Abstract
The aim of the study was to highlight the sex dependent differences in the electrophoretic protein patterns of male and female Haemonchus contortus worms SDS based polyacrylamide gels of both male and female worms were run side by side for comparison. A total of 33 and 35 polypeptides were detected in polyacrylamide gels stained with coomassie brilliant blue R-250, respectively. Besides many of the fundamental homologies in protein profile, some of the polypeptides specific to either sex were also observed. Most of the characteristic polypeptides were of low molecular weight. These polypeptides needs deeper unrevealing regarding the nature of protein, through well planned zymographic studies, so as to ascertain the true nature and/or type of protein involved in those bands. This will help us in better understanding of parasite immunology and sex influenced differences amongst the worm and the possible variations in their pathogenesis contributed thereof, if any.
Keywords: CBBR-250, Haemonchus contortus, Protein profile, Sex differences
Introduction
Haemonchosis caused by a voracious blood sucking nematode, Haemonchus contortus, is a highly pathogenic and economically significant parasitic entity of small ruminant. The adult parasite attaches itself to the mucosa of host abomasum and feeds mainly on blood and mucosal epithelium thereby, causing severe losses in terms of severe anaemia, oedema, diarrhoea, dehydration, loss in milk, meat and wool production and frequent deaths particularly in young animals, thereby causing great production losses in sheep and goats. At present, the control haemonchosis mainly relies on use of anthelmintics and pasture management. However, depletion of clean pastures, emergence of resistant strains against anthelmintics and increasing concern of drug residues in the environment and food chain have highlighted the need for alternative control strategies such as the use of vaccines (Coles 2002; Jaiswal et al. 2013). Need for development of a successful vaccine against haemonchosis have been highlighted since long(Smith 1999), for which knowledge about protein profile of the parasite is very vital. Biochemical approaches have been tried since long when the application of electrophoresis was used as an analytical tool for solving the taxonomic problems for the identification of parasite (Sibley 1960). The present study was undertaken to generate some basic data on the polypeptide profiles of male and female H. contortus that could be subsequently used for the identification of immunodiagnostic antigens and for gender identification.
Materials and methods
The male and female H. contortus were collected from naturally infected goats slaughtered, at local abattoir houses and brought to the Department of Parasitology for routine epidemiological studies. The parasites were processed as per the procedure described by Shoiab and Irshadullah (2009). Briefly, the parasites were washed several times in Hanks’ medium to remove all the debris and host derived materials. For evacuation of eggs, the female worms were incubated at 37 ± 1 °C in Hanks’ medium for 24 h. Thereafter, both male and female worms were separately homogenized in 0.1 M sodium phosphate buffer (pH 7.4) containing 0.25 M sucrose. Later on, the worms were put in 2X lysis buffer and were boiled for 5 min as per the procedure of Sudan et al. (2014). The soluble proteins were isolated by centrifugation at 1,000×g for 10 min and used for electrophoresis.
Sodium-dodecyl sulphate (SDS) gradient polyacrylamide gel electrophoresis (SDS- gradient PAGE) was performed according to method of Laemmli (1970) with some minor modifications. The soluble protein samples as well as standard molecular weight markers (Genei Chemical Co., Bangalore, India) were boiled for 5 min in sample buffer in the ratio of 2:1 volume containing 50 % stacking gel buffer (pH 6.8), 20 % glycerol, 10 % SDS, 20 % β-mercaptoethanol and 0.05 % (w/v) aqueous bromophenol blue as the marker dye. The electrophoresis was carried out at 90 V in vertical slab gel system (Genei Chemical Co., Bangalore, India). After electrophoresis, the gels were stained in 0.25 % (w/v) coomassie brilliant blue R-250 (CBBR-250) dye (Hi Media).
Results and discussion
The result of SDS gradient PAGE revealed some fundamental homologies and sex-dependent differences in the polypeptide profile of male and female H. contortus. A total of 33 and 35 polypeptides of different molecular weights were resolved by CBBR-250 staining (Fig. 1) in male and female worms, respectively. Most of the polypeptides were common but some specific polypeptides were detected in both male and female counterparts. The two bands at 75 and 52 kDa were found to be very specific in male worms while bands at 90 and 31 kDa correspond to female worms only. Almost similar observations had also been reported earlier also by Shoiab and Irshadullah (2009).
Fig. 1.
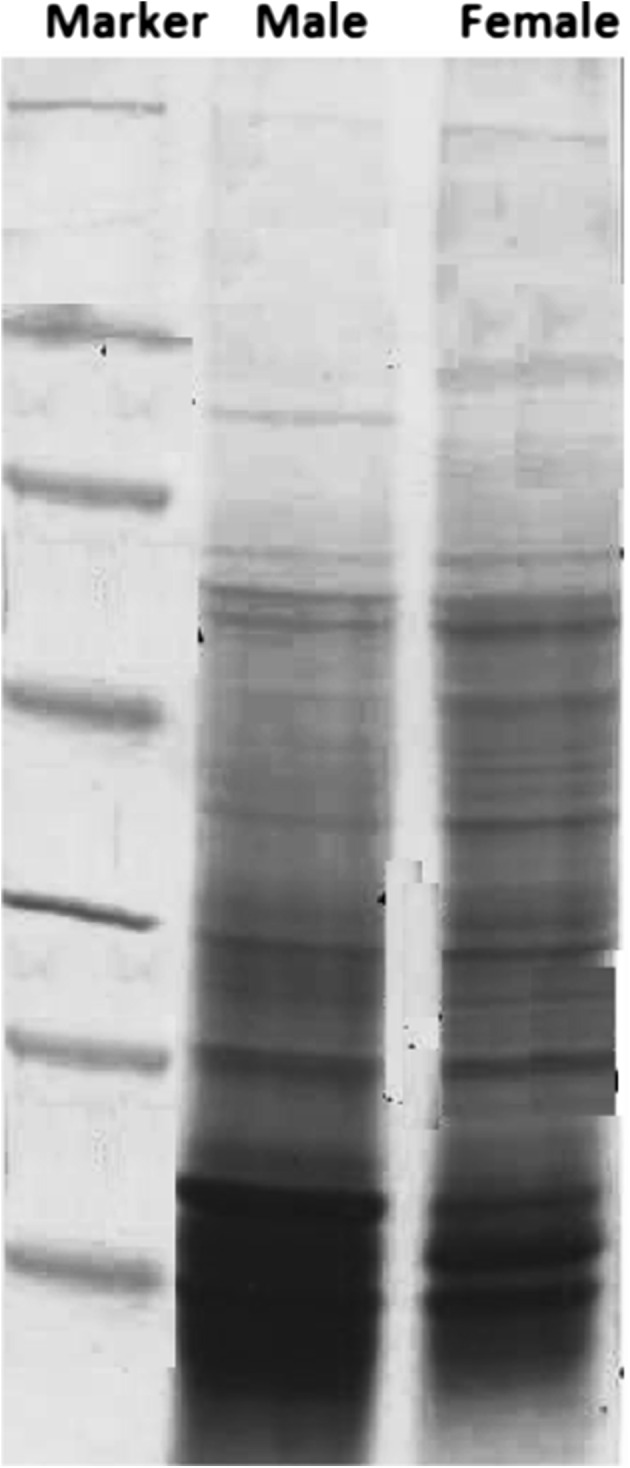
SDS based protein characterization profile of male and female Haemonchus contortus upon coomassie brilliant blue R-250 staining
Comparatively more polypeptides were observed in female as compared to male H. contortus. The extra polypeptides in female worm could be due the fact that, for egg production they required some additional proteins. Likewise, differences in protein profile have been reported in male and female Ascaridia galli in correlation with egg production (Saifullah et al. 1993). Most of the characteristic sex-linked polypeptides were of low molecular weight which, can be very much exploited for the preparation of diagnostic probes, development of vaccine and in designing of new drugs after detailed studies. Even antigenic differences have also been demonstrated between phenotypically different females of H. contortus (Sood and Kapur 1982).
Silver staining could detect more polypeptides be due to its higher sensitivity as well as staining property of conjugated proteins like lipo, phosphor or glycol protein (Dzandu et al. 1984). Similarly, CBBR-250 dye can detect Calmodulin and Troponin C which cannot be detected with silver stain (Irie et al. 1982). Hence it is very much desired that a complete set of different stains could be employed for accurate and more precise demonstration of various peptides in this parasite. Further research is also warranted on comparative study of egg, larval and adult stages different phenotypically different isolates of this parasite so as to find out a highly conservative reactive moiety that may serve as a functional immunodiagnostic and/or immunoprophylactic molecule. It is suggested that these polypeptides could be used/exploited for gender identification, preparation of diagnostic probes, development of vaccine and in designing of new drugs. Moreover, the bands found in the instant study which were sex specific needs deeper unrevealing regarding the nature of protein through well planned zymographic studies so as to ascertain the true nature and/or type of protein involved in those bands. This will help us in better understanding of parasite immunology and sex dependent differences amongst the worm and possible variations in their pathogenesis, if any.
Acknowledgments
The authors are highly thankful to the Vice Chancellor, DUVASU for the facilities provided.
References
- Coles GC. Cattle nematodes resistant to anthelmintics: why so few cases? Vet Res. 2002;33:481–489. doi: 10.1051/vetres:2002034. [DOI] [PubMed] [Google Scholar]
- Dzandu JK, Mercy ED, Eenise LB, Garry EW. Detection of erythrocyte membrane protein, sialoglycoprotein, and lipids in the same polyacrylamide gel using a double staining technique. Proc Natl Acad Sci. 1984;81:1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie S, Seizaki M, Kato Y. A faithful double stain of proteins in the polyacrylamide gels with coomassie blue and silver. Anal Biochem. 1982;126:350–354. doi: 10.1016/0003-2697(82)90526-7. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK, Sudan V, Shanker D, Kumar P. Emergence of ivermectin resistance in gastrointestinal nematodes of goats in a semi-organized farm of Mathura district—India. Vet Arhiv. 2013;83(3):275–280. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Saifullah MK, Ali A, Abidi SMA, Nizami WA. Sex dependent differences in transaminases and polypeptides of Ascaridia galli. J Parasit Appl Anim Biol. 1993;2:19–25. [Google Scholar]
- Shoiab M, Irshadullah M. Protein profile of male and female Haemonchus contortus. J Vet Parasitol. 2009;23(2):147–149. [Google Scholar]
- Sibley CG. The electrophoretic patterns of avian egg white proteins as taxonomic characters. IBIS. 1960;102:215–284. doi: 10.1111/j.1474-919X.1960.tb07114.x. [DOI] [Google Scholar]
- Smith WD. Prospects for vaccines of helminth parasites of grazing ruminants. Int J Parasitol. 1999;29:17–24. doi: 10.1016/S0020-7519(98)00195-7. [DOI] [PubMed] [Google Scholar]
- Sood ML, Kapur J. Electrophoretic analysis of proteins of knobbed and linguiform morphs of female Heamonchus contortus Nematoda: (Trichostrongylidae) Helminthologia. 1982;19:273–278. [Google Scholar]
- Sudan V, Tewari AK, Singh H, Sarvanan BC. Prokaryotic expression and molecular characterization of surface antigen 3 (SAG 3) protein of Toxoplasma gondii. Indian J Anim Sci. 2014;84(8):824–828. [Google Scholar]


