Abstract
Oncolytic viruses are multifunctional anticancer agents with huge clinical potential, and have recently passed the randomized Phase III clinical trial hurdle. Both wild-type and engineered viruses have been selected for targeting of specific cancers, to elicit cytotoxicity, and also to generate antitumor immunity. Single-agent oncolytic virotherapy treatments have resulted in modest effects in the clinic. There is increasing interest in their combination with cytotoxic agents, radiotherapy and immune-checkpoint inhibitors. Similarly to oncolytic viruses, the benefits of chemotherapeutic agents may be that they induce systemic antitumor immunity through the induction of immunogenic cell death of cancer cells. Combining these two treatment modalities has to date resulted in significant potential in vitro and in vivo synergies through various mechanisms without any apparent additional toxicities. Chemotherapy has been and will continue to be integral to the management of advanced cancers. This review therefore focuses on the potential for a number of common cytotoxic agents to be combined with clinically relevant oncolytic viruses. In many cases, this combined approach has already advanced to the clinical trial arena.
Keywords: oncolytic virotherapy, chemotherapy, immunogenic cell death
Introduction
The conventional cancer treatments of surgery, chemotherapy, and radiotherapy remain the mainstay of current therapeutic approaches to cancer. They have been used successfully in combination with one another in the neoadjuvant, concomitant, and adjuvant context for many years. However, despite their utility and curative potential, each modality has its limitations in terms of limited efficacy, significant toxicity, lack of durability of response, and in the case of chemotherapy the emergence of drug resistance. In addition to the release of neoantigens after tumor-cell destruction, exposure of cancer cells to cytotoxic agents may induce innate and adaptive immune responses against the cancer in other ways. Certain modes of cancer cell death are associated with immunogenicity through the induction of immunogenic cell-death (ICD) proteins, such as calreticulin, HSP70, ATP, and HMGB proteins.1 A number of cytotoxic agents have been shown to induce ICD,2 while others are capable of modulating the tumor microenvironment by reducing the function or number of suppressive immune cells (regulatory T cells [Tregs] and myeloid-derived suppressor cells [MDSCs]) or generating inflammatory cytokines (Table 1).3–137 Tumors treated with chemotherapy have also been shown to be more sensitive to cytotoxic T-lymphocyte (CTL) killing.51 Most of the evidence for ICD has been derived from murine models of human cancer. Relatively little is known about the immunogenicity of chemotherapy in cancer patients. The combination of “immunogenic” or ICD-inducing chemotherapy with other anticancer treatment modalities capable of priming and/or propagating immune responses is now being evaluated. While the obvious candidates for combination are cancer vaccines, low doses of radiotherapy and immune-checkpoint inhibitors, there is an increasingly compelling case for combination of chemotherapy with oncolytic viruses (OVs).138
Table 1.
Mechanisms of immunomodulation caused by chemotherapy (chemo) alone, and synergy seen when combined with oncolytic virus
| Chemotherapy drug | Mechanism of immunomodulation caused by chemo alone | Immunomodulation reference | Oncolytic virus–chemo synergy |
|---|---|---|---|
| Cyclophosphamide | Triggers TRAIL CD8+ T cell-mediated apoptosis | 3 | |
| Induces proinflammatory production/induction of ICD marker calreticulin/HMGB1 | 4–6 | ||
| Decreased Treg function | 7–9 | 10,11 | |
| CD8+ T cell-specific tumor activity | 7 | ||
| Induces T-helper type 1 or 17 immunity | 12 | 11 | |
| Decreases complement function | 13 | ||
| Suppression of immune cell types | 14,15 | ||
| Inhibits or delays viral neutralization response | 14–23 | ||
| Increases MDSCs | 24,25 | ||
| Enhances DC function | 26 | ||
| Synergy, but unknown immune function, if any | 27,28 | ||
| Gemcitabine | Decreases MDSCs | 29 | 29–31 |
| Decreases neutralizing antibodies | 29 | 29 | |
| Induces ICD marker calreticulin | 4 | ||
| Induces ICD marker HMGB1 | 32,33 | ||
| Depletes B cells | 34 | 35 | |
| Synergy, but unknown immune function, if any | 32,36–45 | ||
| Bortezomib | Enhances DC function | 46 | |
| ICD and DAMP release | 14 | ||
| Antitumoral immunity | 47 | ||
| CD8+ T cell-mediated inhibition of tumor growth | 46 | ||
| Synergy, but undefined immune function, if any | 48,49 | ||
| Doxorubicin | Induces ICD marker calreticulin | 4 | 50 |
| Granzyme B released by CTLs | 51 | ||
| Induces type I IFN response | 52 | ||
| Increases Treg cells and significantly decreases NK cells | 53 | ||
| Decreases B7-H1/PD-L1 from cell surface | 54 | ||
| Synergy, but undefined immune function, if any | 55–59 | ||
| Mitoxantrone | Induces DC/T-cell tumor infiltrate | 60 | |
| Releases ATP | 60 | ||
| Ecto-CRT, ecto-HSP70, and HMGB1 | 61,62 | ||
| Tumor antigen-specific CD8+ and CD4+ T-cell activity | 60,63,64 | 65 | |
| Enhances DC function | 66 | ||
| Temozolomide | Decreases Treg function | 67 | |
| Tumor-specific T-cell responses | 68 | 68 | |
| Synergy, but undefined immune function, if any | 69–74 | ||
| Docetaxel | Decreases MDSCs, increases CD8+ T cells | 75 | |
| Enhances DC function | 75 | ||
| Synergy, but unknown immune function, if any | 76–82 | ||
| Paclitaxel | Granzyme B released by CTLs | 83 | |
| Induces ICD marker calreticulin | 4 | ||
| Induces MHC | 84 | ||
| Decreases Treg function | 85–87 | ||
| Induces T-helper type 1 immunity | 12 | ||
| Type I IFN and HMGB1 release in vitro | 88 | ||
| NK cells essential for strong synergy | 10 | ||
| Slows neutralizing antibodies (with carboplatin) | 89 | ||
| Synergy, but unknown immune function, if any | 90–99 | ||
| 5-Fluorouracil | CD8+ T cell-mediated apoptosis | 100 | |
| Induces carcinoembryonic antigen (CEA) | 101 | ||
| Decreases MDSCs | 102 | ||
| Synergy, but unknown immune function, if any | 103–105 | ||
| Cisplatin | Decreases Treg function | 106 | |
| CD8+ T cell-specific tumor activity | 106 | ||
| Granzyme B released by CTL | 50 | ||
| Enhances DC function, cytokine release, and cytotoxic T-cell activation | 107 | ||
| Synergy, but unknown immune function, if any | 108–118 | ||
| Mitomycin C | Enhances DC function | 71,119 | |
| Synergy, but unknown immune function, if any | 120–124 | ||
| Azadeoxycytidine Irinotecan | Enhances DC function | 71 | |
| Decreases Treg function | 125 | ||
| NK cells essential | 126 | 126 | |
| Synergy, but unknown immune function, if any | 126–129 | ||
| Rapamycin/everolimus | Inhibition of T-cell proliferation | 130 | 131 |
| Decreases DC maturation | 130 | ||
| Increases Treg cells | 130 | ||
| Decreases cellular IFN | 132 | ||
| Decreases cytokine release | 131 | ||
| Decreases antiviral antibody production | 131,133 | ||
| Synergy, but unknown immune function, if any | 132–136 | ||
| 5-Aza | Induces cancer testis antigen | 137 | |
| Induces MHC | 137 |
Abbreviations: ICD, immunogenic cell death; Treg, regulatory T cell; MDSCs, myeloid-derived suppressor cells; DC, dendritic cell; DAMP, danger-associated molecular pattern; CTLs, cytotoxic T lymphocytes; NK, natural killer; MHC, major histocompatibility complex; TRAIL, TNF-related apoptosis inducing ligand; Ecto-CRT, ecto calreticulin.
Despite being recognized as having the potential to treat cancer since the beginning of the 20th century, OVs are only now entering the clinical arena for certain cancers, following the successful evaluation of talimogene laherparepvec (T-vec) in malignant melanoma.139 OVs are live viruses that are selectively toxic to cancer cells. The basis of selectivity for cancer versus normal cells is based on cell entry (tumor cells expressing a receptor the virus uses to gain entry), impaired IFN response in cancer cells, or dysregulation in key signaling pathways, such as the RAS pathway, which would otherwise (eg, through the phosphorylation of PKR) allow the cell to negate the virus. Clinical trials involving OVs as single agents have largely been safe, demonstrated minimal toxicity, and in certain studies shown signs both of efficacy by radiological evaluation and the presence of live virus in tumor biopsies a week or more after treatment.140,141 However, the overall efficacy of single-agent OV therapy has at best been modest. The true potential of OVs may yet be realized through their combination with other treatment modalities, such as chemotherapy. As well as synergistic mechanisms of tumor-cell killing, combination with chemotherapeutics through careful sequencing may help to overcome some of the barriers in the tumor microenvironment thought to limit the efficacy of OVs. These include large tumor size,142 poor vasculature,143 elevated interstitial pressure,144 and physical barriers.145 One potential limitation of OVs that is regularly debated is the rapid generation of antiviral antibody responses a week or so following OV administration. There have been attempts to attenuate this response using such agents as cyclophosphamide (CPA; discussed later), but it is clear that despite high levels of neutralizing antibodies, further administrations of the same OV can traffic to the tumor environment and cause tumor kill. The OV is most likely protected from neutralizing antibodies by carriage (hitchhiking) on granulocytes, lymphocytes, and platelets to tumor cells in metastatic deposits.146 Recent preclinical and clinical studies have shown that combining chemotherapy with OVs may potentially be highly synergistic, improving on the efficacy of each modality alone (Table 1).
In this review, we explore the many ways in which chemotherapy and OVs have been considered in combination. The methods used by researchers have been based on cell lines using classical isobologram analysis, murine models, and in humans with a number of completed clinical trials.
Cell-death mechanisms: immunogenic cell death is vitalfor cancer therapy
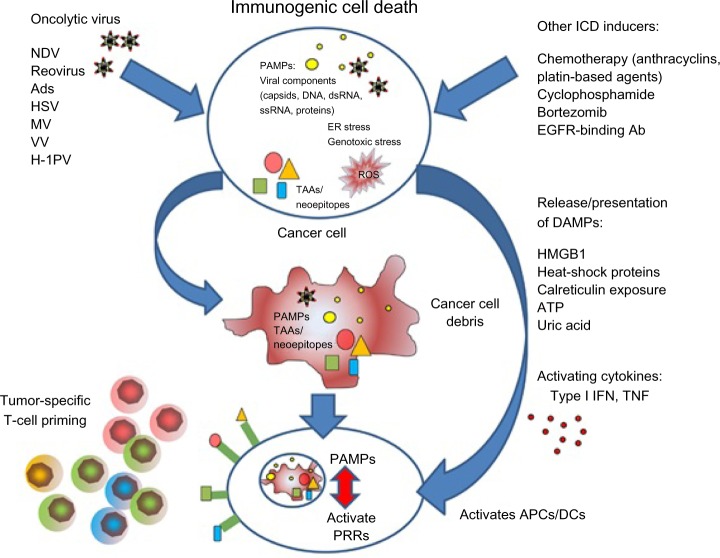
OV-mediated cell death does not fit exactly into one of the three classical categories of cell death (apoptosis, necrosis, and autophagy), and likewise cell-death pathways induced by chemotherapy can vary from agent to agent. Due to the physiological consequences associated with cell death, enormous effort has been invested into understanding the three main mechanisms. Apoptosis is vital for development and the maintenance of tissue homeostasis, and is generally considered to be a nonimmunogenic form of cell death, while necrosis, which is less coordinated and results in the release of proinflammatory cytokines, has been regarded as immunogenic.147 However, it is now clear that the boundaries between each classical cell-death pathway are not defined and there is often overlap. This has been demonstrated by the discovery of “immunogenic” apoptosis in tumor cells, which can be induced by specific chemotherapies, such as the anthracyclines and oxaliplatin (Figure 1).148,149 Similarly, OV-mediated cell death does not fit into either apoptosis or necrosis, but displays features of both, with variations between oncolytic viral types. In general, the immunogenic death (apoptosis, necrosis, autophagy, etc) of cancer cells involves a multistep process, beginning with the recognition of pathogen-associated molecular components, such as viral components, which cause such molecules as fractalkine, nucleotides, and ATP to be released, which in turn attract phagocytes or dendritic cells (DCs), and the expression of such signals as phosphatidylserine and calreticulin that aid recognition by phagocytes or DCs. Finally, danger-associated molecular patterns (DAMPs), such as HMGB1, are expressed. This enables dying tumor cells to lose the ability to induce tolerance and to stimulate powerful anticancer immune responses (Figure 1). Scientists have investigated many ways to increase the immunogenic effects seen with OVs, but it is becoming clearer that one way to complement the ICD mechanisms and the immunomodulatory effects (Table 1) seen with either therapy alone is to combine both OVs and chemotherapy to achieve either at least an additive or (even better) a synergistic result.
Figure 1.
A summary of immunogenic cell death (ICD) caused by oncolytic virus and/or chemotherapy.
Note: Reproduced from Woller N, Gürlevik E, Ureche C-I, Schumacher A, Kühnel F. Oncolytic viruses as anticancer vaccines. Front Oncol. 2014;4:188. doi: 10.3389/fonc. 2014.00188.148
Abbreviations: Ads, adenoviruses; APCs, antigen-presenting cells; DAMPs, danger-associated molecular patterns; DCs, dendritic cells; dsRNA, double-stranded RNA; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; HSV, herpes simplex virus; IFN, interferon; MV, measles virus; NDV, Newcastle disease virus; PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors; PV, parvovirus; ROS, reactive oxygen species; ssRNA, single-stranded RNA; TAAs, tumor-associated antigens; TNF, tumor necrosis factor; VV, vaccinia virus.
Combining chemotherapeutic drugs with OV therapy
Cyclophosphamide
CPA is an alkylating agent that causes cross-linking of DNA, and is used in the management of countless tumor types. In itself, CPA is not an active drug. It requires metabolic activation by aldehyde dehydrogenase, producing the active compound 4-hydroxycyclophosphamide. The release of HMGB1 and ecto-CRT is seen with CPA treatment, which results in DC activation, proinflammatory cytokine production, and T-cell proliferation.5,6 Synergy in vivo has been shown using a variety of OVs and CPA, including herpes simplex virus (HSV)-1, adenovirus, vaccinia,27,28 reovirus, measles, myxoma virus,15 and vesicular stomatitis virus (VSV).23 The combination of CPA with reovirus has been investigated in in vivo models, and these studies have demonstrated safety and efficacy using a carefully titrated CPA schedule, including administration 24 hours before reovirus.20 However, significant normal-tissue toxicity was seen at higher doses, similar to the administration of reovirus to B-cell knockout mice.20 Therefore, careful titration of any immunomodulatory effect is required to optimize efficacy without augmenting viral replication and toxicity in normal tissues. Studies with oncolytic HSV-1 and adenovirus in combination with CPA have shown a fall in the magnitudes of antiviral immune cells, which prevents, inhibits, or delays viral neutralization.14,17,19,21 CPA has been shown in vivo to deplete the complement response to HSV.13 With oncolytic measles virus and VSV, CPA has been shown to strongly damp down the antiviral host immune response,23,150 but in the case of a VSV combination resulted in reduced therapeutic efficacy compared to CPA alone.150 Zemp et al showed that the removal of the tumor-resident macrophage population in an orthotopic glioma model by CPA substantially increased the survival of mice with myxoma virus post-treatment.15
Additional studies imply that a CPA/viral combination can also boost antitumor immunity by inhibiting Tregs.10,11 These data were confirmed in a Phase I clinical trial that showed that metronomic dosing of CPA decreased Tregs in solid tumors treated with adenovirus granulocyte macrophage colony-stimulating factor, without compromising the stimulation of antitumor responses.11 In contrast, Phase I clinical trials showed that reovirus (where CPA dose was escalated from 25 to 1,000 mg/m2) or Seneca Valley virus coadministration with CPA were safe, but did not attenuate host antiviral responses.151,152
Gemcitabine
Gemcitabine is a fluorinated deoxycytidine analog that has two forms: the gemcitabine diphosphate form, which impedes the ribonucleotide reductase enzyme, resulting in a reduction in the pool of deoxynucleotide available for DNA synthesis; whereas the second form, gemcitabine triphosphate, is incorporated into DNA, causing chain termination and resulting in apoptosis and cell death. Gemcitabine has also been shown to deplete MDSCs and promote antitumor immune responses.153 Both gemcitabine and CPA can decrease neutralizing antibodies in cancer patients.29 An increase in antitumor activity was seen with a wide array of OVs in combination with gemcitabine, including adenovirus,38–44 parvovirus,32,33 reovirus,30,37 VSV,35 HSV,31 vaccinia,45 and myxoma virus.36 Gemcitabine alone fails to trigger HMGB1 release; in contrast, parvovirus does induce HMGB1.32 Combination treatment of both parvovirus and gemcitabine results in a high level of tumor cytotoxicity without impeding ICD activities.32
In vivo studies with either HSV or reovirus in combination with gemcitabine improved the survival compared with either treatment alone.30,31 These therapeutic combinations also demonstrate that gemcitabine limits the reovirus/HSV-1-induced accumulation of MDSCs in the tumor microenvironment.30,31 Gemcitabine treatment in a Phase I clinical trial showed greatly reduced levels of reovirus-neutralizing antibodies, and 80% of patients exhibited either a partial response or stable disease.29
Bortezomib
Bortezomib is a peptide-based, reversible proteasome inhibitor. Potent immunomediated antitumor effects were seen after treatment with bortezomib in the form of enhanced DC function and upregulation of the HSP60 and HSP90 proteins.46 Bortezomib has been shown to generate reactive oxygen species, which are believed to cause ICD and DAMP release, increasing cellular stress.154–157 A number of OVs have been studied in combination with bortezomib, including HSV-1,60 reovirus,49 adenovirus,47 and VSV.48 Both HSV-1 and reovirus have shown synergy, but the contribution of immunomodulatory effects of bortezomib and antitumor immune responses in vivo was not examined.49,156 Combining VSV and bortezomib resulted in antagonism in vitro, but in contrast synergy was seen in vivo.48 This may have been due to immune cells in vivo that were not present in the in vitro setting. The authors of this study cited an ovarian tumor mouse study that showed reduction in tumor growth facilitated by CD8+ T-cell function with bortezomib alone.46 In a hepatocellular carcinoma in vivo model, treatment with bortezomib and an adenovirus expressing human telomerase reverse-transcriptase resulted in caspase-dependent apoptosis and a reduction in the antiviral immune responses.47
Doxorubicin
Doxorubicin (Dox) is an anthracycline antibiotic that intercalates into the DNA double-helical structure. This intercalation process hinders unwinding and resealing of DNA for transcription, and thus inhibits cellular DNA replication. Dox also stimulates the rapid production of type I IFNs by tumor cells after activation of TLR3, resulting in the release of chemokine (CXCL10).52 A type I IFN gene signature-predicted response to Dox therapy has been seen in breast cancer patients.52
These data suggest that Dox-mediated immune responses mimic those induced by viral pathogens. In addition to inducing ICD4 and type I IFN secretion, Dox and other chemotherapeutics also increase the susceptibility of tumors to CTLs by increasing tumor-cell permeability to granzyme B released by the CTLs.51 The addition of Dox to adenovirus resulted in significantly increased expression of calreticulin in vitro.50 Synergy between Dox and other OVs, such as HSV-1,55 measles,56 vaccinia,57 Coxsackie virus 21,50 and VSV59 has been seen in vitro and in vivo, but no immunocomponent effects have yet been defined.
Mitoxantrone
Mitoxantrone (MTX) is a synthetic anthracenedione antineoplastic agent derived from the anthraquinone dye ametantrone,158 which is frequently used to manage prostate, leukemia, and breast cancer.159,160 It is structurally similar to Dox, with both drugs having a planar aromatic ring structure that enables them to interact with DNA by intercalation between base pairs. MTX can inhibit the activity of the nuclear enzyme DNA topoisomerase (II), interfere with RNA and cause the cross-linking of DNA and strand breaks, and produce reactive oxygen species. MTX is believed to lack cell-cycle phase specificity, because it has cytocidal effects on both proliferating and nonproliferating cells.161 MTX also has immunosuppressive properties, resulting in the inhibition of proinflammatory cytokines, such as TNF, IL-2, and IFNγ. It is therefore used in the management of multiple sclerosis.162 Cancer cells undergoing immunogenic apoptosis and autophagy after treatment with MTX express various DAMPs, such as ecto-HSP70, ATP, and HMGB-1,160,161 and stimulate the peripheral relocation of CRT.163 MTX treatment also increases uptake of tumor-associated antigens by antigen-presenting cells, resulting in establishment of antitumor activity by antigen-specific CD8+ and CD4+ T cells.59,63,64 Both in murine models76,77 and in human patients with cancer,146 antitumor immune responses induced by cancer cells undergoing ICD are associated with better clinical responses. A combination of HSV-1 with MTX failed to increase cytotoxicity or halt virus replication in vitro.65,164 In contrast, in vivo, the same combination provided significant survival benefit when administered locally to HER-2/neu subcutaneous tumors.65 This protective effect was facilitated by enhanced levels of tumor antigen-specific CTL cells and an increase in intratumoral infiltration of neutrophil cells.65 These results were confirmed by depleting CD4-, CD8-, and Ly6G-expressing cells from the model, showing that these cells are essential for enhanced efficacy.65
Irinotecan
Irinotecan is an antineoplastic enzyme inhibitor and shows activity against colorectal, lung, esophageal, and gastric cancers, leukemia, and lymphomas. Irinotecan inhibits the topoisomerase I-DNA complex and causes double-strand DNA breakage that results in cell death. In the clinic, irinotecan is used in combination with fluorouracil and leucovorin (FOLFIRI) in colon cancer patients.125 Treatment with the FOLFIRI combination significantly reduced the amount of CD4+FoxP3+ Tregs in patients, without altering the total number of lymphocytes or the population of CD4+ T lymphocytes.125 Irinotecan has been shown to inhibit HSV-1 viral replication and lytic oncolysis in colon cancer cell lines.164 In contrast, other groups show synergy with OVs/irinotecan, including HSV-1 encoding CYP2B1,127 reovirus,128 and Sindbis virus.126 However, only the study on Sindbis virus looked at immune components in irinotecan synergy, concluding that natural killer cells are essential for the process.126
Temozolomide
Temozolomide (TMZ) is an alkylating agent currently used as first-line therapy for glioma treatment, due to its DNA-damaging effect.165,166 Advanced melanoma patients treated with low-dose TMZ followed by DC (autologous tumor lysate) vaccination showed a reduction in circulating immunosuppressive FoxP3+ Tregs.67
Synergy has been recorded between TMZ and both HSV69,71 and adenovirus.68,72–74 An unconventional patient study on various cancers treated with oncolytic adenovirus and a low dose of TMZ showed an upregulation of ICD signal HMGB1 and specific tumor T-cell responses, which resulted in disease control in 67%68 of cases. These results are interesting, but are difficult to interpret, due to the large number of different types of adenoviruses used in this study and differences in doses of TMZ.
PI3K–Akt–mTOR pathway inhibitors
The PI3K–Akt–mTOR signaling cascade is well characterized and plays a crucial role in a variety of physiologic processes, including cell-cycle progression, differentiation, transcription, translation, apoptosis, motility, autophagy, anabolic processes (including protein and lipid synthesis), and metabolic processes (including normal glucose homeostasis). Activation of the PI3k–Akt–mTOR signaling pathway is implicated in tumorigenesis, and PI3K–Akt–mTOR is the most frequently mutated pathway in cancer. PI3K/Akt inhibitors show synergy with HSV-MG18L71 and adenovirus ZD55-TRAIL166, but immunocomponents were not studied. mTOR is a master regulator of cellular translation and also impacts translation of viral proteins. Rapamycin is able to inhibit mTOR167,168 by forming a complex with FKBP12.141,169 This inhibits proliferation, which results in the induction of autophagy in cancer cells.130,170 T and B lymphocytes also show a decrease in cell function in the presence of rapamycin.171–175 Also, rapamycin exhibits significant antiangiogenesis and anticancer properties.133 Studies with an oncolytic HSV show that rapamycin enhances viral replication in vitro.134 A possible mechanism for this enhanced viral replication may be the reduction of cellular IFN, which has been seen with VSV/rapamycin in an in vivo glioma model.136 Studies with adenovirus have shown that rapamycin/everolimus can suppress the adenovirus innate response (TNF, IL-1β, IL-6, IL-8, IL-10, IL-12, and IFNδ) reduce T-cell infiltration and decrease anti-Ad antibody production and T-cell function.131,133 This suppression by rapamycin/everolimus of the host viral immune response may explain the improved efficacy of oncolytic HSV,134 VSV,136 adenovirus,131,135 and myxoma virus15 in a number of in vivo models.
Mitomycin C
Mitomycins are a group of antineoplastic antibiotics, of which mitomycin C (MMC) is the most studied. MMC is an alkylating agent that cross-links DNA and is produced by Streptomyces caespitosus. Apoptosis can be induced by MMC, either by the caspase 3- and 8-dependent Fas–FasL pathway or via the activity of the NFκB pathway.176 MMC has also been shown to maintain innate and adaptive immune responses in a major subpopulation of human blood DCs (slan DC). This has encouraged the design of clinical trials for tumor patients that are based on the simultaneous administration of tumor antigen-loaded DCs and MMC.119 Intracellular adhesion molecule-1 and decay accelerating factor, the viral entry receptors for Coxsackie virus A21, have been shown to be upregulated in the presence of MMC, leading to synergy between virus and drug.120 HSV-1,121–123 vaccinia,124 and adenovirus177 have all shown synergy with MMC, but these studies have not identified any immune-function mechanism.
Docetaxel
Docetaxel (Doc) has been shown to have a number of inhibitory functions on tumor cells, including inducing apoptosis, angiogenesis, and impeding gene-expression processes,178 but its primary anticancer function is via microtubule stabilization. Doc has been shown to decrease MDSCs and thus increase CD8+ T-cell activity in a murine model of breast cancer.179 Oncolytic adenovirus,76–79 reovirus,80,81 and HSV-182 have all shown synergy with Doc, but these studies have not identified any immune-function mechanism. Doc had no effect on the production of neutralizing antibodies to reovirus in a Phase I clinical trial.81
5-Fluorouracil
5-Fluorouracil (5-FU) is an antimetabolite drug that inhibits the enzyme thymidylate synthase and the incorporation of its metabolites into RNA and DNA.180 5-FU did not suppress the production of neutralizing antibodies against G207, but increased viral spread in subcutaneous hamster gallbladder tumors.105 Antitumor effects of 5-FU are mediated, at least in part, by its selective cytotoxic action on MDSCs.102 Exposure of colon and pancreatic cancer cells to 5-FU significantly antagonizes both wild-type HSV-1 replication and lytic oncolysis.164 In contrast, an HSV-1 mutant missing one copy of its ICP0, ICP4, and ICP34.5 gene (NV1066) resulted in enhanced viral replication.
Cisplatin
Cisplatin is a well-characterized alkylating agent used for the management of a wide range of cancers. As with other alkylating agents, its main mode of action is its ability to cross-link with the purine bases on the DNA. Cisplatin also interferes with DNA-repair mechanisms, which causes DNA damage, and subsequently induces apoptosis in tumor cells.181 Cisplatin can decrease Tregs and enhance antigen-specific CD8+ T-cell activity in murine models,106 and almost completely abrogate the inflammatory cytokine gene upregulation induced by reovirus.115 In contrast, a parvovirus–cisplatin combination induced higher cytokine release than either agent alone, and also resulted in pronounced DC maturation and cytotoxic T-cell activation.107
Discussion
OVs have been shown to be safely combined with conventional cytotoxic agents and evaluation in clinical trials justified on the basis of potential synergy through direct cytotoxicity, indirect immunogenicity, and/or alteration of the tumor microenvironment. The number of agents in clinical trials reflects the potential for this approach, which has recently focused away from delivery of live viruses to tumor sites, tumor lysis, and debulking to the induction of antitumor immunity through local induction of ICD, which ultimately will result in abscopal effects on distant metastases. Although not yet formally addressed in studies, there would most likely be low likelihood of cross-resistance to either treatment modality.
A number of human studies have already exploited this potential, as exemplified by the US Food and Drug Administration approval of the agent T-Vec for the treatment of malignant melanoma. Ongoing human studies are evaluating both DNA and RNA viruses and wild-type agents, as well as modified agents expressing immunostimulatory gene products. Combination with immune-checkpoint inhibitors has swiftly followed, with signals already of increased response rates compared to virus or checkpoint inhibitor alone.182 This follows evidence in a preclinical in vivo melanoma model, the oncolytic Newcastle disease virus, in combination with an anti-CTLA-4 antibody (ipilimumab), that showed enhanced tumor infiltration by activated CD8+ and CD4+ T cells and a reduction in Tregs.183 This model also showed a nearly 70% rate of cure with combination treatment compared to less than 25% for agents alone. Prolonged survival was also seen in the same in vivo melanoma model (B16.F10) when treated with a combination of anti PD-1 antibody and reovirus.184 It is most likely that in the near future, combination studies with OVs will focus largely on immune-checkpoint modulation, but this may be tempered in term of toxicities and high cost.
While the earliest combination studies of OVs with chemotherapeutic agents were focused on attenuation of the expected brisk neutralizing antiviral antibody response, there is huge potential for combinations based on the immunostimulatory effects of common cytotoxic agents. Recent studies have shown convincingly that many OVs can hitchhike on circulating blood cells, are protected from neutralizing antibodies, and reach tumor sites, so this end point of chemotherapy–OV combination is now being considered less important. A key factor that may allow combination studies to evolve is that almost all human OV studies have been associated with minimal toxicity, and actual dose-limiting toxicities rarely achieved. Therefore, patients will not be expected to face new and additional side effects and lower quality of life beyond the known chemotherapeutic agent-toxicity profile.
Historically, chemotherapy has been thought to prompt cancer cell death in an immunogenically silent way, but extensive studies have shown that such treatment can induce humoral and cellular antitumor immunity and break immune tolerance to tumors. The more subtle detail of this potential centers around the dose and sequencing of agents: CPA is myelosuppressive at conventional doses, but immunomodulatory as a single dose in combination with immunotherapy, or may be used to delete Tregs by metronomic dosing. Furthermore, combination studies will logically exploit the natural tropism of certain OVs for tumor vasculature with chemotherapeutic agents with antiangiogenic potential or those that may cause vascular leakage to allow OV into that tumor microenvironment.185
There is huge potential for the combination of OVs with chemotherapeutics, but success will entail careful selection of the OV, the tumor model, the molecular dysregulation harbored by the malignancy, and the transgenes the OV carries, together with the best dose and sequencing with the most appropriate cytotoxic. The ideal disease setting and virus is not clear as yet, and further challenges will be evaluation of response to combination therapy and the contribution of an OV added to a classical three-drug regimen in a common setting, such as advanced breast or gastrointestinal cancers. Our wealth of experience with single- and multiagent chemotherapy regimens at least allows us a head start with clinical translation of combinations with OVs.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Most RG, Currie AJ, Cleaver AL, et al. Cylophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8+ T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 5.Schiavoni A, Sistigu M, Valentini F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;77:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 6.Bracci L, Moschella F, Sestili P, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 7.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 8.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+CD25+ T regulatory cell function implicated in enhanced immune response by low dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 9.Taieb J, Chaput N, Schartz N, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 10.Kottke T, Chester J, Ilett E, et al. Precise scheduling of chemotherapy primes VEGF-producing tumors for successful systemic oncolytic virotherapy. Mol Ther. 2011;19:1802–1812. doi: 10.1038/mt.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerullo V, Diaconu I, Kangasniemi L, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–1746. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 13.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–7475. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zemp FJ, McKenzie BA, Lun X, et al. Cellular factors promoting resistance to effective treatment of glioma with oncolytic myxoma virus. Cancer Res. 2014;74:7260–7273. doi: 10.1158/0008-5472.CAN-14-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 17.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 19.Currier MA, Gillespie RA, Sawtell NM, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhar D, Spencer JF, Toth K, Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moschella F, Valentini M, Aricò E, et al. Unraveling cancer chemoimmunotherapy mechanisms by gene and protein expression profiling of responses to cyclophosphamide. Cancer Res. 2011;71:3528–3539. doi: 10.1158/0008-5472.CAN-10-4523. [DOI] [PubMed] [Google Scholar]
- 23.Peng KW, Myers R, Greenslade A, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20:255–261. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem ML, El-Naggar SA, Cole DJ. Cyclophosphamide induces bone marrow to yield higher numbers of precursor dendritic cells in vitro capable of functional antigen presentation to T cells in vivo. Cell Immunol. 2010;261:134–143. doi: 10.1016/j.cellimm.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2008;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakahara T, Uchi H, Lesokhin AM, et al. Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood. 2010;115:4384–4392. doi: 10.1182/blood-2009-11-251231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann E, Weibel S, Szalay AA. Combination treatment with oncolytic vaccinia virus and cyclophosphamide results in synergistic antitumor effects in human lung adenocarcinoma bearing mice. J Transl Med. 2014;12:197. doi: 10.1186/1479-5876-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 29.Lolkema MP, Arkenau HT, Harrington K, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–588. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 30.Gujar SA, Clements D, Dielschneider R, Helson E, Marcato P, Lee PW. Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms. Br J Cancer. 2014;110:83–93. doi: 10.1038/bjc.2013.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esaki S, Goshima F, Kimura H, Murakami S, Nishiyama Y. Enhanced antitumoral activity of oncolytic herpes simplex virus with gemcitabine using colorectal tumor models. Int J Cancer. 2013;132:1592–1601. doi: 10.1002/ijc.27823. [DOI] [PubMed] [Google Scholar]
- 32.Angelova AL, Aprahamian M, Grekova SP, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15:511–519. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 33.Angelova AL, Grekova SP, Heller A, et al. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J Virol. 2014;88:5263–5276. doi: 10.1128/JVI.03688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 35.Hastie E, Besmer DM, Shah NR, et al. Oncolytic vesicular stomatitis virus in an immunocompetent model of MUC1-positive or MUC1-null pancreatic ductal adenocarcinoma. J Virol. 2013;87:10283–10294. doi: 10.1128/JVI.01412-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wennier ST, Liu J, Li S, Rahman MM, Mona M, McFadden G. Myxoma virus sensitizes cancer cells to gemcitabine and is an effective oncolytic virotherapeutic in models of disseminated pancreatic cancer. Mol Ther. 2012;20:759–768. doi: 10.1038/mt.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sei S, Mussio JK, Yang QE, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitner S, Sweeney K, Oberg D, et al. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin Cancer Res. 2009;15:1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D, Kojima T, Ouchi M, et al. Preclinical evaluation of synergistic effect of telomerase-specific oncolytic virotherapy and gemcitabine for human lung cancer. Mol Cancer Ther. 2009;8:980–987. doi: 10.1158/1535-7163.MCT-08-0901. [DOI] [PubMed] [Google Scholar]
- 40.Onimaru M, Ohuchida K, Nagai E, et al. Combination with low-dose gemcitabine and hTERT-promoter-dependent conditionally replicative adenovirus enhances cytotoxicity through their crosstalk mechanisms in pancreatic cancer. Cancer Lett. 2010;294:178–186. doi: 10.1016/j.canlet.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharyya M, Francis J, Eddouadi A, Lemoine NR, Halldén G. An oncolytic adenovirus defective in pRb-binding (dl922-947) can efficiently eliminate pancreatic cancer cells and tumors in vivo in combination with 5-FU or gemcitabine. Cancer Gene Ther. 2011;18:734–743. doi: 10.1038/cgt.2011.45. [DOI] [PubMed] [Google Scholar]
- 42.Cherubini G, Kallin C, Mozetic A, et al. The oncolytic adenovirus Ad∆∆ enhances selective cancer cell killing in combination with DNA-damaging drugs in pancreatic cancer models. Gene Ther. 2011;18:1157–1165. doi: 10.1038/gt.2011.141. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Satoh M, Chen GP, Li DC, Hamada H, Arai Y. E1A, E1B double-restricted adenovirus enhances the cytotoxicity and antitumor activity of gemcitabine to renal cell carcinoma. Chin Med J (Engl) 2011;124:1082–1087. [PubMed] [Google Scholar]
- 44.Kangasniemi L, Parviainen S, Pisto T, et al. Effects of capsid-modified oncolytic adenoviruses and their combinations with gemcitabine or silica gel on pancreatic cancer. Int J Cancer. 2012;131:253–263. doi: 10.1002/ijc.26370. [DOI] [PubMed] [Google Scholar]
- 45.Yu YA, Galanis C, Woo Y, et al. Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Mol Cancer Ther. 2009;8:141–151. doi: 10.1158/1535-7163.MCT-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CL, Hsu YT, Wu CC, et al. Immune mechanism of the antitumor effects generated by bortezomib. J Immunol. 2012;189:3209–3220. doi: 10.4049/jimmunol.1103826. [DOI] [PubMed] [Google Scholar]
- 47.Boozari B, Mundt B, Woller N, et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut. 2010;59:1416–1426. doi: 10.1136/gut.2009.196519. [DOI] [PubMed] [Google Scholar]
- 48.Yarde DN, Nace RA, Russell SJ. Oncolytic vesicular stomatitis virus and bortezomib are antagonistic against myeloma cells in vitro but have additive anti-myeloma activity in vivo. Exp Hematol. 2013;41:1038–1049. doi: 10.1016/j.exphem.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carew JS, Espitia CM, Zhao W, et al. Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer. Cell Death Dis. 2013;4:e728. doi: 10.1038/cddis.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siurala M, Bramante S, Vassilev L, et al. Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumor activity against soft-tissue sarcoma. Int J Cancer. 2015;136:945–954. doi: 10.1002/ijc.29048. [DOI] [PubMed] [Google Scholar]
- 51.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer chemotherapy in mice. J Clin Invest. 2010;120:1111–1114. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sistgu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Q, Zhang W, Ning Z, et al. A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity. PLoS One. 2014;9:e93103. doi: 10.1371/journal.pone.0093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebeh H, Lehe C, Barhoush E, et al. Doxorubicin down-regulates cell surface B7-H1 expression and up-regulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12:R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolyard C, Yoo JY, Wang PY, et al. Doxorubicin synergizes with 34.5ENVE to enhance antitumor efficacy against metastatic ovarian cancer. Clin Cancer Res. 2014;20:6479–6494. doi: 10.1158/1078-0432.CCR-14-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiland T, Lampe J, Essmann F, et al. Enhanced killing of therapy-induced senescent tumor cells by oncolytic measles vaccine viruses. Int J Cancer. 2014;134:235–243. doi: 10.1002/ijc.28350. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz-Hernández E, Hess M, Melen GJ, et al. PEG-pHPMAm-based polymeric micelles loaded with doxorubicin prodrugs in combination antitumor therapy with oncolytic vaccinia viruses. Polym Chem. 2014:1674–1681. doi: 10.1039/C3PY01097J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skelding KA, Barry RD, Shafren DR. Enhanced oncolysis mediated by Coxsackievirus A21 in combination with doxorubicin hydrochloride. Invest New Drugs. 2012;30:568–581. doi: 10.1007/s10637-010-9614-0. [DOI] [PubMed] [Google Scholar]
- 59.Schache P, Gürlevik E, Strüver N, et al. VSV virotherapy improves chemotherapy by triggering apoptosis due to proteasomal degradation of Mcl-1. Gene Ther. 2009;16:849–861. doi: 10.1038/gt.2009.39. [DOI] [PubMed] [Google Scholar]
- 60.Michaud M. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 61.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 62.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 63.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 64.Obeid M. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 65.Workenhe S, Pol JG, Lichty BD, Cummings DT, Mossman KL. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immune tolerance and improves therapeutic efficacy. Cancer Immunol Res. 2013;1:1–11. doi: 10.1158/2326-6066.CIR-13-0059-T. [DOI] [PubMed] [Google Scholar]
- 66.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by antineoplastic agents in low non-cytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridolfi L, Petrini M, Granato AM, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. Transl Med. 2013;11:135. doi: 10.1186/1479-5876-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liikanen I, Ahtiainen L, Hirvinen ML, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 70.Yokoyama T, Iwado E, Kondo Y, et al. Autophagy-inducing agents augment the antitumor effect of telomerase-selve oncolytic adenovirus OBP-405 on glioblastoma cells. Gene Ther. 2008;15:1233–1239. doi: 10.1038/gt.2008.98. [DOI] [PubMed] [Google Scholar]
- 71.Kanai R, Rabkin SD, Yip S, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104:42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alonso MM, Gomez-Manzano C, Jiang H, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14:756–761. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- 73.Holzmüller R, Mantwill K, Haczek C, et al. YB-1 dependent virotherapy in combination with temozolomide as a multimodal therapy approach to eradicate malignant glioma. Int J Cancer. 2011;129:1265–1276. doi: 10.1002/ijc.25783. [DOI] [PubMed] [Google Scholar]
- 74.Kostova Y, Mantwill K, Holm PS, Anton M. An armed, YB-1-dependent oncolytic adenovirus as a candidate for a combinatorial anti-glioma approach of virotherapy, suicide gene therapy and chemotherapeutic treatment. Cancer Gene Ther. 2015;22:30–43. doi: 10.1038/cgt.2014.67. [DOI] [PubMed] [Google Scholar]
- 75.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miranda E, Pineda HM, Oberg D, et al. Adenovirus-mediated sensitization to the cytotoxic drugs docetaxel and mitoxantrone is dependent on regulatory domains in the E1ACR1 gene-region. PLoS One. 2012;7:e46617. doi: 10.1371/journal.pone.0046617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radhakrishnan S, Miranda E, Ekblad M, et al. Efficacy of oncolytic mutants targeting pRb and p53 pathways is synergistically enhanced when combined with cytotoxic drugs in prostate cancer cells and tumor xenografts. Hum Gene Ther. 2010;21:1311–1325. doi: 10.1089/hum.2010.019. [DOI] [PubMed] [Google Scholar]
- 78.Mantwill K, Köhler-Vargas N, Bernshausen A, et al. Inhibition of the multidrug-resistant phenotype by targeting YB-1 with a conditionally oncolytic adenovirus: implications for combinatorial treatment regimen with chemotherapeutic agents. Cancer Res. 2006;66:7195–7202. doi: 10.1158/0008-5472.CAN-05-2339. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Ramesh N, Chen Y, et al. Identification of human uroplakin II promoter and its use in the construction of CG8840, a urothelium-specific adenovirus variant that eliminates established bladder tumors in combination with docetaxel. Cancer Res. 2002;62:3743–3750. [PubMed] [Google Scholar]
- 80.Heinemann L, Simpson GR, Boxall A, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Comins C, Spicer J, Protheroe A, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–5572. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–560. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer chemotherapy in mice. J Clin Invest. 2010;120:1111–1114. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Luo J, Shurin MR. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr) 2011;116:222–233. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Dermawan K, Jin M, et al. Differential impairment of regulatory T cells rather than effector cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129:219–229. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 86.Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 87.Correale P, Cusi MG, Tsang KY, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 88.Huang B, Sikorski R, Kirn DH, Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2011;18:164–172. doi: 10.1038/gt.2010.121. [DOI] [PubMed] [Google Scholar]
- 89.Karapanagiotou EM, Roulstone V, Twigger K, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimoyama S, Goshima F, Teshigahara O, et al. Enhanced efficacy of herpes simplex virus mutant HF10 combined with paclitaxel in peritoneal cancer dissemination models. Hepatogastroenterology. 2007;54:1038–1042. [PubMed] [Google Scholar]
- 91.Lin SF, Gao SP, Price DL, et al. Synergy of a herpes oncolytic virus and paclitaxel for anaplastic thyroid cancer. Clin Cancer Res. 2008;14:1519–1528. doi: 10.1158/1078-0432.CCR-07-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng WG, Li JJ, Hu P, Lei L, Wang JN, Liu RB. An oncolytic herpes simplex virus vector, G47∆, synergizes with paclitaxel in the treatment of breast cancer. Oncol Rep. 2013;29:2355–2361. doi: 10.3892/or.2013.2359. [DOI] [PubMed] [Google Scholar]
- 93.Zeng W, Hu P, Wu J, et al. The oncolytic herpes simplex virus vector G47∆ effectively targets breast cancer stem cells. Oncol Rep. 2013;29:1108–1114. doi: 10.3892/or.2012.2211. [DOI] [PubMed] [Google Scholar]
- 94.Roulstone V, Twigger K, Zaidi S, et al. Synergistic cytotoxicity of oncolytic reovirus in combination with cisplatin-paclitaxel doublet chemotherapy. Gene Ther. 2013;20:521–528. doi: 10.1038/gt.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheong SC, Wang Y, Meng JH, et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rognoni E, Widmaier M, Haczek C, et al. Adenovirus-based virotherapy enabled by cellular YB-1 expression in vitro and in vivo. Cancer Gene Ther. 2009;16:753–763. doi: 10.1038/cgt.2009.20. [DOI] [PubMed] [Google Scholar]
- 97.Ingemarsdotter CK, Baird SK, Connell CM, Öberg D, Halldén G, McNeish IA. Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene. 2010;29:6051–6063. doi: 10.1038/onc.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rein DT, Volkmer A, Bauerschmitz G, et al. Combination of a MDR1-targeted replicative adenovirus and chemotherapy for the therapy of pretreated ovarian cancer. J Cancer Res Clin Oncol. 2012;138:603–610. doi: 10.1007/s00432-011-1135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng JC, Granot T, DiGiacomo V, Levin B, Meruelo D. Enhanced specific delivery and targeting of oncolytic Sindbis viral vectors by modulating vascular leakiness in tumor. Cancer Gene Ther. 2010;17:244–255. doi: 10.1038/cgt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang S, Haluska FG. Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fas-mediated pathways. J Immunol. 2004;172:4599–4608. doi: 10.4049/jimmunol.172.7.4599. [DOI] [PubMed] [Google Scholar]
- 101.Correale P, Aquino A, Giuliani A, et al. Treatment of colon and breast carcinoma cells with 5-fluorouracil enhances expression of carcinoembryonic antigen and susceptibility to HLA-A*02/01-restricted, CEA-peptide specific cytotoxic T cells in vitro. Int J Cancer. 2003;104:437–445. doi: 10.1002/ijc.10969. [DOI] [PubMed] [Google Scholar]
- 102.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 103.Sagawa T, Yamada Y, Takahashi M, et al. Treatment of hepatocellular carcinoma by AdAFPep/rep, AdAFPep/p53, and 5-fluorouracil in mice. Hepatology. 2008;48:828–840. doi: 10.1002/hep.22420. [DOI] [PubMed] [Google Scholar]
- 104.Ma G, Kawamura K, Li Q, et al. Combinatory cytotoxic effects produced by E1B-55kDa-deleted adenoviruses and chemotherapeutic agents are dependent on the agents in esophageal carcinoma. Cancer Gene Ther. 2010;17:803–813. doi: 10.1038/cgt.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakano K, Todo T, Zhao G, et al. Enhanced efficacy of conditionally replicating herpes simplex virus (G207) combined with 5-fluorouracil and surgical resection in peritoneal cancer dissemination models. J Gene Med. 2005;7:638–648. doi: 10.1002/jgm.700. [DOI] [PubMed] [Google Scholar]
- 106.Tseng CW, Hung CF, Alvarez RD, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moehler M, Sieben M, Roth S, et al. Activation of the human immune system by chemotherapeutic or targeted agents combined with the oncolytic parvovirus H-1. BMC Cancer. 2011;11:464. doi: 10.1186/1471-2407-11-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon AR, Kim JH, Lee YS, et al. Markedly enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in combination with cisplatin. Hum Gene Ther. 2006;17:379–390. doi: 10.1089/hum.2006.17.379. [DOI] [PubMed] [Google Scholar]
- 109.Takakura M, Nakamura M, Kyo S, et al. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010;17:11–19. doi: 10.1038/cgt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang S, Readinger JA, DuBois W, et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. 2011;117:1228–1238. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.You L, Wang Y, Jin Y, Qian W. Downregulation of Mcl-1 synergizes the apoptotic response to combined treatment with cisplatin and a novel fiber chimeric oncolytic adenovirus. Oncol Rep. 2012;27:971–978. doi: 10.3892/or.2012.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma B, Wang Y, Zhou X, et al. Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol. 2015;141:419–429. doi: 10.1007/s00432-014-1835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ganesh S, Gonzalez-Edick M, Gibbons D, et al. Combination therapy with radiation or cisplatin enhances the potency of Ad5/35 chimeric oncolytic adenovirus in a preclinical model of head and neck cancer. Cancer Gene Ther. 2009;16:383–392. doi: 10.1038/cgt.2008.90. [DOI] [PubMed] [Google Scholar]
- 114.Hingorani P, Zhang W, Lin J, Liu L, Guha C, Kolb EA. Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts. Cancer. 2011;117:1764–1774. doi: 10.1002/cncr.25741. [DOI] [PubMed] [Google Scholar]
- 115.Pandha HS, Heinemann L, Simpson GR, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–6166. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 116.Sung CK, Choi B, Wanna G, Genden EM, Woo SL, Shin EJ. Combined VSV oncolytic virus and chemotherapy for squamous cell carcinoma. Laryngoscope. 2008;118:237–242. doi: 10.1097/MLG.0b013e3181581977. [DOI] [PubMed] [Google Scholar]
- 117.Mace AT, Harrow SJ, Ganly I, Brown SM. Cytotoxic effects of the oncolytic herpes simplex virus HSV1716 alone and in combination with cisplatin in head and neck squamous cell carcinoma. Acta Otolaryngol. 2007;127:880–887. doi: 10.1080/00016480601075381. [DOI] [PubMed] [Google Scholar]
- 118.Adusumilli PS, Chan MK, Chun YS, et al. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol Ther. 2006;5:48–53. doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wehner R, Bitterlich A, Meyer N, et al. Impact of chemotherapeutic agents on the immunostimulatory properties of human 6-sulfo LacNAc+ (slan) dendritic cells. Int J Cancer. 2013;132:1351–1359. doi: 10.1002/ijc.27786. [DOI] [PubMed] [Google Scholar]
- 120.Annels NE, Simpson G, Arif M, et al. Oncolytic immunotherapy for the treatment of non-muscle invasive bladder cancer using intravesical coxsackievirus A2. Journal for ImmunoTherapy of Cancer. 2015;3(2):331. [Google Scholar]
- 121.Bennett JJ, Adusumilli P, Petrowsky H, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a γ134.5 deleted oncolytic herpes virus (G207) FASEB J. 2004;18:1001–1003. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 122.Mullerad M, Bochner BH, Adusumilli PS, et al. Herpes simplex virus based gene therapy enhances the efficacy of mitomycin C for the treatment of human bladder transitional cell carcinoma. J Urol. 2005;174:741–746. doi: 10.1097/01.ju.0000164730.38431.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simpson GR, Horvath A, Annels NE, et al. Combination of a fusogenic glycoprotein, pro-drug activation and oncolytic HSV as an intravesical therapy for superficial bladder cancer. Br J Cancer. 2012;106:496–507. doi: 10.1038/bjc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sturm JB, Hess M, Weibel S, et al. Functional hyper-IL-6 from vaccinia virus-colonized tumors triggers platelet formation and helps to alleviate toxicity of mitomycin C enhanced virus therapy. J Transl Med. 2012;10:9. doi: 10.1186/1479-5876-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maeda K, Hazama S, Tokuno K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011;31:4569–4574. [PubMed] [Google Scholar]
- 126.Granot T, Meruelo D. The role of natural killer cells in combinatorial anti-cancer therapy using Sindbis viral vectors and irinotecan. Cancer Gene Ther. 2012;19:588–591. doi: 10.1038/cgt.2012.33. [DOI] [PubMed] [Google Scholar]
- 127.Tyminski E, Lerpy S, Terada K, et al. Brain tumor oncolysis with replication conditional herpes simplex type 1 expressing the prodrug activating genes CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res. 2005;65:6850–6857. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- 128.Maitra R, Seetharam R, Tesfa L, et al. Oncolytic reovirus preferentially induces apoptosis in KRAS mutant colorectal cancer cells, and synergizes with irinotecan. Oncotarget. 2014;5:2807–2819. doi: 10.18632/oncotarget.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ottolino-Perry K, Acuna SA, Angarita FA, et al. Oncolytic vaccinia virus synergizes with irinotecan in colorectal cancer. Mol Oncol. 2015;9:1539–1552. doi: 10.1016/j.molonc.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang ZK, Johnson M, Moughon DL, Kuo J, Sato M, Wu L. Rapamycin enhances adenovirus-mediated cancer imaging and therapy in pre-immunized murine hosts. PLoS One. 2013;8:e73650. doi: 10.1371/journal.pone.0073650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lun X, Alain T, Zemp FJ, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70(2):598–608. doi: 10.1158/0008-5472.CAN-09-1510. [DOI] [PubMed] [Google Scholar]
- 133.Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65:6882–6890. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- 134.Fu X, Tao L, Rivera A, Zhang X. Rapamycin enhances the activity of oncolytic herpes simplex virus against tumor cells that are resistant to virus replication. Int J Cancer. 2011;129:1503–1510. doi: 10.1002/ijc.25808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lukashev AN, Fuerer C, Chen MJ, Searle P, Iggo R. Late expression of nitroreductase in an oncolytic adenovirus sensitizes colon cancer cells to the prodrug CB1954. Hum Gene Ther. 2005;16:1473–1483. doi: 10.1089/hum.2005.16.1473. [DOI] [PubMed] [Google Scholar]
- 136.Alain T, Lun X, Martineau Y, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci U S A. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother. 2009;58:589–601. doi: 10.1007/s00262-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Touchefeu Y, Franken P, Harrington KJ. Radiovirotherapy: principles and prospects in oncology. Curr Pharm Des. 2012;18:3313–3320. doi: 10.2174/1381612811209023313. [DOI] [PubMed] [Google Scholar]
- 139.Appleton ES, Turnbull S, Ralph C, et al. Talimogene laherparepvec in the treatment of melanoma. Expert Opin Biol Ther. 2015;15(10):1517–1530. doi: 10.1517/14712598.2015.1084280. [DOI] [PubMed] [Google Scholar]
- 140.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 141.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 142.Cordaro TA, de Visser KE, Tirion FH, et al. Tumor size at the time of adoptive transfer determines whether tumor rejection occurs. Eur J Immunol. 2000;30:1297–1307. doi: 10.1002/(SICI)1521-4141(200005)30:5<1297::AID-IMMU1297>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 143.Bilbao R, Bustos M, Alzuguren P, et al. A blood-tumor barrier limits gene transfer to experimental liver cancer: the effect of vasoactive compounds. Gene Ther. 2000;7:1824–1832. doi: 10.1038/sj.gt.3301312. [DOI] [PubMed] [Google Scholar]
- 144.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 145.Li ZY, Ni S, Yang X, Kiviat N, Lieber A. Xenograft models for liver metastasis: relationship between tumor morphology and adenovirus vector transduction. Mol Ther. 2004;9:650–657. doi: 10.1016/j.ymthe.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 146.Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Woller N, Gurlevik E, Ureche CI, Schumacher A, Kuhnel F. Oncolytic viruses as cancer vaccines. Front Oncol. 2014;4:188. doi: 10.3389/fonc.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 150.Willmon C, Diaz RM, Wongthida P, et al. Vesicular stomatitis virus-induced immune suppressor cells generate antagonism between intra-tumoral oncolytic virus and cyclophosphamide. Mol Ther. 2011;19:140–149. doi: 10.1038/mt.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roulstone V, Khan K, Pandha HS, et al. Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors. Clin Cancer Res. 2015;21:1305–1312. doi: 10.1158/1078-0432.CCR-14-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Burke MJ, Ahern C, Weigel BJ, et al. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62:743–750. doi: 10.1002/pbc.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 154.Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 155.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 156.Milani M, Rzymski T, Mellor HR, et al. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with bortezomib. Cancer Res. 2009;60:4415–4423. doi: 10.1158/0008-5472.CAN-08-2839. [DOI] [PubMed] [Google Scholar]
- 157.Yoo JY, Hurwitz BS, Bolyard C, et al. Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects. Clin Cancer Res. 2014;20:3787–3798. doi: 10.1158/1078-0432.CCR-14-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dunn CJ, Goa KL. Mitoxantrone: a review of its pharmacological properties and use in acute nonlymphoblastic leukaemia. Drugs Aging. 1996;9:122–147. doi: 10.2165/00002512-199609020-00007. [DOI] [PubMed] [Google Scholar]
- 159.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 160.Crespi MD, Ivanier SE, Genovese J, Baldi A. Mitoxantrone affects topoisomerase activities in human breast cancer cells. Biochem Biophys Res Commun. 1986;136:521–528. doi: 10.1016/0006-291x(86)90471-7. [DOI] [PubMed] [Google Scholar]
- 161.Wiseman LR, Spencer CM. Mitoxantrone: a review of its pharmacology and clinical efficacy in the management of hormone-resistant advanced prostate cancer. Drugs Aging. 1997;10:473–485. doi: 10.2165/00002512-199710060-00007. [DOI] [PubMed] [Google Scholar]
- 162.Cocco E, Marrosu MG. The current role of mitoxantrone in the treatment of multiple sclerosis. Expert Rev Neurother. 2014;14(6):607–616. doi: 10.1586/14737175.2014.915742. [DOI] [PubMed] [Google Scholar]
- 163.Pol J, Vacchelli E, Aranda F, et al. Trial watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kulu Y, Kawasaki H, Donahue JM, et al. Concurrent chemotherapy inhibits herpes simplex virus-1 replication and oncolysis. Cancer Gene Ther. 2013;20:133–140. doi: 10.1038/cgt.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wei W, Chen X, Ma X, Wang D, Guo Z. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: a systematic review with meta-analysis. J Neurooncol. 2015;125(2):339–349. doi: 10.1007/s11060-015-1920-0. [DOI] [PubMed] [Google Scholar]
- 166.Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876. doi: 10.1038/cddis.2013.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tong Y, Zhu W, Huang X, et al. PI3K inhibitor LY294002 inhibits activation of the Akt/mTOR pathway induced by an oncolytic adenovirus expressing TRAIL and sensitizes multiple myeloma cells to the oncolytic virus. Oncol Rep. 2014;31:1581–1588. doi: 10.3892/or.2014.3020. [DOI] [PubMed] [Google Scholar]
- 168.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 169.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 170.Takeuchi H, Kondo Y, Fujiwara K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 171.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 172.Aagaard-Tillery KM, Jelinek DF. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol. 1994;156:493–507. doi: 10.1006/cimm.1994.1193. [DOI] [PubMed] [Google Scholar]
- 173.Hleb M, Murphy S, Wagner EF, et al. Evidence for cyclin D3 as a novel target of rapamycin in human T lymphocytes. J Biol Chem. 2004;279:31948–31955. doi: 10.1074/jbc.M400638200. [DOI] [PubMed] [Google Scholar]
- 174.Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 2004;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 175.Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from anti-tumor efficacy. Transplantation. 2002;73:1565–1572. doi: 10.1097/00007890-200205270-00008. [DOI] [PubMed] [Google Scholar]
- 176.Kang YH, Lee KA, Ryu CJ, et al. Mitomycin C induces apoptosis via Fas/FasL dependent pathway and suppression of IL-18 in cervical carcinoma cells. Cancer Lett. 2006;237:33–44. doi: 10.1016/j.canlet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 177.Zhao GZ, Tan WL, Zheng SB, Wu YD, Xie Y, Zhu WH. Cytotoxic effect of oncolytic virus combined with mitomycin against human bladder cancer cells in vitro and in vivo. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1623–1625. 1628. Chinese. [PubMed] [Google Scholar]
- 178.Herbst RS, Khuri FR. Mode of action of docetaxel – a basis for combination with novel anticancer agents. Cancer Treat Rev. 2003;29:407–415. doi: 10.1016/s0305-7372(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 179.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 181.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy. 2015;7:611–619. doi: 10.2217/imt.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rajani K, Parrish C, Kottke T, et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther. 2015 Aug 27; doi: 10.1038/mt.2015.156. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]