Abstract
The gill histology and bacterial load of skin of the grass carp juveniles were investigated in relation to various concentrations of copper sulfate and potassium permanganate. For this purpose, the sublethal doses were determined after a pre-test and then the experiment was done in five treatments (for copper sulfate: 1, 1.94, 3.71, 7.07 and 15 mg/l and for potassium permanganate: 0.25, 0.52, 1.91, 2.27 and 5 mg/l) with three replicates inside the glass aquaria. Also, one group without disinfecting product was considered as control for each experiment. The microbial and histopathological investigations were done after 96 h exposure. According to results, the lowest bacterial load (CFU/g) of skin was observed in 15 mg/l copper sulfate treatment and 0.25 mg/l potassium permanganate treatment (P < 0.05). Also, the histological investigation showed a range of histopathological alternations in gills tissue including lamellar necrosis, hyperplasia, lamellar adhesion, haemorrhage, clubbing of gill lamellae. The severity of these alternations increased with increasing of the doses of the copper sulfate and potassium permanganate. In this regard, the highest histological damages were observed in 15 mg/l copper sulfate and 5 mg/l potassium permanganate respectively. Our results showed that low dosage of potassium permanganate has best effect on reducing of bacterial load of skin with lowest adverse effects on gill tissue.
Keywords: Copper sulfate, Potassium permanganate, Histology, Gill, Grass carp
Introduction
With progress of aquaculture industry especially development of intensive aquaculture, the appearance of pathogens is unavoidable. Therefore, nowadays, a wide range of disinfecting products are used to avoid damaging effects of pathogens. At now, various disinfecting chemicals are used commonly for the control of bacterial and fungal pollution in cultured fish and surrounding water (reviewed by Torgersen and Hastein 1995). The disinfecting products such as malachite green, formalin, copper sulfate, potassium permanganate, chloramines, chlorine dioxide and quaternary ammonium compounds. Irrespective of disinfecting role, these chemicals are subsequently released to the aquatic environment and may have impacts on other aquatic organisms and their habitat (Boyd and Massut 1999; Nash 2003; Erondu and Anyanwu 2005). Therefore, to modify the undesirable impacts of disinfecting drugs on fish and aquatic environment, the applied doses of these chemicals should be optimized. In fact, in addition to have a disinfecting role on fish and water, a disinfecting chemical should be safe for fish and natural environment. The grass carp, Ceteopharyngodon idella is one of the main produced species of aquaculture in many countries including Iran. Grass carp is a basically herbivorous fish that naturally feeds on certain aquatic weeds. However, the juvenile or larvae feed on zooplankton. Under culture conditions, grass carp can well accept artificial feed and obtains good growth.
In Iran, annually, millions of grass carp juveniles are produced by means of the artificial propagation and then injected to culture systems. At now, some disinfecting chemicals such as malachite green, formalin, copper sulfate and potassium permanganate are used routinely for disinfection of fish and water in carp hatcheries of Iran. Nevertheless, there is very little information on the toxicity and histopathological impacts of these chemicals on carps. Therefore, we investigated the impacts of two disinfecting products i.e. copper sulfate and potassium permanganate on histopathological alternations of gill and on bacterial load of skin in grass carp.
Materials and methods
In this experiment, we prepared grass carp juveniles (3–5 g) from International Sturgeon Research Institute, Rasht, Iran. Before the experiment, a pre-text was done to determine the sublethal doses of copper sulfate and potassium permanganate. In this regard, the values of LC10, LC50 and LC90 were measured after 24, 48, 72 and 96 h expose of juveniles with various concentrations of copper sulfate and potassium permanganate. After pre-test, a range of 1–15 mg/l copper sulfate and 0.25–5 potassium permanganate were considered as sublethal range and used for the experiment. The experiment was conducted in 36 glass aquaria (18 aquaria for copper sulfate treatment and 18 aquaria for potassium permanganate treatment) containing 20 l of dechlorinated and gentle aerated water in each of them. Dissolved oxygen and temperature were 7–8 mg/l and 22 °C respectively. For each disinfecting product, 5 treatments and one control group with three replicate were considered. The treatments were: T1: 1, T2: 1.94, T3: 3.71, T4: 7.07 and T5: 15 mg/l copper sulfate and: T1: 0.25, T2: 0.52, T3: 1.91, T4: 2.27 and T5: 5 mg/l potassium permanganate. The control group was water without disinfecting product. To each experimental aquarium, a set of 10 grass carp juveniles were added. After 96 h, the histology of gill and also the bacterial load of skin were investigated.
Sample preparation for bacterial study
From each experimental aquarium, one fish was sampled for bacterial study of skin according Ampofo and Clerk (2010). To remove the bacterial flora of water of culture, fish was washed with 0.9 % physiological serum. After that, small pieces (1 cm) of skin were separated by a scalpel. Then, each tissue piece was diluted 9 times its weight with 0.9 % physiological serum. After homogenization, 1 ml of the diluents was added into sterile Petri dishes containing tryptic soy agar (TSA) and then incubated at 25 °C for 24–48 h. After incubation, the bacterial load [Colony Forming Units/g (CFU/g)] was counted from each bacterial culture plates.
Gill histology
The gill samples were fixed in Bouin’ssolution, embedded in paraffin wax, sectioned at 5–7 mm thick, and stained with hematoxylin and eosin. The slides of gill tissue samples were investigated under a light microscope at 200× magnification.
Statistical analysis
The SPSS software (version 16) was used for data analysis. All data had normal distribution according to Kolmogorov–Smirnov test. One-way analysis of variance (ANOVA) was employed to compare the means. When significant F-ratios were calculated by ANOVA, the Duncan test was applied to identify which means were different.
Results
The lowest bacterial load (CFU/g) of skin was observed in response to 15 mg/l copper sulfate (T5) (Fig. 1) and 0.25 mg/l potassium permanganate (T1) (Fig. 2; P < 0.05).
Fig. 1.
Bacterial load (CFU/g) of skin of the grass carp juveniles after expose to various doses of copper sulfate. T1 1, T2 1.94, T3 3.71, T4 7.07 and T5 15 mg/l copper sulfate. Bars (Mean ± SD) with different letters are significantly different (p < 0.05)
Fig. 2.
Bacterial load (CFU/g) of skin of grass carp juveniles after expose to various doses of potassium permanganate. T1 0.25, T2 0.52, T3 1.91, T4 2.27 and T5 5 mg/l potassium permanganate. Bars (Mean ± SD) with different letters are significantly different (p < 0.05)
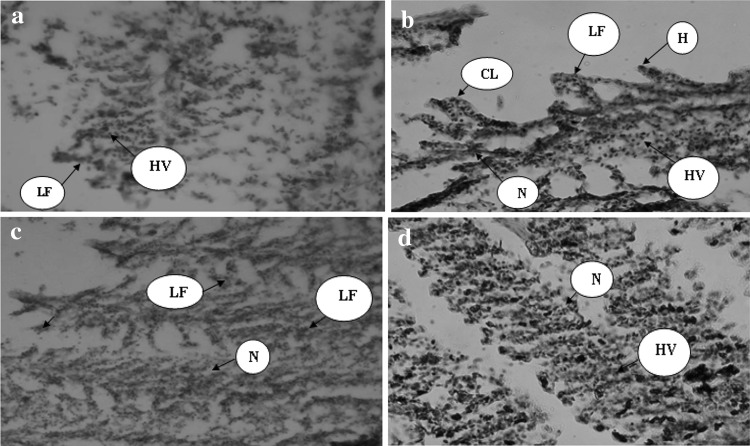
After 96 h expose of grass carp juveniles to various doses of copper sulfate and potassium permanganate, a range of histopathological alternations were observed in gill tissue including: lamellar necrosis, hyperplasia, lamellar adhesion, haemorrhage, clubbing of gill lamellae (Figs. 3, 4). The severity of these alternations increased with increasing of the doses of the copper sulfate and potassium permanganate. In this regard, the highest histological damages were observed in 15 mg/l copper sulfate and 5 mg/l potassium permanganate respectively.
Fig. 3.
Cross section (H.E. ×40) of gill of grass carp juveniles exposed to various doses of copper sulfate (a 1 mg/l; b 1.94 mg/l; c 3.71 mg/l; d 7.07). (H hyperplasia, HV haemorrhage, LF adhesion of gill lamellae, N cell necrosis, He haemorrhage)
Fig. 4.
Cross section (H.E. ×40) of gill of grass carp juveniles exposed to various doses of potassium permanganate (a 0.25 mg/l; b 0.52 mg/l; c 1.91 mg/l; d 2.27 mg/l; e 5 mg/l). H haemorrhage, HV haemorrhage, LF adhesion of gill lamellae, N cell necrosis, CL clubbing of gill lamellae
Discussion
Development of aquaculture industry especially intensive culture has been always along with increasing of pathogenic risks. The disinfecting products are chemicals that used routinely against pathogens in fish culture systems. In addition to have disinfecting effects, these chemicals should be safe for aquatic ecosystems. Correct use of disinfecting products can effectively control many bacterial, parasitic and fungal agents before systemic infections become established, often eliminating the need for antibiotic therapy. In the present study, the effects of sublethal doses of two disinfecting products including copper sulfate and potassium permanganate were investigated on bacterial load of skin of the grass carp juveniles. Potassium permanganate (KMnO4) is an oxidizing agent that has been used for many years in aquaculture. Also, the copper sulfate is a chemical compound used to control bacterial and fungal diseases of fish and surrounding water. Several studies demonstrated the disinfecting role of potassium permanganate and copper sulfate in aquaculture (Plumb 1992; Phillips 1996; Hasan and Ahmed 2002; Faruk et al. 2005; Mitchell et al. 2008). Nevertheless, the disinfecting action of these chemicals is dependent to the water alkalinity, water hardness, pH, dosage and duration of treatment (Palmer 1962; Tucker and Robinson 1990; Peres and Pihan 1991; Straus and Tucker 1993; Erickson et al. 1996; Straus 2004; Carvalho and Fernandes 2006). In our study, the bacteria load of the skin reduced as the copper sulfate concentrations elevated from 1 to 15 mg/l. This shows that the disinfecting impact of the copper sulfate is dose-dependent with best results in high doses. In contrast, disinfecting impacts of potassium permanganate was not dose-dependent since it was observed no changes in bacterial load of the skin with increasing of potassium permanganate from 0.52–5 mg/ml. Even, it was interest that the lowest bacterial load was observed in T1 with lowest concentration of potassium permanganate i.e. 0.25 mg/ml. This shows that the doses more than 0.25 mg/l have not additional effects on bacterial load, indicating a threshold level of effectiveness for potassium permanganate. Different impacts of potassium permanganate and copper sulfate on bacterial flora of skin may be due to their different chemical nature and influencing power. In addition to disinfecting power, the effectiveness of a disinfecting product is also dependent to water hardness, alkalinity and pH. As instance, it was observed that the disinfecting effect of copper sulfate reduced with increasing of water alkalinity and hardness. Therefore, the reduced disinfecting power of potassium permanganate in doses more than 0.25 mg/l may be in relation to changes of some physiochemical parameters of water over the course of the experiment.
In our study, a wide range of histopathological alternations were observed in gills tissue including lamellar necrosis, hyperplasia, lamellar adhesion, haemorrhage, clubbing of gill lamellae. The severity of these alternations increased with increasing of the doses of the copper sulfate and potassium permanganate. Similar histopathological alternations have been reported for various fish species after expose to disinfecting chemicals (Peyghan et al. 2007). These alterations may play a defensive role against disinfecting chemicals. However, these modifications can produce adverse effects on fish health, and may increase their susceptibility to secondary infectious diseases and even death (Hawkins et al. 1984). Therefore, these histopathological alternations should be in normal limits. This gains when the disinfecting chemicals are used in safe doses.
In conclusion, our results showed that the copper sulfate and potassium permanganate with different doses have disinfecting effects on bacterial load of skin, although the applying of them is along with some histopathological alternations. It seems that the potassium permanganate has higher disinfecting power than copper sulfate since the potassium permanganate had good disinfecting role in lower dosage than (15 mg/l copper sulfate vs. 0.25 mg/l potassium permanganate). Generally, information about the influencing doses of disinfecting products can prevent from their adverse environmental harmful impacts arising from excessive use of them.
References
- Ampofo JA, Clerk GC. Diversity of bacteria contaminants in tissues of fish cultured in organic waste-fertilized ponds: health implications. Open Fish Sci J. 2010;3:142–146. doi: 10.2174/1874401X01003010142. [DOI] [Google Scholar]
- Boyd CE, Massut L. Risks associated with the use of chemicals in pond aquaculture. Aquac Eng. 1999;20:113–132. doi: 10.1016/S0144-8609(99)00010-2. [DOI] [Google Scholar]
- Carvalho CS, Fernandes MN. Effect of temperature on copper toxicity and hematological responses in the neotropical fish Prochilodus scrofa at low and high pH. Aquaculture. 2006;251:109–117. doi: 10.1016/j.aquaculture.2005.05.018. [DOI] [Google Scholar]
- Erickson RJ, Benoit DA, Mattson VR, Nelson HP, Leonard AN. The effect of water chemistry on the toxicity of copper to fatheadminnows. Environ Toxicol Chem. 1996;15:181–193. doi: 10.1002/etc.5620150217. [DOI] [Google Scholar]
- Erondu ES, Anyanwu PE. Potential hazards and risks associated with the aquaculture industry. Afr J Biotechnol. 2005;4:1622–1627. [Google Scholar]
- Faruk MAR, Sultana N, Kabir MB. Use of chemicals in aquaculture activities in Mymensingh area, Bangladesh. Bangladesh J Fish. 2005;29:1–10. [Google Scholar]
- Hasan MR, Ahmed GU (2002) Issues in carp hatcheries and nurseries in Bangladesh, with special reference to health management. In: Arthur JR, Phillips MJ, Subasinghe RP, Reantaso MB, MacRae IH (eds) Primary aquatic animal health care in rural, small-scale, aquaculture development. FAO Fisheries Technical Paper No. 406
- Hawkins WE, Overstreet RM, Provancha MJ. Effects of space shuttle exhaust plumes on gills of some estuarine fishes: a light and electron microscopic study. Gulf Res Rep. 1984;7:297–309. [Google Scholar]
- Mitchell AJ, Darwish AM, Fuller SA (2008) Comparison of tank treatments of copper sulfate and potassium permanganate on sunshine bass infested with ichthyobodosis (costiosis) [abstract]. Book of Abstracts Aquaculture America, p 246
- Nash CE. Interactions of Atlantic salmon in the Pacific northwest VI. A synopsis of the risk and uncertainty. Fish Res. 2003;62:339–347. doi: 10.1016/S0165-7836(03)00068-7. [DOI] [Google Scholar]
- Palmer CM (1962) Algae in water supplies, U. S. Public Health Service, Publication No. 657, 88 pp
- Peres I, Pihan JC. Copper LC50 to Cyprinus carpio. Influence of hardness, seasonal variation, proposition of maximum acceptable toxicant concentration. Environ Technol. 1991;12:161–167. doi: 10.1080/09593339109384992. [DOI] [Google Scholar]
- Peyghan R, Oskoizadeh K, Emailzadeh S, Rasekh A. Pathological study of effect of short and long-term copper sulfate bath on gill in grass carp, Ctenopharyngodon idella. Asian Fish Sci. 2007;20:109–116. [Google Scholar]
- Phillips M. The use of chemicals in carp and shrimp aquaculture in Bangladesh, Cambodia, Lao PDR, Nepal, Pakistan, Sri Lanka and Viet Nam. In: Arthur JR, Lavilla-Pitogo CR, Subasinghe RP, editors. Use of chemicals in aquaculture in Asia. Iloilo: Southeast Asian Fisheries Development Center, Aquaculture Department Tigbauan; 1996. pp. 75–84. [Google Scholar]
- Plumb JA. Disease control in aquaculture. In: Shariff IM, Subasinghe RP, Arthur JR, editors. Disease in Asian aquaculture. Manila: Fish Health Section of the Asian Fisheries Society; 1992. pp. 3–17. [Google Scholar]
- Straus DL. Comparison of the acute toxicity of potassium permanganate to hybrid striped bass in well water and diluted well water. J World Aquacult Soc. 2004;35:55–60. doi: 10.1111/j.1749-7345.2004.tb01059.x. [DOI] [Google Scholar]
- Straus DL, Tucker CS. Acute toxicity of copper sulphate andchelated copper to channel catfish Ictalurus punctatus. J World Aquac Soc. 1993;24:390–395. doi: 10.1111/j.1749-7345.1993.tb00170.x. [DOI] [Google Scholar]
- Torgersen Y, Hastein T. Disinfection in aquaculture. Rev Sci Tech Off Int Epiz. 1995;14:419–434. doi: 10.20506/rst.14.2.845. [DOI] [PubMed] [Google Scholar]
- Tucker CS, Robinson EH. Channel catfish farming handbook. New York: Van Nostrand-Reinhold; 1990. [Google Scholar]