Abstract
Interactions between the environment, the gut microbiome, and host characteristics that influence bone health are beginning to be explored. This is the first area where functional benefits from diet-induced changes in the gut microbiome have been reported for healthy people. Several prebiotics that reach the lower intestine have resulted in an altered gut microbiome that is thought to enhance fermentation of the fibers to produce short-chain fatty acids. These changes are positively correlated with increases in fractional calcium absorption in adolescents and with increases in measures of bone density and strength in animal models. New methodologies are available to explore mechanisms and to refine intervention strategies.
Keywords: Prebiotic fiber, Gut microbiome, Calcium absorption, Bone, Short-chain fatty acids
Gut Mirobiome—a New Frontier?
The potential for gut microbiota to contribute nutrition and bioactive compounds that affect human health including bone is a rather new area of investigation. It has been known for some time that over 90 % of the cells in the human body are microbes, principally located in the distal gut. The gut microbiome is a highly heterogeneous population comprised of 1014 bacteria representing 5000 species and 5 million genes (collectively known as the metagenome) [1]. However, exploring the relationships between the gut microbiome and human health was a slow progress until new molecular biology tools became available. The majority of gut microbiota are anaerobic. Therefore, traditional approaches involving culturing microorganisms in media were not only time-consuming but missed most of the species present. Moreover, only live bacteria could be assessed. With new approaches that determine microbial communities present in the gut by DNA extracted from stools [2, 3], all microbiota present can be detected regardless if they are living or dead. Progress on methods to detect microbial communities and shifts due to interventions open the door for the discovery of strategies to improve health and ameliorate disease including osteoporosis.
Mechanisms for Effect of Diet on Gut Microbiome that Influence Bone Health
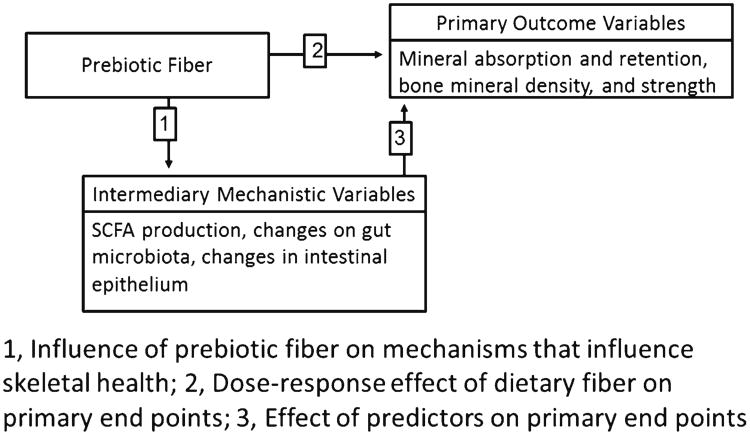
The influence of gut microbiota on bone and how the effects are modulated by diet are poorly understood. A popular theory illustrated in Fig. 1 is that dietary or prebiotic fiber (resistant to digestion) reaches the lower gut where resident microbiota ferment the fiber to short-chain fatty acids (SCFA). The resulting reduced pH environment has been thought to reduce complexation of minerals such as formation of calcium phosphates. Thus, more calcium would be absorbed to support bone accrual or retention. However, that explanation may be oversimplified. SCFAs are indeed produced upon consumption of prebiotic fibers [4]. Yet, the resultant amount of total SCFAs or individual SCFAs such as butyric acid, acetate, and propionate in response to fibers are not highly correlated with calcium absorption and bone parameters [4]. The presence of SCFAs increased calcium transport across rat colonic segments in an Ussing chamber, whereas treating with HCl did not [5]. Thus, a simple lowering of pH to solubilize minerals seems unlikely to be the mechanism. Butyrate is the preferred energy source of colonic mucosal cells; thus, SCFA may work to improve the health of the gut [6].
Fig 1. Model for role of prebiotic fiber effects.
Another possible mechanism is that SCFAs influence signaling pathways which may regulate mineral absorption or alter intestinal cell metabolism or proliferation. For example, SCFAs increased uptake of calcium by breast cancer MCF-7 cells unless G-protein GPR43 was silenced [7]. In addition, increasing SCFA production, and butyrate in particular, may act by metastable epigenetic regulation of the chromatin structure which results in differential gene expression. Butyrate altered the epigenome of skeletal muscle cells through nucleosome positioning [8]. Furthermore, butyrate was shown to induce histone acetylation that repletes miRNA expression [9]. This suggests that fiber-induced changes to the microbiome that enhance fermentation may produce potent signaling molecules that could have far-reaching effects, possibly including altering mineral absorption that enhances bone mass.
An entirely different theory for a role of the gut microbiota on bone is through modulation of the immune system. Sex steroid deficiency creates a chronic inflammatory state that is at least partially responsible for osteoporosis [10]. Furthermore, the Western diet that is high in fat and the overuse of antibiotics increase the inflammatory environment of the gut microbiome. The gut microbiota are in constant communication with the immune system; thus, they help suppress immune response to harmless commensal microbes and help protect the host from invading pathogens. The gut microbiota help shape systemic immunity as germ-free animals have immature mucosal immune systems [11]. The skeleton is a source of mesenchymal and hematopoietic progenitors. Hematopoietic stem cells generate both T cells and osteoclasts. Using germ-free control mice and mice that were colonized with gut microbiota from donor mice, Sjögren et al. [12•] showed that the germ-free mice had increased bone mass (associated with reduced osteoclasts) and altered immune system (reduced TNF α expression and T cells), which were normalized by colonization. However, further research is needed on whether diet can alter the effects of the gut microbiome on the immune system and ultimately bone mass.
Enhancing Calcium Absorption as a Strategy for Osteoporosis Prevention
Regardless of the mechanism, alterations in the gut microbiome that enhance calcium absorption, and perhaps other bone-related minerals as well, is an attractive strategy to reduce risk of osteoporosis. Calcium is the dominant mineral in bone and is recognized as a shortfall nutrient by the Dietary Guidelines for Americans [13]. Increasing calcium intake has been associated with increased bone accrual [14] and suppression of PTH and bone resorption [15, 16]. In fact, in pubertal girls, for every milligram of additional absorbed calcium, 1 mg less of calcium was resorbed from bone [17]. Calcium absorption across the gut can occur via active, vitamin D-dependent, facilitated diffusion and through passive absorption down a chemical gradient by paracellular diffusion. The former takes place largely in the small intestine, although the rate-limiting, vitamin D-regulated transport protein, calbindin D9k, is also found in the rat cecum and large intestine [17]. Passive absorption occurs throughout the intestine. The proportion of passive absorption increases as calcium intake increases and transcellular transporter proteins become saturated.
Despite much public health awareness of the importance of calcium intake for osteoporosis prevention, both milk consumption, the major dietary source, and calcium supplement use have declined in recent years. The latter may be associated with concern over cardiovascular risk despite inconsistent results and lack of demonstrated mechanism or dose response [18]. Thus, while it may be argued that increasing calcium intake is a simpler approach to correcting calcium deficiency, many do not choose calcium-rich foods. Prebiotic fibers offer an alternative strategy to enhancing calcium nutrition with the possibility of stimulating the bone microenvironment.
Prebiotic Fibers as a Dietary Strategy to Alter the Gut Microbiome
Prebiotics are food ingredients that serve as substrates for the gut microbiota. Prebiotic fibers by definition are resistant to absorption and are hydrolyzed and fermented by colonic microbiota in the lower gut. The desired outcome when incorporated into the diet in sufficient amounts is that they will selectively stimulate the growth of some bacteria that have beneficial effects to the host. Currently, we know little about properties of novel fibers that enhance bone health beyond their resistance to digestion.
Prebiotic fibers vary in types and lengths of sugars and their chemical bonds. Disaccharides have two sugar units such as lactulose or lactitol. Lactulose is created during heat sterilization of milk. It was one of the first prebiotics to be linked to gut microbial populations [19].
Oligosaccharides have ∼3−10 sugar units. These include short-chain fructo-oligosaccharides (FOS), soybean oligosaccharides, xylo-digosaccharides, and galacto-oligosaccharides (GOS). The longer chained prebiotics of >10 units include inulin (long-chain FOS) and resistant starches. The length of the chain may influence location of fermentation in the gut with longer chained prebiotics more likely to reach the lower gut [20].
All of these types of prebiotics have been associated with increases in bifidobacteria in doses up to 20 g/day given for 7-to 64-day interventions across the life span as reviewed by Ariefdjohan et al. [21]. A few studies such as Ito et al. [22] and Ballongue et al. [23] found increases in lactobacilli and decreases in coliforms as well, but these studies targeted only a few bacterial populations. We recently used pyrosequencing to determine more comprehensive changes in bacterial populations in postmenopausal women fed dietary soy bar supplements for 1 week [24]. As with other studies, increases in Bifidobacterium were observed. Changes in bacteria taxa were correlated with changes in soy isoflavone metabolites. Soy isoflavones, i.e., genistin and daidzin, are phytoestrogens that are converted to their aglycones during digestion and then to metabolites such as equol and O-desmethylangolensin (ODMA) by gut microbiota. Bifidobacterium and Eubacterium were significantly greater in the 4 women out of 17 studied who produced equol.
Evidence for Modulating Bone Health
Many studies have evaluated the effect of prebiotics on bone health [reviewed in 2, 25], but few have studied the relationship in the context of manipulating the gut microbiome. We have conducted a series of studies in rats and humans that have specifically studied the interrelationship of dietary prebiotics, microbiota, and calcium absorption or bone measures (described below).
Animal Models
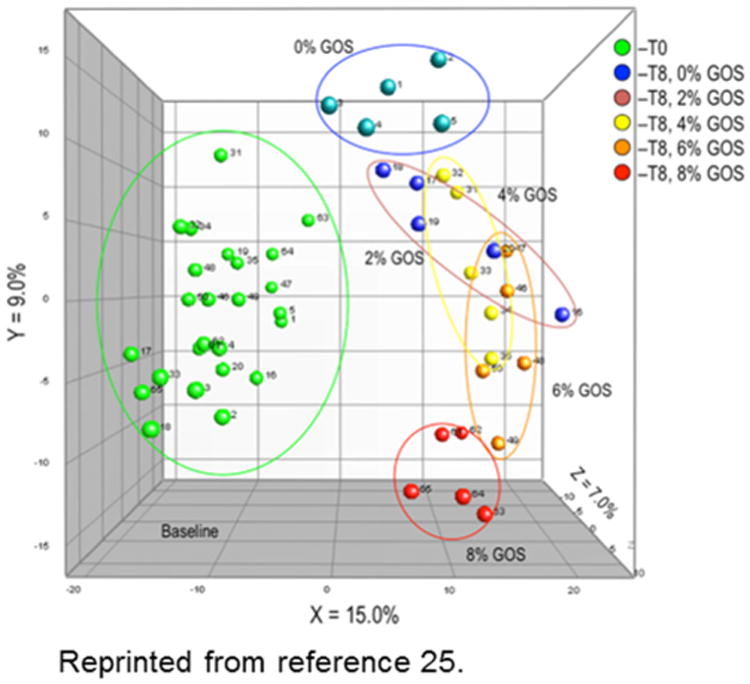
Animal models have the advantage of being able to provide controlled diets for sufficiently long periods to determine bone outcomes. From an 8-week feeding study [26] of 0, 2, 4, 6, or 8 % of the prebiotic fiber, GOS, in growing male Sprague-Dawley rats, we developed a regression model using the framework depicted. The results are shown in Fig. 2. Dietary GOS significantly decreased cecal pH and increased cecal wall and content weight in a dose-dependent manner (P<0.0001). Bacteria communities were altered by GOS treatment from baseline with separation due to GOS dose (Fig. 3). Quantitative PCR of fecal DNA showed an increased proportion of bifidobacteria with GOS (P=0.0001). Calcium and magnesium absorption and retention and femur and tibia breaking strengths, distal femur total and trabecular vBMD and area and proximal tibia vBMD increased (P<0.02) with GOS supplementation.
Fig 2. Mechanistic outcomes predicted calcium absorption and BMD in rats. Adapted from [24].
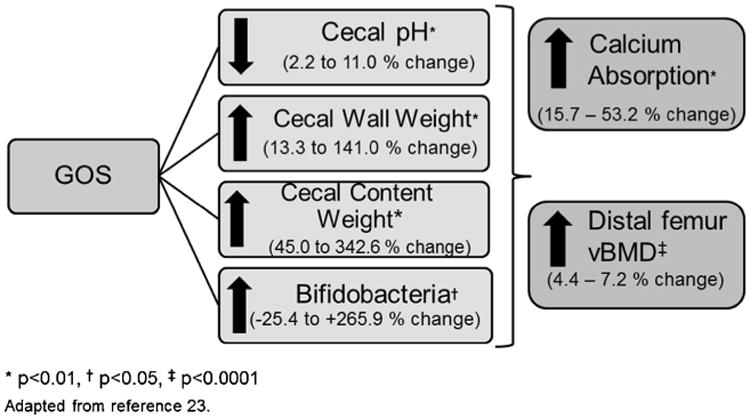
Fig 3. Bacterial communities differ by GOS treatment. Reprinted with permission from [26]. Copyright (2011) American Chemical Society.
In an evaluation of eight prebiotic fibers at 4 % by weight of diet for 12 weeks in a male weanling rat model, we saw increases in SCFA, mineral absorption, and bone density and strength [3]. All fibers except resistant starches resulted in increased total SCFA and butyrate and acetate. SCFA were not significantly correlated with bone parameters, but cecal content weight did correlate with BMD and strength. Soluble corn fiber (SCF) and soluble fiber dextrin had the greatest benefit to bone properties including whole body bone mineral content (BMC) and distal femur vBMD, cortical thickness and area, and peak breaking strength. We did not analyze the changes in gut microbiota in this study but did in the human trials with SCF described below.
Human Studies
Only one prebiotic feeding study in humans has been of sufficient duration to determine changes in bone using DXA [27]. In that 1-year RCT of 8-g inulin plus FOS in adolescent girls, gain in whole body BMC and BMD was greater in supplemented than non-supplemented girls. Gut microbiota were not profiled in that study.
The effects of prebiotics on calcium absorption and metabolism are mixed [25]. This could be due to a variety of factors including methods of assessing calcium absorption that do not reflect lower gut absorption, dose and type of prebiotic, duration of intervention, and host characteristics including calcium status, age, and gut microbiome.
In our first attempt in humans to relate prebiotic consumption to changes in gut microbiota and subsequently to calcium absorption, we studied the effect of GOS at 0, 2.5, or 5 g/day in a smoothie drink for 3-week periods given in randomized order to 31 healthy adolescent girls [28••]. Fractional calcium absorption using dual stable isotopes was increased about ∼10 % with both levels of GOS; a dose-response effect was not observed. The increase in fractional calcium absorption occurred in the late phase between 24 and 36 h consistent with lower gut absorption. Fecal bifidobacteria numbers, determined by quantitative PCR using biofidobacteria-specific primers, increased as a result of GOS feeding.
Using more sophisticated analysis of microbial communities, 454 pyrosequencing of the PCR-amplified 16S ribosomal RNA gene, we related changes due to feeding SCF to fractional calcium absorption measured with dual stable calcium isotopes [29••]. This short-term controlled feeding study in 24 adolescent boys and girls used a crossover, random order design of 2–3-week balance periods with 12-g SCF or 0-g SCF (control). Fractional calcium absorption was 12 % higher (P=0.02) with SCF treatment, which if persistent over 1 year would account for an additional 15.1 g of bone Ca or 1.8 % of total body Ca or BMC. The association between differences after SCF and control feeding in bacterial genera and late phase fractional calcium absorption is shown in Table 1. Several bacteria that correlated with Ca absorption with SCF feeding are known fiber fermenters including Bacteroides in the phylum Bacteroidetes and Butyricicoccus, Oscillibacter, and Dialister in the phylum Firmicutes.
Table 1. Correlations between calcium absorption and bacteria genera that may affect lower gut mechanisms (n=21).
| Genera differences at end of each treatment | Difference in calcium absorption between treatment and control | |
|---|---|---|
|
| ||
| Coefficient | P value | |
| Bacteroides | 0.483 | 0.027 |
| Actinomyces | −0.553 | 0.009 |
| Pseudomonas | −0.473 | 0.03 |
| Butyricicoccus | 0.454 | 0.039 |
| Oscillibacter | 0.565 | 0.008 |
| Dialister | 0.619 | 0.003 |
| Other Erysipelotrichaceae | −0.463 | 0.034 |
Pearson's correlations and Spearman's rank correlations
Future Research
The prebiotic feeding-induced changes in the gut microbiome in genera known to enhance SCFA production from fiber fermentation were significantly correlated with increased fractional calcium absorption (humans and animal models) and bone density and strength (animal models). These effects reported here are meaningful as well as accepted dietary strategies for potentially reducing the risk of osteoporosis. The ability to effect a change in the gut microbiome associated with a functional outcome measure is believed to be the first such association in healthy people. As promising as that is, further validation is needed. In humans, a prebiotic intervention resulted in greater whole body bone accrual in teens [25], but this link to changes in the gut microbiome was not studied.
Some basic methodological assumptions need to be evaluated. For example, does the gut microbiome from fecal measurements reflect the microbiome at the active site of the intestine for mineral absorption or other functions? We have not yet estimated the minimal characteristics of a healthy microbiome.
The mechanisms of action need to be worked out. Transcriptome analysis will facilitate identification of what signaling pathways are affected. Metagenomic and metabolic analyses can help determine what host and microbiome characteristics and metabolites influence function. In a recent example in healthy adult men, Holscher et al. [30•] demonstrated using whole-genome shotgun sequencing that fiber (SCF or polydextrose) feeding shifted the Bacteroides: Firmicutes ratio, shifted bacterial genes involved in lipid and B vitamin metabolism, and depleted bacterial butyrate metabolism.
The advances in methodology offer a promising future to explore the role of this new frontier, the gut microbiome, on bone health. Diet is one means of altering the gut microbiome for health, but we have a great deal to learn about what works and how.
Acknowledgments
Conflict of Interest: CM Weaver has received research support from Tate & Lyle and FrieslandCampina.
Footnotes
Compliance with Ethics Guidelines: Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JL. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whisner CM, Weaver CM. In: Probiotics and Prebiotics in Food, Nutrition and Health: Interactions of probiotics and prebiotics with minerals. Semih Ötleş., editor. CRC Press; Boca Rotan FL: 2013. pp. 200–231. [Google Scholar]
- 3.Bongers A, van de Heuval EGHM. Prebiotics and the bioavailability of minerals and trace elements. Food Rev Int. 2003;19:397–422. [Google Scholar]
- 4.Weaver CM, Martin BR, Story JA, Hutchinson I, Sanders L. Novel fibers increase bone calcium content and strength beyond efficiency of large intestine fermentation. J Agric Food Chem. 2010;58:8952–7. doi: 10.1021/jf904086d. [DOI] [PubMed] [Google Scholar]
- 5.Mneo H, Hara H, Tomita F. Short-chain fatty acids enhance diffusional Ca transport in the epithelium of the rat cecum and colon. Life Sci. 2001;69:517–26. doi: 10.1016/s0024-3205(01)01146-8. [DOI] [PubMed] [Google Scholar]
- 6.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–26. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonezawa T, Kobayashi Y, Obara Y. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathw2ay via CPR43 in the MCF-7 human breast cancer cell line. Cell Signal. 2007;19:1885–193. doi: 10.1016/j.cellsig.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Henagan TM, Navard AM, Ye J. Sodium butyrate remodels whole genome nucleosome maps and attenuates high fat diet-induced mitochondrial dysfunction in skeletal muscle from C57BL6/J mice. FASEB J. 2014;28:1072.1. [Google Scholar]
- 9.Li CJ, Li RW, Elsasser TH. McroRNA (miRNA) expression is regulated by butyrate-induced epigenetic modulation of gene expression in bovine cells. Genet Epigenetics. 2010;3:23–32. [Google Scholar]
- 10.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2012;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 12•.Sjögren K, Endhal C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–67. doi: 10.1002/jbmr.1588. This was the first study to offer a mechanism for how the gut microbiota might control bone mass. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2011. [Google Scholar]
- 14.Palacios C, Martin BR, McCabe GP, McCabe L, Peacock M, Weaver CM. Dietary calcium requirements do not differ between Mexican American boys and girls. J Nutr. 2014;144:1167–73. doi: 10.3945/jn.113.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wastney ME, Martin BR, Peacock M, Smith D, Jiang XY, Jackman LA, et al. Changes in calcium kinetics in adolescent girls induced by high calcium intake. J Clin Endocrin Metab. 2000;85:4470–5. doi: 10.1210/jcem.85.12.7004. [DOI] [PubMed] [Google Scholar]
- 16.Phang JM, Berman M, Finerman GA, Neer RM, Rosenberg LE, Hahn TJ. Dietary perturbation of calcium metabolism in normal man: compartmental analysis. J Clin Invest. 1969;48:67–77. doi: 10.1172/JCI105975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronner F, Pansu D. Nutritional aspects of calcium absorption. J Nutr. 1999;129:9–12. doi: 10.1093/jn/129.1.9. [DOI] [PubMed] [Google Scholar]
- 18.Weaver CM. Calcium supplementation: is protecting against osteoporosis counter to protecting against cardiovascular disease. Curr Osteoporos Rep. 2014;12:211–8. doi: 10.1007/s11914-014-0208-1. [DOI] [PubMed] [Google Scholar]
- 19.Seki N, Hamano H, Iiyama Y, Asano Y, Kokubo S, Yamauchi K, et al. Effect of lactulose on calcium magnesium absorption: a study using stable isotopes in adult men. J Nutr Sci Vitaminol. 2007;53:5–12. doi: 10.3177/jnsv.53.5. [DOI] [PubMed] [Google Scholar]
- 20.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 21.Ariefdjohan MW, Brown-Esters ON, Savaiano DA. Ch. 38 Intestinal microflora and diet in health. In: Coulston AM, Boushey CJ, Ferruzzi MG, editors. Nutrition in the prevention and treatment of disease. 3rd. Elsevier, Inc.; 2013. pp. 719–738. [Google Scholar]
- 22.Ito M, Deguchi Y, Miyamori A, Matsumoto K, Kikuchi H, Matsumoto K, et al. Effects of administration of galactooligosaccarides on the human faecal microflora, stool weight and abdominal sensation. Microb Ecol Health Dis. 1990;3:285–92. [Google Scholar]
- 23.Ballongue J, Schumann C, Quignon P. Effects of feeding lactitol on colonic microbiota and enzymatic activity. Scand J Gastroenterol. 1997;32:41–4. doi: 10.1080/00365521.1997.11720716. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsu CH, Weaver CM, Martin BR, Clavijo A, Barnes S. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PONE. 2014;9:e108924. doi: 10.1371/journal.pone.0108924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park CY, Weaver CM. Calcium and bone health: influence of prebiotics. Funct Food Rev. 2011;3:62–72. [Google Scholar]
- 26.Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, McCabe LD, et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem. 2011;59(12):6501–10. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 27.Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, et al. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82:471–6. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- 28••.Whisner CM, Martin BR, Schoterman MHC, Nakatsu CH, McCabe LD, McCabe GP, et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double blind crossover trial. Br JNutr. 2013;110:1292–303. doi: 10.1017/S000711451300055X. This study evaluated the dose response effect of GOS on calcium absorption and specific gut bacteria in healthy pubertal girls. The benefit of this fiber affirmed the benefit of nondigestible fibers on bone in a similar age group [26] [DOI] [PubMed] [Google Scholar]
- 29••.Whisner CM, Martin BR, Nakatsu CH, McCabe GP, McCabe LD, Peacock M, et al. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: a randomized controlled trial using dual stable isotopic tracers. Br J Nutr. 2014;112:446–56. doi: 10.1017/S0007114514000981. This controlled feeding trial was unique in removing the confounding of a variable diet on changes in microbial communities. It was the first study to link changes in diet-induced microbiota with a functional outcome in healthy volunteers. [DOI] [PubMed] [Google Scholar]
- 30•.Hoelster HD, Caporaso JG, Hooda S, Brulc JM, Fahey GC, Jr, Swanson KS. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.114.092064. This paper represents a well-executed, appropriately controlled human intervention that adds metagenomics analysis to previously reported fiber-induced shifts in microbial communities. This revealed some unpredicted changes that greatly expanded an understanding of the role of the gut microbiome in human health, though not specifically relevant to bone. [DOI] [PubMed] [Google Scholar]