activity to convert the aldehyde groups to carboxylic acids in the metal ions. Further, amide groups are also responsible for the presence of the enzymes and these enzymes are responsible for the reduction synthesis and stabilization of the metal ions, further, polyphenols are also proved to have potential reducing agent in the synthesis of the silver nanoparticles (Asmathunisha et al. 2010; Prasad and Elumalai 2011; Mukunthan et al. 2011). Previously, Baun et al. (2008) reported that, the toxicity of C60-carbon nanotubes and titanium dioxide to an aquatic invertebrate Daphnia magna. The coastal plants are generally rich in polyphenolic compounds (Kathiresan and Veera Ravi 1990). Thus several factors may determine the nanoparticle synthesis by saltmarsh plant and however, the exact mechanism is yet to be elucidated. It is concluded from the present findings that, the biosynthesised silver nanoparticles of leaf aqueous extract of A. marina provided potential killing effect on mosquito larvae’s which could be used for prevention of several dreadful diseases.
Conclusion
The biosynthesized silver nanoparticles using A. marina leaves extract proved excellent mosquitocidal activity. Hence, the biological approach appears to be cost efficient alternative to conventional physical and chemical methods of silver nanoparticles synthesis and would be suitable for developing a biological process for large-scale production.
Acknowledgments
The author thanks the authorities of Annamalai University for providing the necessary facilities and the INCOIS-SATCORE Project (G4/515/2008), Ministry of Earth Sciences (Government of India) for financial support. We also thank the anonymous referees for the valuable comments, which greatly improved our manuscript.
Conflict of interest
All authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the Journal. We declare that we have no conflict of interest.
References
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Abeysinghe PD, Wanigatunge RP, Pathirana RN. Evaluation of antibacterial activity of different mangrove plant extracts. Ruhuna J Sci. 2006;1:108–116. [Google Scholar]
- Ahmad A, Mukherjee P, Senapati S. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B. 2003;28:313–318. doi: 10.1016/S0927-7765(02)00174-1. [DOI] [Google Scholar]
- Ali BH, Bashir AK. Toxicological studies on the leaves of Avicennia marina (mangrove) in rats. J Appl Toxicol. 1998;18:111–116. doi: 10.1002/(SICI)1099-1263(199803/04)18:2<111::AID-JAT481>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Almehmadi RM. Larvicidal, histopathological and ultra-structure studies of Matricharia chamomella extracts against the rift valley fever mosquito Culex quinquefaciatus (Culicidae: Diptera) J Entomol. 2011;8:63–72. doi: 10.3923/je.2011.63.72. [DOI] [Google Scholar]
- Asmathunisha N, Kathiresan K, Anburaj K. Synthesis of antimicrobial silver nanoparticles by callus leaf extracts from saltmarsh plant Sesuvium portulacastrum L. Colloids Surf B Biointer. 2010;79:488–493. doi: 10.1016/j.colsurfb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Balakrishnan S, Indira K, Srinivasan M. Mosquitocidal properties of Bacillus species isolated from mangroves of the Vellar estuary, Southeast coast of India. J Para Dis. 2013 doi: 10.1007/s12639-013-0371-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl Ecol Manag. 2002;10:421–452. doi: 10.1023/A:1021397624349. [DOI] [Google Scholar]
- Baun A, Hartmann NB, Grieger K. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17:387–396. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- Becker N, Petric D, Zgomba M. Mosquitoes and their control. New York: Kluwer Academic/Plenum Publishers; 2003. pp. 5–23. [Google Scholar]
- Chakkaravarthy VM, Ambrose T, Vincent S. Bioefficacy of Azadirachta indica (A. juss) and Datura metel (Linn.) leaves extracts in controlling Culex quinquefaciatus (Diptera: Culicidae) Trends Appl Sci Res. 2011;8:191–197. [Google Scholar]
- Dhanasekaran D, Sakthi V, Thajuddin N. Preliminary evaluation of Anopheles mosquito larvicidal efficacy of mangrove actinobacteria. Int J Appl Biol Pharm Tech. 2010;1(2):374–381. [Google Scholar]
- Finney DJ. Probit analysis. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- Ishibashi F, Satasook C, Isman MB. Insecticidal 1H-Cyclopentatrahydro[b] Benzofurans from Aglaia odorata. Phytochemistry. 1993;32:307–310. doi: 10.1016/S0031-9422(00)94986-0. [DOI] [Google Scholar]
- Kathiresan K, Veera Ravi A. Seasonal changes in tannin content of mangrove leaves. Indian For. 1990;116(5):390–392. [Google Scholar]
- Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B. 2009;7:133–137. doi: 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Khafagi I, Gab-Alla A, Salama W. Biological activities and phytochemical constituents of the gray mangrove Avicennia marina (Forssk.) Vierh. Egypt J Bot. 2003;5:62–69. [Google Scholar]
- Kong H, Jang J. One-step fabrication of silver nanoparticle embedded polymer nano fibers by radical-mediated dispersion polymerization. Chem Commun. 2006;28:3010–3012. doi: 10.1039/b605286j. [DOI] [PubMed] [Google Scholar]
- Kuber CB, D’Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B. 2006;47:160–164. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Mukunthan KS, Elumalai EK, Patel TN. Catharanthus roseus: a natural source for the synthesis of silver nanoparticles. Asian Pac J Trop Biomed. 2011;1(4):270–274. doi: 10.1016/S2221-1691(11)60041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladimeji HO, Nia R, Kalu N. In vitro biological activities of Carica papaya. Res J Med Plant. 2007;1:92–99. doi: 10.3923/rjmp.2007.92.99. [DOI] [Google Scholar]
- Oladimeji HO, Ubulom PM, Akpabio EI. Larvicidal and anti-microbial potentials of Nymphaea odorata. J Pharmacol Toxicol. 2008;3:357–362. doi: 10.3923/jpt.2008.357.362. [DOI] [Google Scholar]
- Parashar UK, Saxenaa PS, Srivastava A. Bio inspired synthesis of silver nanoparticles. Dig J Nanomater Biostruct. 2009;4:159–166. [Google Scholar]
- Petit C, Lixon MP, Pileni MP. In situ synthesis of silver nanocluster in AOT reverse micelles. J Phys Chem. 1993;97:12974–12983. doi: 10.1021/j100151a054. [DOI] [Google Scholar]
- Prasad TNVKV, Elumalai EK. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed. 2011;1(6):439–443. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premanathan M, Kathiresan K, Yamamoto N. In vitro anti-human immunodeficiency virus activity of polysaccharide from Rhizophora mucronata Poir. Biosci Biotechnol Biochem. 1999;63(7):1187–1191. doi: 10.1271/bbb.63.1187. [DOI] [PubMed] [Google Scholar]
- Rao DR, Mani TR, Rajendran R. Development of high level of resistance to Bacillus sphaericus in a field population of C. quinquefasciatus Say (Diptera: Culcidae) Afr J Biomed Res. 1995;8:31–33. [PubMed] [Google Scholar]
- Ravikumar S, Ramanathan G, Subhakaran M. Antimicrobial compounds from marine halophytes for silkworm disease treatment. Int J Med Sci. 2009;5:184–191. [Google Scholar]
- Ravikumar S, Ramanathan G, Jacob Inbaneson S. Antiplasmodial activity of two marine polyherbal preparations from Chaetomorpha antennina and Aegiceras corniculatum against Plasmodium falciparum. Parasitol Res. 2011;108(1):107–113. doi: 10.1007/s00436-010-2041-5. [DOI] [PubMed] [Google Scholar]
- Service MV. Biological control of mosquitoes has it a future? Mosq News. 1983;43:113–120. [Google Scholar]
- Shin SH, Ye MK, Kim HS. The effects of nanosilver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int Immuno pharmacol. 2007;7:1813–1818. doi: 10.1016/j.intimp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Syed Ali M, Ravikumar S, Margaret Beula J. Bioactivity of seagrass against the dengue fever mosquito Aedes aegypti larvae. Asian Pac J Trop Biomed. 2012;2:570–573. doi: 10.1016/S2221-1691(12)60099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangam TS, Kathiresan K. Mosquito larvicidal activity of marine plant extracts with synthetic insecticides. Bot Mar. 1991;34:537–539. [Google Scholar]
- Thangam TS, Kathiresan K, Mabbett T. Mosquito larvicidal activity of seaweed extract against A. aegypti and C. quinquefasciatus. Int Pest Control. 1993;35:94–95. [Google Scholar]
- Vinayagam A, Senthilkumar N, Umamaheshwari A. Larvicidal activity of seaweed extract against A. aegypti and C. quinquefasciatus. Int Pest Control. 2008;35:94–95. [Google Scholar]
- WHO (1975) Instructions for determining the susceptibility of resistance mosquito larvae to insecticides. Mimeographed Document WHO/VBC/75 583
- WHO (2010) Antiretroviral treatment working group treatment white paper
- Zandi K, Taherzadeh M, Yaghoubi R. Antiviral activity of Avicennia marina against herpes simplex virus type 1 and vaccine strain of poliovirus (An in vitro study) J Med Plan Res. 2009;3(10):771–775. [Google Scholar]