Abstract
Opioid abuse and dependence have evolved into an international epidemic as a significant clinical and social problem with devastating consequences. Repeated exposure to the opioid, for example morphine, can induce profound, long-lasting behavioral sensitization and physical dependence, which are thought to reflect neuroplasticity in neural circuitry. Central serotonin (5-HT) neurotransmission participates in the development of dependence on and the expression of withdrawal from morphine. Serotonin 5-HT2C receptor (5-HT2CR) agonists suppress psychostimulant nicotine or cocaine-induced behavioral sensitization and drug-seeking behavior; however, the impact of 5-HT2CR agonists on behaviors relevant to opioid abuse and dependence has not been reported. In the present study, the effects of 5-HT2CR activation on the behavioral sensitization and naloxone-precipitated withdrawal symptoms were examined in mice underwent repeated exposure to morphine. Male mice received morphine (10 mg/kg, s.c.) to develop behavioral sensitization. Lorcaserin, a 5-HT2CR agonist, prevented the induction and expression, but not the development, of morphine-induced behavioral sensitization. Another cohort of mice received increasing doses of morphine over a 7-day period to induce morphine-dependence. Pretreatment of lorcaserin, or the positive control clonidine (an alpha 2-adrenoceptor agonist), ameliorated the naloxone-precipitated withdrawal symptoms. SB 242084, a selective 5-HT2CR antagonist, prevented the lorcaserin-mediated suppression of behavioral sensitization and withdrawal. Chronic morphine treatment was associated with an increase in the expression of 5-HT2CR protein in the ventral tegmental area, locus coeruleus and nucleus accumbens. These findings suggest that 5-HT2CR can modulate behavioral sensitization and withdrawal in morphine-dependent mice, and the activation of 5-HT2CR may represent a new avenue for the treatment of opioid addiction.
Keywords: Serotonin, 5-HT2C receptor, Morphine, Behavioral sensitization, Withdrawal, Ventral tegmental area, Nucleus accumbens, Locus coeruleus
1. Introduction
The off-prescription use of pharmaceutical opioids, or the abuse of illicitly obtained heroin, has evolved into an international epidemic of opioid abuse and dependence, which represents a significant clinical and societal problem with devastating consequences (Cicero et al., 2005). In 2011, 4.2 million Americans aged 12 or older (or 1.6 percent of the population) had used heroin at least once. It is estimated that about 23 percent of individuals who use heroin become dependent (NIH, 2014). Repeated exposure to an opioid can induce a profound long-lasting behavioral sensitization, which reflects neuroplasticity associated with exposure to the addictive drug. Persistent physical and psychological dependence is another characteristic of opioid addiction which is considered to play a pivotal role in the reinstatement of drug-seeking behavior after a period of abstinence (Robinson and Berridge, 1993).
Repeated exposure to an opioid, such as morphine, results in a progressive and enduring enhancement in the drug’s motor stimulant effects elicited by a subsequent drug challenge. This phenomenon, termed behavioral sensitization, is thought to underlie certain biological aspects of drug addiction. Behavioral sensitization is the consequence of drug-induced neuroadaptive changes in a circuit involving dopaminergic and glutamatergic interconnections between the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC) and amygdala (Vanderschuren and Kalivas, 2000). There are three distinct, operationally defined phases of behavioral sensitization: induction, development and expression. Induction refers to the immediate neurophysiological consequences that are associated with the administration of the drug. Development refers to a protracted alteration in gene expression and neuroplasticity within the reward circuit. Expression is the behavioral reflection of the long-term consequences of the initial drug-induced neuroplastic changes within the reward circuitry (Kalivas and Stewart, 1991). The VTA is implicated as being critical for the induction phase, also referred to as initiation, while the NAc is considered to be a critical site for the expression phase (Steketee and Kalivas, 2011). The persistence of physical dependence is another characteristic of opioid addiction. It is generally accepted that behavioral sensitization and physical dependence contribute to relapse, compulsive drug-seeking and drug-taking phenotypes (Robinson and Berridge, 1993). Thus, there is a significant medical and societal need for elucidation of mechanisms underlying behavioral sensitization and the development of effective pharmacotherapies for opioid addiction.
Central serotonin (5-HT) neurotransmission participates in the development of dependence on and the expression of withdrawal from morphine (el-Kadi and Sharif, 1995). Accumulating evidence demonstrates that 5-HT2C receptor (5-HT2CR), a G protein-coupled receptor, plays a critical role in drug-seeking and drug-taking phenotypes in rodents (Devroye et al., 2013; Filip et al., 2012; Zaniewska et al., 2007; Zaniewska et al., 2010a). Stimulation of the 5-HT2CR increases intracellular Ca2+ level, and extracellular-signal-regulated kinase (ERK) phosphorylation via a Gαq-PLC signaling pathway (Di Giovanni, 2011). Histological studies have revealed that 5-HT2CRs are widely expressed in mesolimbocortical regions related to drug addiction, for example VTA, NAc, locus coeruleus (LC), and PFC. In rodents, pharmacological activation of 5-HT2CR with lorcaserin or Ro60-0175 suppresses impulsive food intake (Fletcher et al., 2007; Thomsen et al., 2008), nicotine self-administration (Higgins et al., 2012), cocaine self-administration (Fletcher et al., 2002), and the reinstatement of cocaine-seeking after a period of abstinence (Fletcher et al., 2008). 5-HT2CR activation suppresses the expression of nicotine-induced behavioral sensitization (Zaniewska et al., 2009; Zaniewska et al., 2010a) and depression-like behavior during nicotine withdrawal (Zaniewska et al., 2010b). To date there has been little attention paid to the participation of 5-HT2CR in morphine-induced behavioral sensitization, physical dependence and withdrawal phenotypes.
In the present study we investigated the effect of 5-HT2CR activation on behavioral sensitization and withdrawal symptoms in morphine-dependent mice and further explored the effects of chronic morphine exposure on 5-HT2CR expression in the VTA, NAc and LC. Our results indicate that a selective 5-HT2CR agonist suppresses behavioral sensitization and withdrawal in morphine-dependent mice.
2. Materials and Methods
2.1. Animals
Adult male Kunming mice weighing 22 ± 2 g were obtained from the Experimental Animal Center of Anhui Medical University (Hefei, China) and housed in groups of four per standard polycarbonate cage with ad libitum access to food and water. Mouse cages were maintained in a temperature- and humidity-controlled colony room with 12-h/12-h light/dark cycle with lights on at 7:00 AM. Mice were acclimated to the laboratory housing condition for one week prior to experiments. All procedures were conducted in accordance with the guidelines as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Anhui Medical University.
2.2. Chemicals and reagents
Morphine hydrochloride was obtained from The First Pharmaceutical Co. Ltd of Shenyang (Shenyang, China), naloxone hydrochloride was purchased from Kangze Pharmaceutical Co. Ltd (Hunan, China), clonidine came from Yuanyang Pharmaceutical Company (Yuanyang, China), lorcaserin, the 5-HT2CR agonist, was purchased from Hebei Pharmaceutical Company (Hebei, China), and SB 242084, a 5-HT2CR antagonist, was purchased from Tocris Bioscience (Minneapolis, MN). SB 242084 was dissolved in sterile saline containing 1% DMSO. All other compounds were dissolved in 0.9% sterile saline. The drug concentration was adjusted to an injection volume of 10 ml/kg body weight. The primary antibody to 5-HT2CR was purchased from Santa Cruz Biotechnology (sc-17797, Dallas, TX). The secondary HRP-conjugated anti-mouse IgG antibody for the Western blot (WB) assay was purchased from Beijing Zhong-Shan Biotechnology Co., Ltd (Beijing, China). Enhanced Bicinchoninic acid (BCA) protein assay kit was purchased from Beyotim Institute of Biotechnology (Haimen, China).
2.3. Morphine-induced behavioral sensitization
Kunming mice were used in this study to study the effect of 5-HT2CR on morphine-induced behavioral changes. Kunming mice are the most widely used outbred colony of mice in China. We have determined that there is no significant difference in morphine-induced behavioral sensitization and withdrawal between Kunming and C57BL/6 mice (Wu and Zhang, manuscript in preparation). Morphine (10 mg/kg) was injected subcutaneously twice a day (8:00 AM and 7:00 PM) for 3 days and then drug treatment was suspended for 5 days. On day 9, a challenge dose of morphine (10 mg/kg) was given and the locomotor activity of the mice was measured for 60 min (Li et al., 2008).
To test the influence of 5-HT2CR activation on the induction of morphine behavioral sensitization, lorcaserin was administered in combination with the twice-daily morphine regimen for 3 days (with lorcaserin given 15 min prior to the morphine injection). To test the influence of lorcaserin on the development of behavioral sensitization, the 5-HT2CR agonist was administered daily for 5 days during the period in which morphine treatment was suspended. To test the influence of lorcaserin on the expression of behavioral sensitization, the 5-HT2CR agonist was administered 15 min prior to the morphine challenge. All mice received the challenge dose of morphine (10 mg/kg) before the locomotor activity test on day 9. To confirm that the effect of lorcaserin on expression of behavioral sensitization was 5-HT2CR-dependent, SB 242084 (1.0 mg/kg, i.p.) was administered 20 min before lorcaserin treatment on day 9 to examine the morphine-induced behavioral change.
2.4. Induction of morphine dependence and withdrawal
Using a previously described protocol (el-Kadi and Sharif, 1995), morphine dependence in mice was achieved by administering morphine subcutaneously 3 times each day starting at a dose of 5 mg/kg and the dose was increased each day until a dose of 100 mg/kg was reached by day 6. On day 7, the mice received 100 mg/kg morphine treatment at 8:00 AM and 2:00 PM (see Table 1). After 2.5 h, the mice received clonidine (0.2 mg/kg, i.p.) or lorcaserin (0.5, 0.75 and 1.0 mg/kg, i.p.) treatment followed by naloxone (5.0 mg/kg, i.p.) 30 min later to precipitate morphine withdrawal. Mice were placed individually into clear square observation chambers (30 cm by 30 cm by 35 cm tall) with bedding at the bottom, and closely observed for 30 min. To test the dependence of the 5-HT2CR on lorcaserin’s effects on morphine withdrawal symptoms, SB 242084 (1.0 mg/kg, i.p.) was administered 20 min prior to lorcaserin treatment.
Table 1.
Induction of morphine dependence
| Day | Morphine (mg/kg, s.c.)
|
||
|---|---|---|---|
| 8:00 AM | 2:00 PM | 8:00 PM | |
| 1 | 5 | 5 | 5 |
| 2 | 10 | 10 | 10 |
| 3 | 20 | 20 | 20 |
| 4 | 40 | 40 | 40 |
| 5 | 80 | 80 | 80 |
| 6 | 100 | 100 | 100 |
| 7 | 100 | 100 | — |
2.5. Behavioral assessment
Behavioral assessment was conducted with the PhenoTyper system (Noldus Information Technology, Wageningen, The Netherlands). The mouse was placed in the center of an arena with clear walls and a black floor. Before each trial, the arena floor and walls were thoroughly cleaned with a 10% ethanol solution to reduce olfactory cues. Locomotor activity and other behaviors were tracked via an overhead video camera interfaced with behavioral tracking software EthoVision XT 5.1. The EthoVision software detected the mouse by static subtraction mode (in this case a white mouse on a black background) in each video frame (29.97 fps) and tracked the movement of the digitized pixels that represent the “center point” of the mouse. Two dependent measures, distance traveled and velocity, were used to assess locomotor behavior. Distance traveled is defined as the recorded movement of the mouse’s center point in cm over the duration of the trial. The withdrawal symptoms quantified included jumping (all feet off the floor), burrowing (escape digging), body grooming, rearing (front feet off the floor), wet dog shakes (whole body shakes), head shakes, digging body, paw licking, face grooming, penile grooming, scratch and extend. The scores of these behaviors (counts/30 min) were recorded for each mouse. The body weight of each mouse was measured before the administration of the vehicle or test drug, before the injection of naloxone, and again 30 min after naloxone injection.
2.6. Western blot
Mice received the twice-daily morphine regimen for 3 days, followed by a 5-day abstinence period to induce behavioral sensitization, or the escalating morphine treatment schedule to induce dependence as described above. To examine the influence of induction of behavioral sensitization on 5-HT2CR expression, brain tissues were rapidly harvested on day 9 and stored at −80° C. To examine the influence of morphine dependence on 5-HT2CR expression, brain tissues were rapidly harvested from the mice on the escalating morphine treatment schedule on day 7 and then stored at −80 °C before processing. Tissue samples obtained from the VTA, NAc and LC regions were homogenized in ice-cold RIPA lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Trition X-100, 1% sodium deoxycholate, 0.1% SDS and 1 mM PMSF. The brain regions from at least 3 mice were pooled together to obtain a sufficient amount of protein required for WB. Samples were kept in ice for 30 min before centrifuging at 15,000×g at 4° C for 10 min. The supernatant was collected as total proteins and the protein concentration of each supernatant sample was determined using a BCA protein assay kit. Equal amounts of protein samples (30 μg) were separated by 12% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% v/v tween-20 (TBST) for 1 h at room temperature, the membrane loaded with protein samples was incubated with the primary antibody (1:100) at 4° C overnight. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-mouse; 1:10,000) at room temperature for 2 h to identify primary antibody. After triple TBST washes, bands were detected by an enhanced chemiluminescent substrate (Thermo Fisher Scientific Inc. Waltham, MA). The Bioshine ChemiQ4600 imaging system (Shanghai Bioshine Scientific instrument Co., Ltd, Shanghai, China) was used to visualize protein bands, and densitometry was performed with ImageJ software. 5-HT2CR densitometry values were normalized with respect to β-actin immunoreactivity in the same lane to correct for any loading and transfer differences among samples. There were 3–4 trials for each group.
2.7. Statistical analysis
Data were expressed as mean ± S.E.M. Statistically significant differences in distance traveled, velocity and behavioral parameters were determined by one-way or two-way analysis of variance (ANOVA). If significance was found, post hoc Bonferroni or Dunnett’s multiple comparison was used. Student’s t-test (for independent samples) was used to analyze the different between two groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Activation of 5-HT2CR inhibits the induction and expression of morphine-induced behavioral sensitization
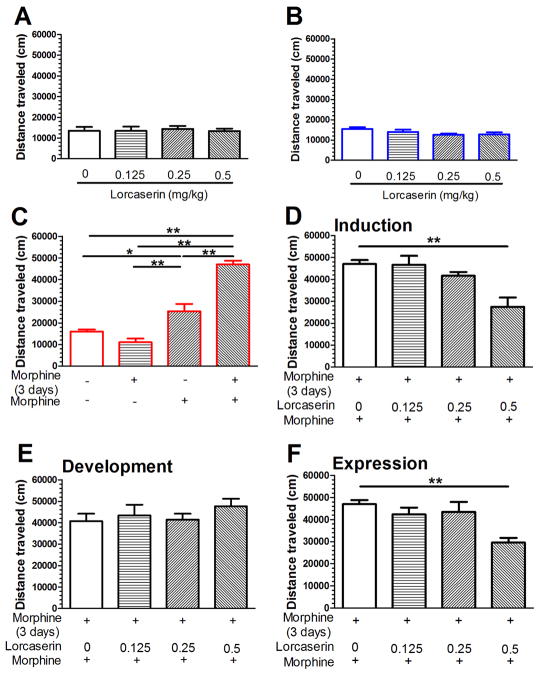
Forty mice were divided randomly into 4 groups (n = 10 for each group). There was no difference in horizontal distance traveled among the groups before drug treatment (F(3,36) = 0.08; n.s., see Fig. 1A). Lorcaserin (0, 0.125, 0.25 and 0.5 mg/kg, i.p.) treatment did not alter the horizontal distance traveled (F(3,36) = 1.99; n.s., see Fig. 1B) during the 60 min peroid immediately after the injection. Morphine (10 mg/kg, s.c.) was administered twice a day (8:00 AM and 7:00 PM) for 3 days and then morphine treatment was suspended for 5 days. On day 9, the challenge dose of morphine (10 mg/kg, s.c.) was administered and locomotor activity was measured for 60 min (see Fig. 1C). A two-way ANOVA on distance traveled revealed a significant main effect on morphine treatment on days 1–3 (F(1,35) = 14.84, P < 0.01), a significant main effect on morphine treatment on day 9 (F(1,35) = 111.77, P < 0.01), and a significant interaction between days 1–3 and day 9 morphine treatment (F(1,35) = 38.26, P < 0.01). As compared to the saline control, repeated morphine treatment on days 1–3 did not alter distance traveled on day 9 (n.s.); however, the morphine challenge on day 9 significantly increased the distance traveled (P < 0.01). Morphine challenge on day 9 to mice treated with repeated saline on days 1–3 produced a significant increase in distance traveled as compared to mice given the saline challenge on day 9, but was lower than those given morphine on days 1–3. These results indicate that the repeated morphine treatment successfully produced a behavioral sensitization to morphine.
Fig. 1. Activation of 5-HT2CR suppresses the induction and expression of morphine-induced behavioral sensitization.
(A) There was no difference in horizontal distance traveled in the 4 groups of mice that would be assigned to the saline and lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) groups. (B) Lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) treatment did not alter the horizontal distance traveled. (C) Morphine (10 mg/kg, s.c.) was administered twice a day (8:00 AM and 7:00 PM) for 3 days and then morphine treatment was suspended for 5 days. On day 9, a challenge dose of morphine (10 mg/kg, s.c.) was administered, resulting in a significant increase in locomotor responding, as measured by distance traveled. (D) Lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) was administered 15 min prior to the morphine injection for 3 days during the induction stage. On day 9, a challenge dose of morphine (10 mg/kg, s.c.) induced significantly less distance traveled in mice pretreated with 0.5 mg/kg lorcaserin. (E) Lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) was administered daily during the 5-day development phase. On day 9, there was no difference in distance traveled between prior treatment groups after a challenge dose of morphine (10 mg/kg, s.c.). (F) Lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) was administered on day 9 after the induction and development phases of morphine behavior sensitization. Lorcaserin at 0.5 mg/kg significantly decreased distance traveled. Data are expressed as mean ± S.E.M.; n = 10; * P < 0.05, ** P < 0.01.
Lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p., 15 min prior to the morphine injection) was given in combination with morphine twice daily for 3 day during the induction phase. A morphine challenge dose was given on day 9 to examine the expression of behavioral sensitization. Lorcaserin given during the induction phase altered the distance traveled in morphine sensitization mice (F(3.36) = 8.13; P < 0.01, see Fig. 1D); post hoc Dunnett’s multiple comparisons indicated that lorcaserin at 0.5 mg/kg significantly suppressed the distance traveled (P < 0.01) compared to the morphine alone group. A second cohort of mice received lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) each day during the 5 day period in which morphine treatment was suspended to examine the influence of the 5-HT2CR agonist on the development of behavioral sensitization. There was no difference in distance traveled between the lorcaserin-treated and saline-treated mice to morphine challenge on day 9 (F(3,36) = 0.69; n.s., see Fig. 1E). Finally, the third cohort of mice received the repeated morphine injection on days 1–3, followed by a 5-day suspension from morphine. On day 9, mice received saline or lorcaserin (0.125, 0.25 and 0.5 mg/kg, i.p.) 15 min prior to the morphine challenge to test the effects of the 5-HT2CR agonist on the expression of behavioral sensitization. Lorcaserin treatment altered the distance traveled after morphine challenge (F(3.35) = 6.15; P < 0.05, see Fig. 1F); post hoc Dunnett’s multiple comparisons tests showed that lorcaserin at 0.5 mg/kg significantly suppressed the distance traveled as compared to the control group (P < 0.01).
3.2. Naloxone precipitates withdrawal behaviors in morphine-dependent mice
Mice received morphine for 7 days (see Table 1) to develop morphine dependence. 2.5 hours after the last morphine injection, mice received saline, naloxone or lorcaserin. Naloxone challenge on day 7 significantly increased jumping, burrowing, body grooming, rearing, wet dog shakes, head shaking, digging body posture, pawing licking, face grooming, penile grooming, scratching, and weight loss as compared to saline control (see Table 2).
Table 2.
Naloxone precipitates withdrawal symptoms in morphine-dependent mice. Mice received morphine for 7 days as described in Table 1. 2.5 hours after last injection, mice received saline, naloxone (5.0 mg/kg, i.p.) or lorcaserin (0.5 mg/kg, i.p.). Naloxone treatment precipitated withdrawal symptoms.
| Group | n | Jumping | Burrowing | Body grooming | Rearing | Wet dog shakes |
|---|---|---|---|---|---|---|
| Saline | 9 | 5.78±0.86 | 3.89±0.59 | 7.00±0.60 | 1.00±0.29 | 1.33±0.33 |
| Naloxone | 10 | 22.70±1.36** | 6.10±0.43** | 14.10±0.82** | 4.50±0.43** | 5.00±0.47** |
| Lorcaserin | 9 | 9.00±0.71 | 2.89±0.35 | 6.56±0.71 | 2.00±0.58 | 3.00±0.37* |
| Head shakes | Digging body | Paw licking | Face grooming | Penile grooming | Scratch | Extend |
|---|---|---|---|---|---|---|
| 2.22±0.46 | 1.33±0.24 | 2.89±0.42 | 5.89±0.54 | 0.78±0.28 | 5.00±0.67 | 4.11±0.35 |
| 8.20±0.74** | 3.40±0.34** | 7.40±0.64** | 9.80±0.74** | 4.80±0.51** | 10.20±0.90** | 4.50±0.45 |
| 3.67±0.37 | 2.44±0.38 | 6.78±0.62** | 6.44±0.58 | 3.78±0.40** | 7.78±0.60 | 3.33±0.33 |
P < 0.05,
P< 0.01 vs. saline group; data are expressed as mean ± SEM.
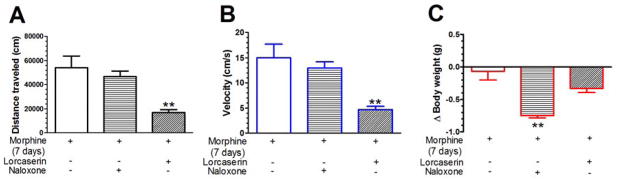
The distance traveled, velocity and change in body weight were analyzed in morphine-dependent mice treated with lorcaserin or naloxone. A one-way ANOVA revealed significant differences in distance traveled (F(2,25) = 9.6; P < 0.01, see Fig. 2A), velocity (F(2,25) = 9.6; P < 0.01, see Fig. 2B) and body weight (F(2,25) = 17.21; P < 0.01, see Fig. 2C). Post hoc Bonferroni’s multiple comparisons indicated that lorcaserin-treated mice presented a significant decrease in distance traveled and velocity, and naloxone-treated mice presented a significant decrease in body weight as compared to the control mice (all P values < 0.01).
Fig. 2. Naloxone precipitates withdrawal behaviors in morphine-dependent mice.
Mice received morphine for 7 days (see Table 1) to develop morphine dependence. 2.5 hours after the last morphine injection, mice received saline, lorcaserin (0.5 mg/kg, i.p.) or clonidine (0.2 mg/kg, i.p.). Lorcaserin suppressed distance traveled (A) and velocity (B) in mice after repeated morphine exposure. (C) Naloxone significantly decreased the body weight of mice after repeated morphine. Data are expressed as mean ± S.E.M.; n = 9–10; ** P < 0.01 vs. morphine alone group.
3.3. Lorcaserin ameliorates naloxone-precipitated withdrawal symptoms in morphine-dependent mice
Mice received morphine for 7 days (see Table 1) to develop morphine dependence. 2.5 hours after the last morphine injection, mice received lorcaserin (0.5, 0.75, and 1.0 mg/kg, i.p.) or clonidine (0.2 mg/kg, i.p.). 30 min later, all mice received naloxone (5.0 mg/kg, i.p.). Lorcaserin and clonidine significantly attenuated naloxone-precipitated withdrawal symptoms, including jumping, burrowing, body grooming, rearing, wet dog shakes, head shakes, paw licking, penile grooming, scratch and extent (all P values < 0.05), but did not affect the digging body posture and facial grooming (see Table 3). In addition, morphine-withdrawn mice presented symptoms of ptosis, piloerection, defecation, urination, irritability to touch and other abnormal behaviors.
Table 3.
Lorcaserin ameliorates naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Mice received morphine for 7 days as described in Table 1. 2.5 hours after last injection, mice received lorcaserin (0.5, 0.75 or 1.0 mg/kg, i.p.) or clonidine (0.2 mg/kg, i.p.). Mice received naloxone 30 min after the lorcaserin or clonidine injection.
| Group | n | Jumping | Burrowing | Body grooming | Rearing | Wet dog shakes |
|---|---|---|---|---|---|---|
| Saline | 10 | 30.40±2.25 | 6.20±0.59 | 14.90±1.59 | 4.30±0.37 | 6.20± 0.65 |
| Lorcaserin | ||||||
| 0.5 mg/kg | 10 | 12.80±0.85** | 3.00±0.39** | 9.50±0.65** | 2.50±0.45* | 3.20±0.44** |
| 0.75 mg/kg | 10 | 8.80±0.99** | 2.10±0.23** | 7.00±0.83** | 2.00±0.39** | 1.80±0.25** |
| 1.0 mg/kg | 10 | 7.00±0.70** | 1.30±0.30** | 5.70±0.52** | 1.80±0.33** | 1.00±0.21** |
| Clonidine | ||||||
| 0.2 mg/kg | 10 | 7.30±0.67** | 1.40±0.16** | 6.10±0.55** | 1.90±0.23** | 0.90±0.23** |
| Head shakes | Digging body | Paw licking | Face grooming | Penile grooming | Scratch | Extend |
|---|---|---|---|---|---|---|
| 6.00±0.56 | 4.50±0.62 | 12.90±1.17 | 15.60±1.41 | 7.40±0.98 | 15.7±1.21 | 3.20±0.39 |
| 3.10±0.43** | 4.20±0.63 | 10.40±0.56 | 7.90±0.81 | 3.30±0.26** | 9.60±0.67** | 3.00±0.37 |
| 2.20±0.44** | 4.40±0.48 | 9.20±0.66* | 4.20±0.39 | 3.00±0.33** | 8.30±0.65** | 3.10±0.31 |
| 1.80±0.44** | 4.00±0.58 | 7.40±0.62** | 2.50±0.27 | 2.50±0.43** | 5.60±0.37** | 3.10±0.35 |
| 1.70±0.42** | 4.70±0.56 | 8.90±0.75** | 2.60±0.45 | 1.10±0.31** | 6.00±0.33** | 2.80±0.39 |
P < 0.05,
P < 0.01 vs. saline group; data are expressed as mean ± SEM.
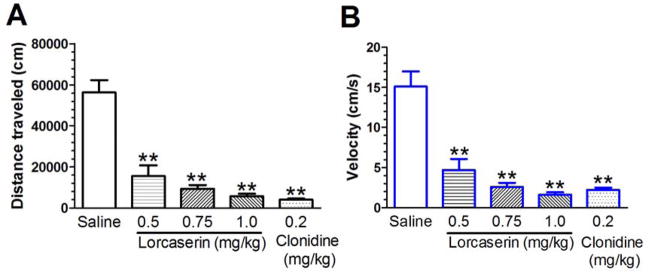
Lorcaserin ameliorates naloxone-precipitated horizontal locomotion in morphine-dependent mice. The distance traveled and velocity measures were recorded for 60 min. A one-way ANOVA revealed a significant difference in distance traveled and velocity (all P values < 0.01, see Fig. 3). Lorcaserin (0.5, 0.75 and 1.0 mg/kg, i.p.) and clonidine (0.2 mg/kg, i.p.) significantly decreased the distance traveled (see Fig. 3A) and velocity (see Fig. 3B) (all P values < 0.01).
Fig. 3. Lorcaserin inhibits naloxone-precipitated locomotor activity in morphine-dependent mice.
Mice received morphine for 7 days to develop the morphine dependence. Morphine withdrawal was precipitated by naloxone (0.5 mg/kg, i.p.). Mice received pre-treatment of lorcaserin, or the positive control clonidine, 30 min before naloxone, and locomotor activities were recorded for 60 min. Lorcaserin (0.5, 0.75 and 1.0 mg/kg, i.p.) and clonidine (0.2 mg/kg, i.p.) significantly suppressed distance traveled (A) and velocity (B). Data are expressed as mean ± S.E.M.; n = 9–10; ** P < 0.01 vs. saline.
3.4. SB 242084 prevents lorcaserin-mediated suppression of behavioral sensitization and naloxone-precipitated withdrawal in morphine-dependent mice
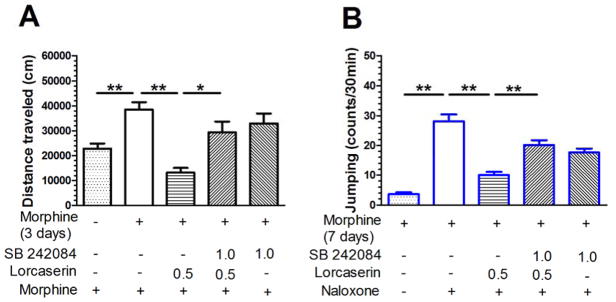
Mice received twice-daily morphine treatment for 3 days and then the drug treatment was suspended for 5 days. Morphine challenge on day 9 to mice treated with repeated morphine on days 1–3 produced a significant increase in distance traveled as compared to mice given saline on days 1–3 (P < 0.01). On day 9, mice received the 5-HT2CR antagonist SB 242042 (1.0 mg/kg, i.p.) 20 min before lorcaserin treatment, followed 20 min later by the morphine challenge dose to test whether the influence of lorcaserin on the expression of morphine-induced behavioral sensitization was dependent on the 5-HT2CR. As shown in Fig. 4A, a one-way ANOVA revealed a significant difference in distance traveled (F(4,58) = 9.2, P < 0.01). SB 242084 prevented lorcaserin-mediated suppression of the expression of behavioral sensitization. Another cohort of mice received repeated morphine treatment followed by naloxone to precipitate withdrawal. Naloxone challenge on day 7 produced a significant increase in jumping behavior as compared to saline treatment in mice that had received repeated morphine treatment (P < 0.01). Mice received SB 242042 (1.0 mg/kg, i.p.) 20 min before lorcaserin injection, followed 30 min later by naloxone. As shown in Fig. 4B, a one-way ANOVA revealed a significant difference in jumping episodes (F(4,61) = 38.92, P < 0.01). SB 242084 prevented lorcaserin-mediated suppression of jumping behavior.
Fig 4. SB 242084 prevents lorcaserin-mediated suppression of behavioral sensitization and naloxone-precipitated withdrawal in morphine-dependent mice.
(A) Mice received morphine for 3 days and then drug treatment was suspended for 5 days. Morphine challenge on day 9 to morphine-dependent mice produced a significant increase in distance traveled as compared to mice given saline on days 1–3. On day 9, SB 242042 (1.0 mg/kg, i.p.) was administered 20 min prior to the lorcaserin treatment. SB 242084 prevented lorcaserin-mediated suppression of the expression of behavioral sensitization. (B) Another cohort of mice received repeated morphine treatment to develop dependence. Naloxone challenge on day 7 elicited a significant increase in jumping behavior as compared to saline-treated mice that had previously received repeated morphine treatment. SB 242042 (1.0 mg/kg, i.p.) was administered 20 min prior to the lorcaserin treatment. SB 242084 prevented lorcaserin-mediated suppression of jumping behavior in morphine withdrawal mice. Data are expressed as mean ± S.E.M. * P < 0.05; n = 10–18; * P < 0.05, ** P < 0.01.
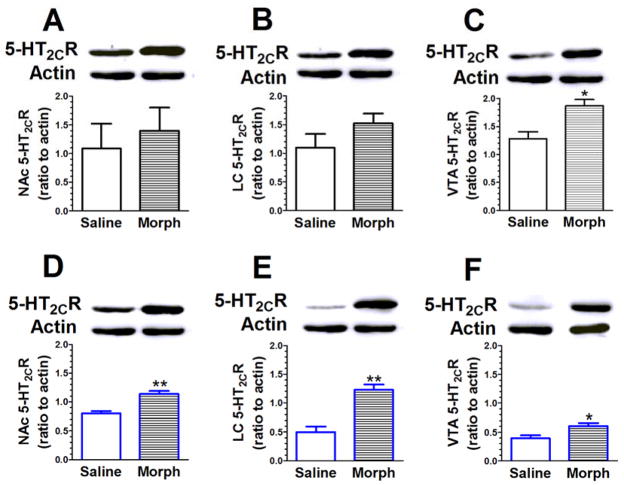
3.5. Chronic morphine treatment is associated with an increase in 5-HT2CR expression
Mice received repeated morphine treatment to develop behavioral sensitization and dependence. The 5-HT2CR protein expression was measured in NAc (see Figs. 5A and 5D), LC (see Figs. 5B and 5E), and VTA (see Figs. 5C and 5F). Student’s t-test revealed a significant increase in the 5-HT2CR expression in VTA (t(4) = 3.48, P < 0.05; see Fig. 5C) in morphine sensitization mice as compared to saline-treated mice. Similarly, 5-HT2CR expressions in NAc (t(4) = 5.08, P < 0.01; see Fig. 5D), LC (t(4) = 5.35, P < 0.01; see Fig. 5D) and VTA (t(4) = 2.90, P < 0.05; see Fig. 5F) were increased in morphine-dependent mice as compared to saline-treated mice.
Fig 5. Chronic morphine treatment is associated with an increase in 5-HT2CR expression in NAc, LC and VTA.
Mice received repeated morphine treatment to develop behavioral sensitization (A – C) or dependence (D – F). The 5-HT2CR expressions in NAc (A, D), LC (B, E) and VTA (C, F) were explored. A Student’s t-test revealed a significant increase in 5-HT2CR expression in the VTA (C) in morphine sensitization mice. Similarly, the expression of 5-HT2CR in NAc (D), LC (E) and VTA (F) were increased in morphine-dependent mice. Data are expressed as mean ± S.E.M.; n = 3–4; * P < 0.05, ** P < 0.01 vs. saline.
4. Discussion
The present study examined the influence of a selective 5-HT2CR agonist on behavioral sensitization and withdrawal symptoms in morphine-dependent mice. Our results indicate that the 5-HT2CR agonist lorcaserin suppressed the induction and expression of morphine-induced behavioral sensitization, and reduced naloxone-precipitated withdrawal symptoms. The 5-HT2CR antagonist SB 242084 prevented the lorcaserin-mediated alleviation of behavioral sensitization and withdrawal in morphine-dependent mice, indicating that the effects of lorcaserin are dependent upon the 5-HT2CR. Repeated morphine treatment increased 5-HT2CR expression in the VTA, NAc and LC. Together, these findings provide the first evidence to support the view that 5-HT2CR modulates opioid dependence and pharmacological activation of 5-HT2CR may be a novel route for the treatment of morphine abuse and dependence.
Our data indicate that activation of 5-HT2CR suppressed the induction and expression of morphine-induced behavioral sensitization. The process of behavioral sensitization that occurs during the repeated exposure to drugs of abuse may be one of the key factors involved in the acquisition and maintenance of compulsive drug-seeking behaviors. The long-lasting nature of behavioral sensitization may be attributed to persistent enhanced responsiveness of neurons that innervate the NAc, such as dopamine neurons from VTA and glutamate neurons from the PFC and the basolateral amygdala (BLA). Ibotenic acid-induced lesions of the mPFC, which encompasses both the prelimbic and infralimbic regions, disrupt the induction of sensitization to cocaine and amphetamine (Wolf et al., 1995). The induction of sensitization refers to the immediate molecular and/or cellular changes induced by drug actions in the VTA, while changes in DA neurotransmission within the NAc are critical for the expression of behavioral sensitization (Kalivas and Stewart, 1991). Morphine induces locomotor hyperactivity by activation of μ-opioid receptors and the resultant acceleration of dopaminergic neurotransmission (Iwamoto, 1981). 5-HT2CRs are expressed on both inhibitory interneurons and principal neurons of the VTA, PFC and amygdala (Nocjar et al., 2015). 5-HT2CR agonist treatment impaired the expression of behavioral sensitization in rats after repeated nicotine exposure (Zaniewska et al., 2009; Zaniewska et al., 2010a). The 5-HT2CR agonist Ro 60-0175 reduced the firing rate of mesolimbic DA neurons originating in the VTA via acting on GABAergic interneurons (Gobert et al., 2000), leading to a reduction in DA release in terminal regions of the NAc and frontal cortex; these effects are reversed by the selective 5-HT2CR antagonist SB 242084 (Di Matteo et al., 2000; Gobert et al., 2000). In addition, SB 242084 alone increased the burst-firing rate of dopaminergic neurons in the VTA leading to increased release of DA in the NAc (Di Giovanni et al., 1999). These findings suggest that the suppression of mesolimbic dopaminergic neurotransmission by the activation of 5-HT2CRs results in the inhibition of the induction and expression of behavioral sensitization; an interpretation which is further supported by the results of our SB 242084 blockade study (see Fig 4).
Interestingly, activation of 5-HT2CR did not affect the development of behavioral sensitization. The development of behavioral sensitization and addiction behaviors is thought to be attributed to the long-term alteration of gene expression in neurons of the mesocorticolimbic system. Our results suggest that such drug-induced persistent changes in gene expression may not involve 5-HT2CR signaling. The central serotonergic system plays a prominent role in the motor effects of behavioral sensitization to psychostimulants (Devroye et al., 2013; Filip et al., 2012; Steketee and Kalivas, 2011). Activation of 5-HT2CR suppresses the expression of behavioral sensitization induced by nicotine (Zaniewska et al., 2010a) and 3,4-methylenedioxymethamphetamine (MDMA) (Ramos et al., 2005) and the development of behavioral sensitization induced by cocaine (Zayara et al., 2011). The nicotinic ACh receptor antagonist mecamylamine dose-dependently blocked the expression, and to a lesser extent the development, of nicotine sensitization (Bajic et al., 2015). The distinctions between drug effects on the induction, development and expression of sensitization indicate that behavioral sensitization can arise from multiple neuroadaptations across multiple brain nuclei.
Physical dependence and withdrawal symptoms are prominent in morphine addicts. Morphine withdrawal syndrome in rodents is characterized by the expression of multiple behavioral and physiological symptoms such as jumping, ptosis, diarrhea, wet dog shakes and motivational dysfunction. Expression of withdrawal appears to be multisite determined and several neuroanatomical regions are implicated in the expression of the physical signs of morphine withdrawal. Jumping, rearing and locomotor activity are assumed to be regulated by LC; rearing and locomotor activity are related to the periaqueductal gray matter, wet dog shake is involved in the anterior preoptic hypothalamus and nucleus raphe magnus, and is thought to be an adaptation of the body to an increase in set-point temperature in the CNS during withdrawal (Koob et al., 1992). Diarrhea and lacrimation may be dependent on peripheral opiate receptors (Koob et al., 1992; Maldonado et al., 1992). Body weight loss in response to the naloxone challenge is likely related to the significant increase in urination and defection displayed by chronic or acute morphine-dependent rodents.
The central noradrenergic pathways (el-Kadi and Sharif, 1995) and its downstream targets, e.g., the HPA axis (Laorden et al., 2002; Laorden et al., 2000; Wu et al., 2014) play a prominent role in the expression of morphine withdrawal symptoms. Acute morphine treatment inhibits LC neuronal activity, while abrupt termination of morphine treatment disinhibits LC neurons. The increased firing frequency of LC neurons is associated with the expression of withdrawal symptoms (Taylor et al., 1988). The 5-HT2CR exerts a tonic, inhibitory influence upon noradrenergic transmission. The 5-HT2CR agonist Ro 60-0175 markedly suppresses NA levels in the frontal cortex, while 5-HT2CR antagonist, SB 242084 markedly increases NA without modifying 5-HT levels (Millan et al., 1998). Consistently, Ro 60-0175 dose-dependently decreases the firing rate of LC neurons (Gobert et al., 2000). These findings suggest that 5-HT2CR activation suppresses withdrawal symptoms by inhibiting central sympathetic outflow, probably by acting on GABAergic interneurons.
Results from our WB assay provide the first evidence that chronic morphine treatment is associated with an increase in 5-HT2CR expression in the mouse VTA, LC and NAc. These findings suggest that alteration in 5-HT2CR expression is a component of the neuroadaptation to chronic or repeated morphine exposure. The functional significance of the morphine-induced overexpression of 5-HT2CR remains unclear. Further studies will be necessary to identify the subcellular distribution of 5-HT2CR, receptor signaling and functional characteristics.
Our findings contribute to a better understanding of the influence of 5-HT neurotransmission on opioid abuse and dependence. We provide the first evidence that a selective 5-HT2CR agonist suppresses the induction and expression of morphine-induced behavioral sensitization, and ameliorates naloxone-precipitated withdrawal in morphine-dependent mice. Further, repeated morphine treatment is associated with an increase in 5-HT2CR expression within the mesolimbocortical reward circuit. The pattern of results presented here provides strong support for our view that the 5-HT2CR is a potential target for preventing morphine abuse and dependence.
HIGHLIGHTS.
Activation of 5-HT2CR suppresses the induction and expression of behavioral sensitization induced by repeated morphine exposure
Activation of 5-HT2CR ameliorates naloxone-precipitated withdrawal symptoms in morphine-dependent mice
Chronic morphine exposure is associated with an increase in 5-HT2CR expression in ventral tegmental area, locus coeruleus and nucleus accumbens
5-HT2CR may be a drug target for preventing the abuse and dependence of opioid
Acknowledgments
This project was supported by National Natural Science Foundation of China (81271217, 81470432, 81471161), Ph.D. start fund from Anhui Medical University (XJ201405) to G.Z.; the NSF (IBN 0630522) and NIH (MH 086591-01) to R.W.S. We thank Sarah J. Cohen for proofreading.
Footnotes
Author contributions
Gongliang Zhang: Conceived the idea, supervised research, analyzed data and wrote manuscript. Xian Wu: Conceived the idea, conducted research and wrote manuscript. Yong-Mei Zhang: Conceived the idea, analyzed data and wrote manuscript. Huan Liu: Conducted research and analyzed data. Qin Jiang: Supported research. Gang Pang: Conducted research and analyzed data. Xinrong Tao: Conducted research and analyzed data. Liuyi Dong: Conducted research and analyzed data. Robert W. Stackman Jr: Conceived the idea and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajic D, Soiza-Reilly M, Spalding AL, Berde CB, Commons KG. Endogenous cholinergic neurotransmission contributes to behavioral sensitization to morphine. PLoS One. 2015;10:e0117601. doi: 10.1371/journal.pone.0117601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6:662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Devroye C, Filip M, Przegalinski E, McCreary AC, Spampinato U. Serotonin2C receptors and drug addiction: focus on cocaine. Exp Brain Res. 2013;230:537–545. doi: 10.1007/s00221-013-3593-2. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdere P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience. 1999;91:587–597. doi: 10.1016/s0306-4522(98)00655-1. [DOI] [PubMed] [Google Scholar]
- Di Giovanni GEE, Di Matteo V. 5-HT2C Receptors in the Pathophysiology of CNS Disease. Humana Press; New York: 2011. [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- el-Kadi AO, Sharif SI. The role of 5-HT in the expression of morphine withdrawal in mice. Life Sci. 1995;57:511–516. doi: 10.1016/0024-3205(95)00284-d. [DOI] [PubMed] [Google Scholar]
- Filip M, Spampinato U, McCreary AC, Przegalinski E. Pharmacological and genetic interventions in serotonin (5-HT)(2C) receptors to alter drug abuse and dependence processes. Brain Res. 2012;1476:132–153. doi: 10.1016/j.brainres.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Le AD, Higgins GA. Serotonin receptors as potential targets for modulation of nicotine use and dependence. Prog Brain Res. 2008;172:361–383. doi: 10.1016/S0079-6123(08)00918-7. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET. Locomotor activity and antinociception after putative mu, kappa and sigma opioid receptor agonists in the rat: influence of dopaminergic agonists and antagonists. J Pharmacol Exp Ther. 1981;217:451–460. [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Castells MT, Milanes MV. Effects of morphine and morphine withdrawal on brainstem neurons innervating hypothalamic nuclei that control the pituitary-adrenocortical axis in rats. Br J Pharmacol. 2002;136:67–75. doi: 10.1038/sj.bjp.0704684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laorden ML, Fuertes G, Gonzalez-Cuello A, Milanes MV. Changes in catecholaminergic pathways innervating paraventricular nucleus and pituitary-adrenal axis response during morphine dependence: implication of alpha(1)- and alpha(2)-adrenoceptors. J Pharmacol Exp Ther. 2000;293:578–584. [PubMed] [Google Scholar]
- Li JX, Chen SQ, Deng YP, Liang JH. Effects of 5-hydroxytryptophan on morphine-induced sensitization in mice. Journal of Chinese Pharmaceutical Sciences. 2008;17:1–5. [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- NIH. DrugFacts: Heroin. National Institute on Drug Abuse; 2014. http://www.drugabuse.gov/publications/drugfacts/heroin. [Google Scholar]
- Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA. Serotonin-2C and -2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience. 2015;297:22–37. doi: 10.1016/j.neuroscience.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Goni-Allo B, Aguirre N. Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Garcia EJ, Grant SJ, Roth RH, Redmond DE., Jr Clonidine infusions into the locus coeruleus attenuate behavioral and neurochemical changes associated with naloxone-precipitated withdrawal. Psychopharmacology (Berl) 1988;96:121–134. doi: 10.1007/BF02431544. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- Wu P, Shi HS, Luo YX, Zhang RX, Li JL, Shi J, Lu L, Zhu WL. Neuropeptide trefoil factor 3 attenuates naloxone-precipitated withdrawal in morphine-dependent mice. Psychopharmacology (Berl) 2014;231:4659–4668. doi: 10.1007/s00213-014-3615-1. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Filip M. Interactions of serotonin (5-HT)2 receptor-targeting ligands and nicotine: locomotor activity studies in rats. Synapse. 2009;63:653–661. doi: 10.1002/syn.20645. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegalinski E, Filip M. Effects of the serotonin 5-HT2A and 5-HT2C receptor ligands on the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2007;571:156–165. doi: 10.1016/j.ejphar.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Wydra K, Filip M. Differential effects of serotonin (5-HT)2 receptor-targeting ligands on locomotor responses to nicotine-repeated treatment. Synapse. 2010a;64:511–519. doi: 10.1002/syn.20756. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Wydra K, Filip M. Effects of serotonin (5-HT)2 receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology. 2010b;58:1140–1146. doi: 10.1016/j.neuropharm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Zayara AE, McIver G, Valdivia PN, Lominac KD, McCreary AC, Szumlinski KK. Blockade of nucleus accumbens 5-HT2A and 5-HT2C receptors prevents the expression of cocaine-induced behavioral and neurochemical sensitization in rats. Psychopharmacology (Berl) 2011;213:321–335. doi: 10.1007/s00213-010-1996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]