Abstract
Lymphatic vessels have historically been viewed as passive conduits for fluid and immune cells, but this perspective is increasingly being revised as new functions of lymphatic vessels are revealed. Emerging evidence shows that lymphatic endothelium takes an active part in immune regulation both by antigen presentation and expression of immunomodulatory genes. In addition, lymphatic vessels play an important role in uptake of dietary fat and clearance of cholesterol from peripheral tissues, and they have been implicated in obesity and arteriosclerosis. Lymphatic vessels within different organs and in different physiological and pathological processes show a remarkable plasticity and heterogeneity, reflecting their functional specialization. In addition, lymphatic endothelial cells (LECs) of different organs were recently shown to have alternative developmental origins, which may contribute to the development of the diverse lymphatic vessel and endothelial functions seen in the adult. Here, we discuss recent developments in the understanding of heterogeneity within the lymphatic system considering the organ-specific functional and molecular specialization of LECs and their developmental origin.
Keywords: Lymphatic vessel, Lymphatic vascular development, Lymphangiogenesis, Lymphvasculogenesis, Haemogenic endothelium
1. Introduction
The lymphatic vasculature has long been recognized for its vital function as a unidirectional drainage system for the clearance of interstitial fluid and transport of tissue-derived immune cells and antigens to the lymph nodes. However, it is becoming increasingly appreciated that lymphatic endothelial cells (LECs) not only provide a structural framework for transportation. Recent research has shown that LECs can present antigens and express immunoregulatory molecules that modulate immune cell activation and functions (reviewed in1,2). In addition, the lymphatic system plays an essential role in the uptake of dietary fat and has been implicated in clearance of cholesterol from peripheral tissues, and lymphatic dysfunction may thus contribute to the pathogenesis of obesity and arteriosclerosis (reviewed in3–5).
Lymphatic vessels within different organs and in different physiological and pathological processes show remarkable plasticity and heterogeneity, which likely reflects their tissue-specific specialization (Figure 1). In support of this, both blood endothelial cells (BECs) and LECs from different organs show unique expression profiles and transcriptional programmes.6,7 In addition, BECs differentiated from embryonic stem cells adopt different molecular features depending on which tissue they were transplanted into,6 suggesting that endothelium possesses an inherent phenotypic plasticity that allows organ-specific functional specialization. LECs in embryos compared with adult tissues, as well as LECs of different vascular beds, show differences concerning basic growth factor responses, but the molecular basis of this is not well understood. For example, postnatal deletion of vascular endothelial growth factor C (Vegfc),8 which is the critical lymphatic growth factor during embryogenesis9 was shown to result in regression of the specialized lacteal lymphatic vessels within the intestinal villi, without affecting the integrity or function of dermal lymphatic vessels.8 Vascular bed-specific responses are further highlighted by the observations that Vegfc overexpression in the respiratory tract induced lymphatic vessel growth only during a critical period in perinatal development,10 while in the skin Vegfc activated lymphangiogenesis also in adult stages.11,12 To add another level of complexity, LECs in certain organs were recently shown to have multiple cellular origins,13–15 which may contribute to the development of the diverse lymphatic vessel and LEC functions in the adult.
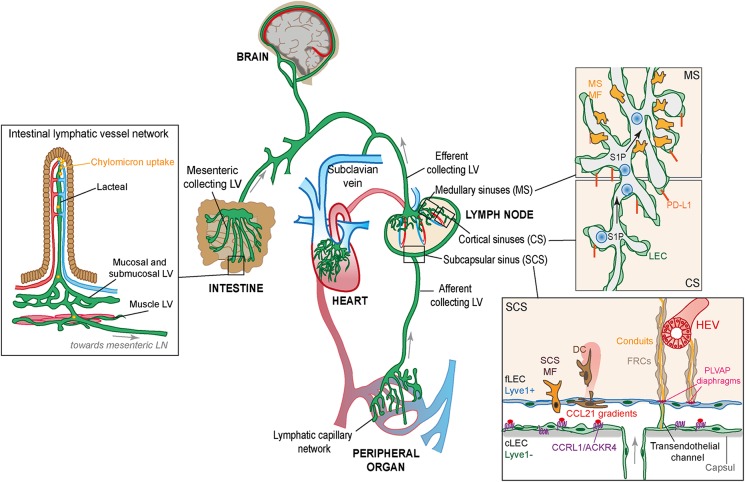
Figure 1.
Organization of the lymphatic vasculature and the specialized lymphatic vessels within the intestine and the lymph node. The lymphatic capillary network (green) absorbs fluid that continuously leaks out from the blood capillary beds and returns it to the blood circulation (blue/red). On the left: The specialized capillary vessels in the intestinal villi called lacteals also take up dietary lipids as chylomicron particles. Both lacteals and capillary vessels in other peripheral organs provide a route for tissue-derived immune cells to the lymph nodes distributed along the collecting vessels; a function that is essential for induction of efficient immune responses and peripheral tolerance. On the right: Molecular and structural features of LECs in different parts of the lymph node (SCS, MS, and CS) are shown. LECs that build the ceiling of the SCSs (cLECs) can be molecularly distinguished from the LECs that form the floor (fLECs) by their low expression of the lymphatic marker Lyve1. cLECs instead express the atypical chemokine receptor CCRL1 (ACKR4) which through scavenging the chemokine CCL21 creates chemokine gradients for migration of tissue-derived DCs (brown) from the SCS into the lymph node parenchyma. Distinct populations of macrophages (MFs; yellow) are associated with SCS and MS but have a common role in scanning the lymph for pathogens and antigens. Small antigens, cytokines, and chemokines can enter the lymph node conduit system that descends from the floor of the SCS in the direction of high endothelial venules (HEVs). The size restriction of the conduit system is determined by PLVAP+ sieve-like diaphragms in transendothelial channels that connect the SCS to the conduits. CS and MS produce S1P essential for immune cell exit from the lymph node and express the immune check point molecule PD-L1 involved in LEC-induced antigen tolerance.
We are only beginning to understand the molecular differences that endow and functionally distinguish different types of lymphatic vessels in different organs and biological contexts. Although some of the signals that regulate the developmental patterning and functional specialization of the lymphatic vasculature have been identified, little is known about how this patterning is maintained and how LEC plasticity is controlled. Here, we review recent insights into the heterogeneity within the lymphatic system focusing on the organ-specific functional and molecular specialization and the developmental origins of LECs.
2. Functional and molecular heterogeneity of lymphatic vessels
2.1 Vessel-type-specific specialization of lymphatic endothelium for fluid absorption and drainage
In most tissues, the return of extravasated tissue fluid back to the blood starts with absorption by specialized blind-ended capillary lymphatic vessels (also known as initial lymphatic vessels) (Figure 2). They converge into progressively larger collecting lymphatic vessels that transport the fluid, which inside the lymphatic vessels is called lymph, into the largest lymphatic vessels; the thoracic and right lymphatic ducts that empty into the subclavian veins. Despite their unique morphological features, lymphatic capillaries and collecting vessels share the expression of many of the genes that distinguish lymphatic from blood endothelium.16–18 These include the master transcriptional regulator of lymphatic differentiation and identity, prospero homeobox 1 (Prox1), the transmembrane O-glycoprotein podoplanin (Pdpn, also known as gp38 and T1alpha), the tyrosine kinase receptor vascular endothelial growth factor 3 (Vegfr3 also known as FLT4), and neuropilin 2 (Nrp2), a receptor for Class III semaphorins, which similar to Vegfr3 binds Vegfc (Figure 2). These genes are not only markers of LECs but also critical regulators of the development of the lymphatic vasculature (reviewed in17,18). All lymphatic vessels also share the expression of the endothelial-specific junctional proteins VE-cadherin, Claudin5, and PECAM-1, but the organization of the cell–cell junctions is distinct. Lymphatic capillaries show discontinuous button-like junctions, while collecting vessels have zipper-like junctions19 (Figure 2). Lymphatic capillaries are surrounded by a thin discontinuous basement membrane. They readily sense changes in interstitial pressure by their anchoring to the extracellular matrix,20 which can modulate the opening of ‘flap valves’ in between the button junctions to allow fluid entry.19 It is also through these flap valves that immune cells enter the lymphatic vessels.19,21
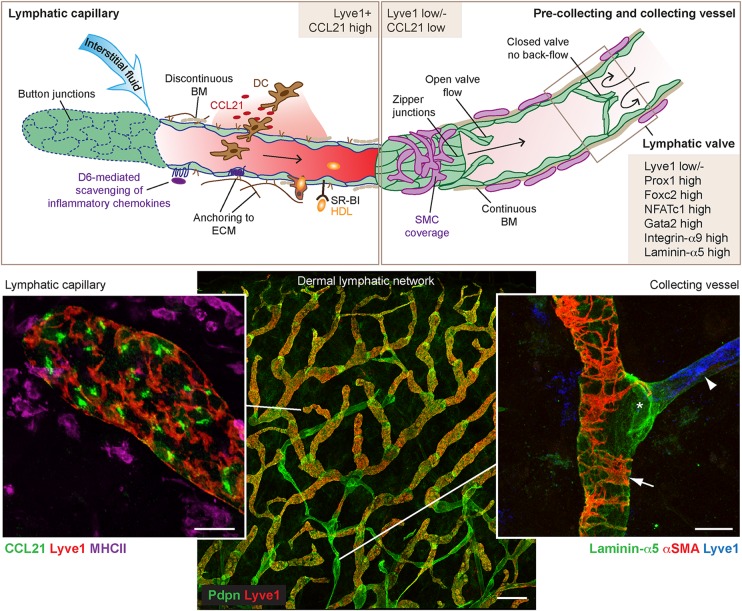
Figure 2.
Structural and molecular features of capillary and collecting lymphatic vessels. The blind-ended lymphatic capillaries, designed to remove fluid and soluble molecules from the interstitial space, are characterized by button-like intercellular junctions, discontinuous basement membrane (BM), and anchoring filaments. High capillary expression of the CCR7-ligand CCL21 allows recruitment of DCs. Collecting lymphatic vessels that transport the lymph instead have zipper-like intercellular junctions, continuous basement membrane, smooth muscle cell coverage, and intraluminal valves. The main distinguishing molecular features of the endothelium of capillary vessels, collecting vessels, and valves are depicted in brown boxed areas. Additional functions of the capillary and collecting lymphatic networks include inflammatory chemokine scavenging through expression of the atypical chemokine receptor D6 (ACKR2) and RCT passively or through expression of the cholesterol carrier SR-BI or indirectly through macrophages. Bottom panels show whole-mount immunofluorescence images of mouse ear skin stained with the indicated antibodies. Arrowhead points to a Lyve1 positive capillary and arrowhead to a Lyve1 negative collecting vessel. Valve at a vessel branch point is indicated by an asterisk. Scale bars: 25 µm (capillary and collecting vessels) and 200 µm (lymphatic network).
Lymphatic capillaries can be distinguished from collecting vessels by their high expression of the hyaluronan receptor Lyve122 and the chemokine CCL2123,24 (Figure 2). The latter allows recruitment of activated CCR7 positive dendritic cells (DCs) both in homeostasis and in inflammation through the formation of haptotactic CCL21 chemokine gradients, established by the immobilization of CCL21 on extracellular heparin sulfate, that guides migration of DCs towards the lymphatic vessels.24 These gradients may act together with soluble gradients produced by LECs or by the DCs themselves as they migrate.25 DC entry is followed by intraluminal crawling inside the lymphatic capillaries, a process which was recently described to depend on immobilized intraluminal CCL21 gradients formed in a flow-dependent manner.26 In contrast, transfer of DCs within the collecting vessels to draining lymph nodes is thought to be passive. In addition to DCs, other immune cells including recirculating memory T-cells27 and neutrophils28 have been reported to enter lymphatic vessels in a CCR7-dependent manner. In contrast to CCL21, the role of Lyve1 in capillary vessels is still not clear. Although Lyve1 is expressed already in the earliest venous-derived LEC progenitors in E9.5-E10.5 embryos,29 deletion of Lyve1 in mice does not result in major defects in lymphatic vessel morphogenesis or mobilization of DCs to lymph nodes when evaluated after FITC dibutyl phthalate skin painting.30
Whereas capillary vessels are designed for uptake of fluid and immune cell recruitment, collecting vessels are specialized for transport. They are surrounded by a continuous basement membrane and perivascular smooth muscle cells, which together with the zipper-like junctions make these vessels relatively impermeable for fluid diffusion19 (Figure 2). Besides morphology and distribution of junctional proteins, collecting vessels can be distinguished by reduced expression of Lyve122 and CCL2123 compared with capillary vessels, and it is generally not thought that collecting vessels allow transmigration of immune cells. However, recent data show that collecting vessels display an increased permeability during inflammation allowing exchange of lymph components with adipose tissue macrophages and DCs, which were shown to enter the lymph node.31 The large collecting vessels and the connecting lymph nodes are embedded in subcutaneous or visceral fat, but the implication of this is largely unknown. In addition to an exchange of lymph components between the adipose tissue immune cells and the collecting vessels, the association may reflect the need for energy and other factors during adaptive immune responses in the lymph nodes32,33 and could be a reservoir of common progenitors of lymph node mesenchymal stromal cells and adipocytes, active in development and possibly regeneration.34 Another special feature of collecting vessels is the luminal valves that are essential for preventing retrograde lymph flow. Endothelial cells forming the valve leaflets show a unique spindle-like morphology and expression profile that is shared with venous valve endothelial cells.35,36 They are characterized by high expression of the transcription factors Foxc2 and GATA2, and Integrin-α9 that are not only expressed by but also critically required for the formation of valves.35,37,38 Valve endothelial cells also express high levels of Prox1, Connexin-37, and they show high calcineurin/NFATc1 signalling, while Lyve1 and Nrp2 levels are low or absent35,39 (Figure 2).
There is not a clear border between capillary and collecting vessels, and sometimes the term pre-collecting vessel is used to describe the part of the lymphatic network in-between the capillary vessels and collecting vessels. Pre-collecting vessels express Lyve1 and contain valves but have no or very few smooth muscle cells. In human tissues, these vessels were described to display lower expression of PDPN together with distinct expression of chemokines and chemokine receptors including CCL27 and the atypical chemokine receptor Duffy antigen chemokine receptor DARC (also known as ACKR1).23
Studies on mouse models have uncovered molecular mechanisms that regulate the remodelling of the embryonic lymphatic vasculature into a fully functional network of capillaries and collecting vessels and acquisition of vessel-type-specific features. In addition, flow-induced mechanosignalling has been implicated in lymphatic vessel remodelling and maintenance of specific features including luminal valves.38–41 For detailed information of the molecular regulation of lymphatic vessel morphogenesis and remodelling, we would like to refer the reader to excellent recent reviews.16–18
2.2 Specialized functions of lymphatic vessels in fat absorption and metabolism
The intestinal lymphatic system consists of two independent vessel networks: the lacteals that drain the intestinal villi interconnected with a submucosal lymphatic network, and the lymphatics that drain the muscular layer within the intestine42 (Figure 1). Both of these connect in the mesentery with larger collecting vessels, which transport the incoming fluid to the mesenteric lymph node. The lacteals play a unique and vital role in the uptake of triglyceride-loaded particles known as chylomicrons and fat-soluble vitamins from the villi, for delivery to the blood via the cisterna chyli and thoracic duct. The mechanism of uptake of the chylomicrons, which can be very large up to 1 μm in diameter, is not entirely clear, and may involve both intercellular transport through LEC junctions and intracellular transport across LECs in vesicles (reviewed in3,4). Active contraction of the lacteals, which is mediated by smooth muscle cell contractions and controlled by the autonomic nervous system, allows drainage of absorbed lipids into the collecting vessel network.43
In many aspects, lacteals display similar features as skin dermal capillary vessels, including high expression of Lyve1 and CCL21,7,44 while mesenteric collecting vessels are characterized by low expression of Lyve1 as well as the presence of valves and layers of extracellular basement membrane and smooth muscle cells.39 Just as in the dermis, the lymphatic vessels within the villi and submucosa provide a path for tissue-derived DCs to reach the draining mesenteric lymph node, which constitutes a fundamental mechanism for induction of oral tolerance towards ingested antigens and the microbiota (reviewed in45). However, lacteals do not have the same uniform button-like junctional organization as, e.g. skin dermal capillary vessel but a mix of both continuous zipper junctions and discontinuous button-like junctions.44 The lacteals in adult intestine in contrast to adult dermal lymphatic vessels display a low but detectable proliferation also under homeostasis.44 Reflecting the more active proliferative state of the lacteals, deletion of the Notch ligand DLL444 or postnatal deletion of Vegfc8 resulted in lacteal regression and reduced uptake of lipids without affecting the integrity of quiescent dermal lymphatic vessels. Cultured human intestinal and dermal LECs display a distinct set of differentially expressed genes, despite sharing the expression of known LEC markers.7 However, further studies profiling specifically the transcriptome of lacteals are needed to understand the molecular mechanisms underlying their functional specialization.
Although the lacteals have a unique role in the uptake of dietary fat, lymphatic vessels outside the gastrointestinal tract also affect fat metabolism and defects in lymph transport may contribute to cholesterol-driven diseases like atherosclerosis (reviewed in3–5). Atherosclerosis is a chronic inflammatory disease of larger arteries and a major cause of myocardial infarction and stroke. It involves formation of plaques in the subendothelial intimal space with recruitment of monocytes that accumulate as lipid-loaded foam cells. Reverse cholesterol transport (RCT) refers to the removal of cholesterol from the tissue and its delivery back to the liver. Lymphatic vessels have been shown to contribute to RCT, either by acting as conduits for macrophages46 or by cell free uptake of cholesterol passively or actively through expression of the HDL receptor SR-BI.47 Hence, lymphatic vessels could be expected to have a protective effect against atherosclerosis by affecting both RCT and the local inflammatory environment. This notion is supported by data from in vivo animal experiments. Mice with lymphatic hypoplasia due to systemic defects in Vegfr3 signalling either due to a kinase inactivating mutation in Vegfr3 (Chy mice) or expression of soluble Vegfr3-Ig show higher levels of lipoprotein and faster progression of atherosclerotic lesions in genetic models of atherosclerosis (LDLR−/−/ApoB100/100).48 However, it should be noted that lymphatic vessels associated with arteries are rarely found in the intima where the arteriosclerotic plaques develop but instead localize to the adventitial layer. Further studies are needed to fully understand the impact of lymphatic vessels on the local atherogenic process.
Accumulation of adipose tissue has been reported as a clinical manifestation in patients suffering from chronic lymphoedema,4,5,49 which suggests direct or indirect roles of lymphatic vessels in local fat metabolism. In support of this notion, haploinsufficient Prox1 mice and the Chy mice carrying a kinase inactivating mutation in Vegfr3 that both develop oedema were also reported to display accumulation of fat.50,51 However, conflicting data for the Chy model exist,5 and other mouse models with lymphatic dysfunction such as K14-Vegfr3-Ig mice do not display increased body weight compared with controls.5,51 It is possible that differences in genetic background of the mice and thereby differences in the immunological phenotype52 contribute to different outcomes on fat metabolism. Inflammatory cytokines such as tumour necrosis factor alpha are known to affect fat metabolism,53 and lymphatic vessels may influence adipose tissue biology indirectly due to their role in clearance of cytokines and immune cells from the tissue environment.
2.3 Fluid homeostasis in the brain and the eye
No lymphatic vessels are found inside the brain in contact with neuronal cells, where an alternative system for fluid drainage, the so-called glymphatic system, exists. The glymphatic system is named after its dependence on glial cells and a similar draining function as the lymphatic system, but there are no structural similarities between the two (reviewed in54). However, in 2015, two groups independently demonstrated that the mouse brain meninges contain a lymphatic network.55,56 Their data confirmed and extended a forgotten anatomical observation by the Italian physician Mascagni in 1787 and observations of dural lymphatic vessels in rat published in 1987.57 The new studies by Louveau et al.55 and Aspelund et al.56 showed that meningeal lymphatic vessels absorb cerebrospinal fluid allowing antigen and immune cell trafficking to cervical lymph nodes but only have a limited role in fluid drainage from the brain.55,56 Phenotypically the meningeal lymphatic vessels were shown to have features of lymphatic capillaries, including expression of Lyve1 and CCL21, with lymphatic valves observed only in vessels near the base of the skull.55,56 The re-discovery of meningeal lymphatic vessels has put new focus on the immune regulation of the brain and could have implications for the understanding of immune-mediated diseases of the brain such as encephalomyelitis.58 It remains to be determined if the brain lymphatic vessels are molecularly different from other lymphatic vessels in the body.
The cornea of the eye is completely avascular, which has been linked to expression of a secreted splice variant of Vegfr2 and truncated soluble Vegfr3 inhibiting the effects of Vegf/Vegfc.59,60 However, lymphatic vessels are present in the ciliary body, and the anterior segment of the eye and abnormal lymphangiogenesis plays a role in pathological eye conditions (reviewed in61). In addition, it was recently shown that the so-called Schlemm's canal (SC) that has an important function in draining the aqueous humour in-between the lens and the cornea, has lymphatic-like properties.62–66 The SC expresses Prox1 and forms by postnatal migration of venous-derived progenitors analogous to the formation of venous-derived lymph sacs during embryogenesis.62–64 ECs lining the SC also express other typical lymphatic markers including Integrin-α9, Vegfr3, and CCL21 and respond to Vegfc with increased sprouting and proliferation. However, they have very low or no expression of Lyve1 and Pdpn,62,63 and the SC is thus not considered a true lymphatic vessel. Interestingly, the expression of Prox1 was found to correlate with SC integrity and function, suggesting that it could be used as a biosensor for pathological changes.62 In addition, due to the stimulatory effect on SC growth, Vegfc treatment was suggested as a potential novel strategy for treatment of the degenerative disease glaucoma, which is coupled to increased intraocular pressure due to inadequate drainage of the aqueous humor.63 Angiopoietin/Tie2 may also represent a target for glaucoma treatment due to a reported selective dependence of the lymphatic vessels in the eye and the SC on this signalling pathway.65 Genetic deletion of the two Tie2 ligands angiopoietin 1 and 2 during late embryogenesis at E16.5 caused a specific loss of eye lymphatic vessels and the SC resulting in development of glaucoma within 3 months after birth.65 It is possible that the late embryonic requirement of angiopoitin/Tie2 may reflect, at least in part, the postnatal formation of the SC, but it could also indicate diversification of the signalling pathways regulating different vascular beds.
2.4 Molecular and functional heterogeneity of the lymph node lymphatic vessel network
The lymphatic vessels are closely integrated with the lymph nodes, which are distributed, often in chains, along the collecting lymphatic vessels. The lymphatic connection to the lymph node is crucial for the establishment of efficient contacts between tissue and blood-borne immune cells, and the lymph nodes are essential not only for induction of effective immune responses but also their resolution and for maintenance of tolerance (reviewed in45,67,68). The lymph nodes consist of a complex network of lymphatic sinuses, surrounding a highly organized parenchyma of immune cells, specialized blood vessels, and stromal cells which together provide an optimal environment for induction and regulation of immune responses67,68 (Figure 1). The lymphatic sinuses of the lymph node can be seen as an extension of the peripheral lymphatic network but differ from both capillary and collecting vessels with unique structural and molecular features.
2.4.1 Lymph node subcapsular sinus
The incoming lymph is drained by afferent lymphatic vessels into the large subcapsular sinus (SCS) located underneath the collagenous capsule that surrounds the lymph node. The lymphatic cell layer forming the floor of the SCS (fLECs) allows cellular trafficking into the lymph node parenchyma but is at the same time highly selective with regard to the molecules transported, excluding those >70 kDa or with a radius >4 nm.69 Smaller antigens and soluble molecules like chemokines and cytokines can gain access through the highly specialized conduit system, which consists of a network of tubular collagen fibres ensheathed by fibroblastic reticular cells (FRCs).69,70 The reticular fibres descend from the fLEC layer in the direction of the high endothelial venules (HEVs), where blood derived immune cells enter the lymph node parenchyma. The conduit system allows crosstalk between lymph and blood71 but still shields the immune cell compartment of the lymph node from direct contact to the lymph. Access of antigens to the lymphoid compartment of the lymph node instead has to go through DCs or other antigen presenting cells70 (reviewed in45,68,72).
The features of the sinus floor that allow exclusion of fluid transport but still permit cellular transmigration have been elusive for a long time. Recently, expression of the glycoprotein plasmalemma vesicle-associated protein (PLVAP) (also known as PV-1 and MECA-32) by lymph node LECs was shown to be essential for the barrier function of the sinus, affecting both the size-selective entry of lymph-borne antigens to the conduits and the cellular transmigration.73 PLVAP was shown to form sieve-like diaphragms in transendothelial channels in LECs that bridge the sinus to the conduits.73 PLVAP was not restricted to fLECs but displayed a widespread expression in lymph node LECs, while no expression was reported in peripheral skin LECs.73 PLVAP has earlier been thought to be confined to BECs and is the only protein that has been associated with formation of diaphragms in endothelial cells.74 The fLECs are also lined by a basement membrane-like extracellular matrix and are in close contact with sinus-lining-specialized stromal cells called marginal reticular cells (MRCs)75 and metallophilic CD169+ macrophages (MMF) that scan the lymph for antigens.76,77
The same chemokine axis CCR7–CCL21 that guides entry of DCs into peripheral lymphatic vessels24,26 have intriguingly also been shown to be essential for their exit from the SCS into the lymph node parenchyma.78 Whereas transendothelial channels restricted by PLVAP diaphragms in fLECs may provide a path for the lymph-borne immune cells into the lymph node, the CCR7-driven directional transit was shown to be regulated by the LECs that form the ceiling of the SCS (cLECs), dependent on their expression of the atypical chemokine receptor CCRL1 (ACKR4).79 CCRL1 is a scavenging receptor for the CCR7-ligands CCL19 and CCL21, efficiently targeting them for internalization and degradation. Expression of CCRL1 was shown to allow formation of CCL21 chemokine gradients reaching from the sinus into the lymph node parenchyma. Conversely, the absence of CCRL1 resulted in a dramatic accumulation of CCL21 at the floor of the SCS, creating a reversed gradient and preventing efficient exit of DCs.79 Expression of CCRL1 is mainly confined to the SCS cLECs, and no expression is found in the primary dermal lymphatic plexus. Another marker that has been specifically assigned to LECs in the SCS is the chemokine CCL1,80,81 which was shown to allow CCR8 chemokine receptor-driven entry of tumour cells into the lymph node.80 The absence of CCL1 still allowed tumour entry into the afferent lymphatics but blocked transmigration of tumour cells across the sinus. The immunological functions of sinus-derived CCL1 is not entirely clear, but CCR8 has been shown to be involved in monocyte-derived DC migration from the skin to lymph nodes.81
2.4.2 Lymph node cortical and medullary sinuses
Most of the lymph that enters the SCS will never enter the conduits but will be channelled out through the cortical and medullary sinuses (CSs and MSs, respectively) and leave the lymph node through the efferent lymphatic vessels. The MSs form a complex irregularly shaped network and are transversed by fine reticular strands. Similar to fLECs, MS LECs intimately interact with macrophages. The medullary macrophages are phenotypically distinct from the macrophages found in the SCS but similarly contribute to clearance of pathogens and antigens from the lymph (reviewed in72). The areas of CSs that connect to the MSs contain fewer macrophages. The factors that determine the distinct spatial relationship between different macrophage populations and different part of the lymph node LEC network are not known.
Both CSs and MSs have been shown to be involved in lymphocyte egress from the lymph node by their production of sphingosine-1-phosphate (S1P). S1P binds the sphingosine-1-phospate receptor type 1 (S1PR1) expressed by T-cells, which is necessary for T-cell egress both from the thymus into the blood and from lymph node into the lymph.82–85 The important source of S1P in the blood is haematopoietic cells,83 whereas the important source of S1P in lymph was shown to be Lyve1 expressing cells,82 which will include fLECs and large parts of the CS and MS network. Deficiency in the production of S1P in Lyve1 expressing cells also affects patterning of the peripheral lymphatic vessels,82 and S1P may thus have a dual role in lymphatic vessel development and LEC-mediated immune regulation. Another molecule involved both in developmental patterning of lymphatic vessels and in the regulation of S1P egress is Integrin-α9.86 Besides its role in the development of lymphatic valves,35 Integrin-α9 was also shown to regulate secretion of S1P from the LECs.86
2.5 Lymph node LECs in antigen presentation and regulation of immune cell activation
Besides expressing proteins that can affect immune cell access to and exit from the lymph node parenchyma, multiple data support that lymph node LECs express both MHC class I and class II molecules and contribute to antigen presentation and regulation of immune cell activation.87–93 Tewalt et al.88 showed that lymph node LECs lack expression of co-stimulatory molecules but express the immune check point molecule programmed cell death 1 (PD-1) ligand (PD-L1), with the highest expression in CSs and MSs. In line with this finding, several studies have linked lymph node LECs to peripheral and tumour-induced tolerance,87–89,91,93 but lymph node LECs may also act as a reservoir for antigens facilitating induction of protective immune responses in vaccination and viral infection, an effect that was shown to be dependent on the proliferative response of LECs in infection.92 Lymph node LECs have been suggested to present antigens directly, but it is also possible that they induce tolerance indirectly by transferring antigens to DCs93 or that they receive antigen complexes from DCs.91 Most studies do not differentiate in vivo effects of antigen presentation by LECs from other lymph node stromal cells, and many questions remain.
The lymphatic network of the peripheral skin-draining lymph node is much simpler in a newborn mouse compared with an adult94 and will grow and remodel extensively during the first postnatal weeks. Neonatal lymph nodes were shown to express much lower levels of PD-L1 compared with adult lymph nodes,95 and it is likely that crosstalk between recruited immune cells and ECs in conjunction with other stromal cells shapes postnatal lymph node lymphatic patterning. It is also an interesting possibility that peripheral lymphatic vessels can adopt a lymph node-like specialization in disease. This may occur during the formation of tertiary lymphoid organs, also known as ectopic lymphoid structures, which are an accumulation of immune cells and stromal cells with a lymph node-like organization in chronically inflamed tissues and tumours.96
2.6 Peripheral lymphatic vessel heterogeneity and plasticity in immune regulation
Reduced peripheral lymphatic drainage will in addition to oedema be expected to lead to increased accumulation of inflammatory mediators, including cytokines, which are normally transported away with the lymph and affect immune cell trafficking to the lymph nodes. This can disrupt the immune regulatory pathways that are dependent on the communication between the tissue and the lymph nodes.1,45,67 Supporting this notion, inhibition of the Vegfc/Vegfr3 growth factor axis has been shown to result in increased and prolonged inflammatory responses,97,98 defects in peripheral immune tolerance,45,99 and can change the resting immunological environment within the tissue.50 It should be noted that the experimental strategies have been based on targeting of the Vegfc/Vegfr3 growth factor axis globally, and it cannot be excluded that part of the effects may be due to effects on other Vegfr3 expressing cells including macrophages100 and BECs.101 Interestingly, the effect on Vegfr3/Vegfc inhibition may also be context dependent, and treatment with soluble Vegfr3 receptor in inflammation-driven carcinogenesis was shown to reduce the inflammatory response and inhibit cancer development.102
Both lymph node and peripheral lymphatic vessels express cytokine and toll-like receptors (TLRs)1,2 in a pattern that differs between LECs in different organs103,104 and respond to inflammatory stimuli not only through changes in proliferation and fluid permeability (reviewed in2). For example, inflamed dermal lymphatic endothelium was reported to suppress DC maturation through up-regulation of Mac-1/ICAM-1.105 Peripheral LECs can also influence leukocyte migration by modulating chemokine availability through expression of the atypical chemokine receptor D6 (ACKR2),106 a scavenging receptor for inflammatory chemokines. Human LECs in the skin, gut, and lymph nodes express D6,107 but any potential differences in the expression of D6 between different types of vessels or organs are unknown. D6 deficient mice display exuberated and prolonged responses in phorbol ester-induced skin inflammation,108 which was linked to accumulation of leukocytes around peripheral lymphatic vessels and in draining lymph nodes leading to lymphatic congestion and impaired fluid and cell drainage.106 Dermal LECs display different gene expression profiles in response to treatment of the skin with a contact sensitizer oxazolone vs. complete Freund’s adjuvant.109 The plasticity of LECs in disease is also supported by reported changes in LEC gene expression profiles in experimental models of tumour-associated lymphatic vessels.110 It will be of high relevance to understand how LECs adapt to different conditions during infection, inflammation, and cancer.
3. Heterogeneous origin of LECs in different organs
3.1 Discovery of LECs of different origins
Florence Sabin, an American anatomist, postulated already in 1902 that mammalian lymphatic vessels originate from the venous ECs.111 Based on ink injection experiments in embryonic pigs, she proposed that two primitive jugular lymph sacs originate from ECs budding from large veins, which is followed by sprouting of vessels from the lymph sacs (the centrifugal model).111 An alternative model by Huntington and McClure in 1910 proposed that LECs form from mesenchymal precursors (the centripetal model).112 Based on the analysis of serial tissue sections, Huntington and McClure concluded that lymphatic vessels initially develop along embryonic veins and centripetal extensions are formed to connect them first with the lymph sacs and then with the venous system.112 The debate on the two competing theories continued until more than 100 years later, in 2007, Srinivasan et al. provided convincing evidence in support of Sabin's theory of the venous origin of lymphatic vessels by genetic tracing of Prox1 lineage cells.113 The venous source of LECs was shown to be evolutionary conserved in teleosts based on studies in zebrafish.114,115 Reports from avian embryos116,117 and amphibians118 suggested an additional mesodermal source of LECs. An alternative origin of LECs was also suggested in the mouse.119 However, these studies were based on marker expression118,119 or chimeric transplantation experiments,116,117 and not lineage tracing, and functional evidence was lacking. In 2015, our group14,15 and Klotz et al.13 surprisingly found compelling evidence for at least two alternative non-venous sources of LECs in the mouse embryo, which contributed, alongside the venous-derived LECs, to the development of the lymphatic vasculature in an organ-specific manner (Figure 3). The new data could be seen as settling the old controversy concerning the origin of lymphatic vessels, but it also opens up many new questions.
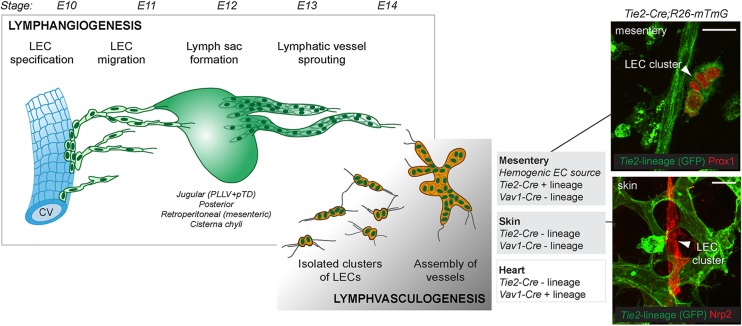
Figure 3.
Key developmental steps in the formation of the embryonic lymphatic vasculature. The first LECs arise through transdifferentiation from venous endothelial cells. Venous-derived LECs exit the veins and form primitive lymphatic structures, so-called lymph sacs, from which vessels sprout further to peripheral organs (lymphangiogenesis). Alongside, in certain organs, non-venous-derived LEC progenitors give rise to clusters of LECs that assemble into vessels (lymphvasculogenesis). Stages (E, embryonic days) of mouse development are shown on the top and key differences in the cellular lineages of non-venous-derived LECs in the mesentery, skin, and heart are depicted. Mesenteric non-venous-derived LECs form from a HemEC source and are traced (GFP+) by the pan-endothelial Tie2-Cre (top panel on the right). In contrast, both the non-venous dermal LECs of lumbar skin (bottom panel on the right) and part of the cardiac lymphatic vasculature form from Tie2 negative lineage (GFP-), and their precise cellular origin within the embryo still remains to be determined. Scale bars: 25 µm.
3.2 Venous origin of lymphatic vessels
During the past decade, the stepwise process of LEC commitment, which is driven by Prox1 expression in a subset of venous EC, and the formation of the first venous-derived lymphatic vessels have been carefully characterized in the mouse113,120,121 and zebrafish.114,115 Although some of the upstream regulators of Prox1 have been identified, it is still unclear how Prox1 expression and thus selection of LECs is restricted into specific veins to allow the formation of six primitive lymph sacs [i.e. paired jugular and posterior lymph sacs, retroperitoneal (mesenteric) lymph sac and cisterna chyli] in precise locations in the mammalian embryos. A recent study in zebrafish demonstrated that the commitment of LECs within the posterior cardinal vein (PCV) is linked with asymmetric division of Prox1 positive BECs, with daughter cells adopting different fates; one becomes LEC and up-regulates Prox1, while the other remains in the vein and down-regulates Prox1 expression.122 Intriguingly, another recent study reported that the zebrafish PCV contains specialized mesodermal-derived angioblasts that can give rise to LECs but also to other endothelial cell populations.123 These angioblasts were shown to reside on the ventral wall of the PCV and transit dorsally within the vein before giving rise to lymphatic sprouts.123 Together, these data demonstrate a previously unknown heterogeneity within the developing cardinal vein and suggest that LEC fate can be specified before budding from the PCV. It remains to be shown whether mammalian veins also harbour such angioblasts with capacity to differentiate into LECs.
3.3 Tie2 lineage negative, non-venous origin of the dermal lymphatic vasculature
Studies on the early development of the murine lymphatic vasculature have been focused on the analysis of the ‘jugular lymph sac’, i.e. the peripheral longitudinal lymphatic vessel (PLLV) and primordial thoracic duct (pTD)120 formation in the cervical and thoracic regions of the embryo. Lineage tracing and high-resolution visualization of the developing vasculature in this region,113,120 together with functional studies assessing the consequence of Prox1 deletion using the endothelial restricted Tie2-Cre line,113 suggested that all LECs descend from a Tie2 lineage endothelial source, consistent with their venous origin. However, we reported that although the cervical and thoracic dermal LECs were efficiently traced with the Tie2-Cre in E13 embryos, isolated clusters of LECs forming in the lumbar region of the skin were negative14 (Figure 3). Fluorescence-activated cell sorting analysis revealed that the Tie2 lineage negative LECs accounted for more than 30% of the entire dermal lymphatic vasculature in E13 embryos. Consistent with the major contribution of non-venous-derived LECs to the vessels of the lumbar skin, LEC clusters in this region could not be initially targeted and depleted by Tie2-Cre-induced deletion of Prox1. This was despite the efficient Tie2-Cre-induced recombination in the blood vasculature and in early venous-derived LEC progenitors, which lead to the conclusion that an alternative Tie2 lineage negative LEC progenitor source gives rise to the isolated clusters of LECs in the lumbar region of the skin.14
The isolated Tie2 lineage negative non-venous-derived LEC clusters rapidly coalesced to continuous vessel networks in a process we termed lymphvasculogenesis,14 analogous to the process of vasculogenesis occurring during early establishment of the blood vasculature system (reviewed in124). The source of the dermal non-venous LEC progenitors still remains to be discovered. Although lineage tracing data suggests a non-endothelial source of dermal LECs, it cannot be excluded that the Tie2 negative lineage reflects vessel formation from a so far non-characterized immature vascular bed. Several studies have suggested a haematopoietic origin of lymphatic progenitors,125–128 mainly based on the expression of shared markers, including, e.g. Pdpn, Lyve1, and Vegfr3, which are expressed by both LECs and haematopoietic cells including monocytes and macrophages. However, our studies excluded transdifferentiation of LECs from haematopoietic progenitors since Vav1-Cre, a pan-haematopoietic Cre line, did not show any tracing of dermal LECs.14 This is consistent with previous studies that also excluded Vav1-Cre lineage tracing of LECs.129
3.4 Non-venous haemogenic endothelial source of the mesenteric lymphatic vasculature
Similar to the lumbar skin, mesenteric lymphatic vessels were reported to form from isolated clusters of LECs that assemble into vessels, in contrast to the sprouting of venous-derived LECs from the mesenteric lymph sac.15 Intriguingly, in contrast to the dermal LEC progenitors, the mesenteric progenitors were efficiently traced by the Tie2-Cre transgene (Lukas Stanczuk and TM, unpublished data; Figure 3), which supports an endothelial origin of these LECs. However, rather than venous endothelium, the origin of the mesenteric LECs was traced to haemogenic endothelial cells (HemECs).15 This conclusion was based on two major observations: (1) Positive lineage tracing using the endothelial-specific Pdgfb-CreERT2 line, which, when induced at embryonic day (E)8-E9, labels efficiently all major haemogenic vessels including the dorsal aorta, vitelline artery, and the yolk sac (YS) vasculature, but not the venous-derived LEC progenitors, and (2) positive lineage tracing of E10–E11-induced cKit-CreERT2. cKit is a marker associated with HemECs and HemEC-derived haematopoietic progenitors from all known haemogenic sites of the embryo and the YS.130 Using these strategies, it was not, however, possible to distinguish between the different haemogenic sites. Like in the skin, the mesenteric LECs could not be traced by Vav1-Cre, which suggests a HemEC-derived lineage separate from known haematopoietic lineages.
Whether distinct mechanisms differentially regulate lymphatic vessel formation from venous and non-venous LEC progenitors is largely unknown. Mahadevan et al.131 reported that in the chick and mouse, gut lymphatic vessel formation relies on prior growth of arteries that coincides with the asymmetric gut tube looping and is restricted to the left side of the developing dorsal mesentery. Asymmetrically expressed Pitx2 drives expression of the Cxcr4 ligand Cxcl12 on the left side, thereby directing asymmetric arteriogenesis, and regulates mesenteric lymphatic vessel formation in an organ-specific manner. In particular, Pitx2 deficiency was shown to specifically inhibit the formation of the mesenteric lymphatic vessels, but not the venous-derived mesenteric lymph sac, which was concluded to indicate the existence of an alternative non-venous-derived LEC progenitor population.131 Organ-specific effects of genetic targeting of the Vegfr3/PI3K kinase pathway further indicated differences in the molecular regulation of mesenteric compared with dermal lymphatic vessel formation. Deletion of p110α catalytic subunit of the PI3K specifically in the LECs, or combined haploinsufficiency for genes encoding p110α and Vegfr3 resulted in organ-specific defects in the development of mesenteric and intestinal lymphatic vessels.15 In addition, global deletion of the p110α regulatory subunits p85α, p55α, and p50α resulted in organ-specific lymphangiectasia of intestinal lymphatic vessels with less pronounced defects in other lymphatic vascular beds.132
Although the dermal and mesenteric non-venous LEC progenitors have distinct embryonic sources, they display similarities in marker expression. Non-venous-derived LECs can be differentiated by their initial low expression of Lyve1 both in the mesentery and skin.14,15 Lyve1 is, however, quickly up-regulated as the clusters assemble, and the non-venous-derived LECs share expression of several markers of venous-derived LECs, including Vegfr2, Vegfr3, Prox1, Nrp2, and Pdpn. Also Tie2, which is initially not expressed in the dermal LEC clusters, is induced as the vessels assemble and mature.14
3.5 Non-venous origin of cardiac LEC progenitors
Klotz et al. reported that part of the lymphatic vasculature in the heart, similar to the dermal LEC clusters, could not be traced by the Tie2-Cre transgene.13 This suggests that local blood vessels, which are presumably efficiently targeted by the Tie2 transgene, are unlikely to provide a source of cardiac LECs, but they are instead derived from a non-endothelial cell origin or a Tie2 negative vascular bed. Based on positive lineage tracing with the haematopoietic Vav1-Cre and E7 induced Csf1r1MeriCreMer, YS HemECs were suggested as the source of cardiac LECs.13 It was previously shown that early induction of Csf1r1MeriCreMer labels erythro-myeloid progenitors (EMPs), which are the major haematopoietic progenitors produced in the YS.133,134 However, YS-derived EMPs can also be labelled in Tie2MeriCreMer by induction at E7.5,134 and it would thus be expected that YS-derived haematopoietic progenitors and their progeny, in contrast to the non-venous-derived cardiac LECs, are similarly targeted by the constitutive Tie2-Cre transgene. Supporting this notion, constitutive Tie2-Cre labels the majority of CD11b+ myeloid cells present in the E10 embryo which at this stage mainly originate from the YS.135 Further evidence for cardiac LEC origin was provided by lineage tracing by Pdgfrb-Cre, which was postulated to support a HemECs YS-derived lineage.13 This was based on an earlier report of Pdgfrβ immunoreactivity in the YS haemogenic endothelium,136 which is in contrast to the absence of Pdgfrβ expression in other BECs.137 Our recent data, however, indicate that the Pdgfrb-Cre transgene does not significantly label YS haemogenic endothelium or YS-derived haematopoietic progenitors135 and the conclusion on the Pdgfrb-Cre-induced tracing of LECs as a proof for a YS-specific lineage of non-venous-derived cardiac LEC progenitors may need re-evaluation. Based on the available data, cardiac non-venous LECs must thus be concluded to derive from a specific haematopoietic Vav1 positive cell lineage with a link to an early YS Csf1r1 lineage originating from a unique, previously unknown Tie2 negative cell population. However, as discussed above, Tie2 negative lineage tracing may alternatively indicate a non-endothelial rather than endothelial origin of cardiac LECs. Klotz et al. excluded several non-endothelial sources, including the WT1+ pro-epicardial organ, Mesp1+ or Nkx2.5+ mesoderm and Wnt1+ neural crest,13 and the precise identity of the cardiac LEC progenitor thus remains to be determined.
4. Concluding remarks
Reducing or increasing lymphatic vessel density and the lymphangiogenic response have been put forward as possible therapeutic tools to modulate inflammatory responses and cancer metastasis. However, in light of our new understanding of lymphatic diversification in the regulation of vessel- and vascular bed-specific functions, new strategies to tune lymphatic responses in disease by regulating the molecular patterning of vessels may prove to be as important as regulation of vessel density alone. However, we still lack an adequate understanding of the organ- and vessel-type-specific molecular differences of endothelium, how they contribute to specific functions of lymphatic vessels, and how the establishment and maintenance of these features are regulated.
The discovery of (an) alternative non-venous origin(s) of LECs that contribute to the lymphatic vasculature of the skin, mesentery, and heart has changed our understanding of the mechanisms of embryonic lymphatic vessel formation. Intriguingly, the source of the non-venous LEC progenitors appears to be different in different organs, potentially contributing to the development of organ-specific features within the lymphatic network. We currently lack tools that allow isolation of non-venous-derived LEC progenitors or their specific tracing in embryonic and postnatal tissues. These tools will be important for assessing the contribution of different types of LEC progenitors to specific vascular beds and for studying their role in lymphatic vessel homeostasis, regeneration, and organ-specific molecular adaptation.
Funding
The work was supported by the European Research Council (ERC-2014-CoG-646849) and the Swedish Research Council. Funding to pay the Open Access publication charges for this article was provided by the European Research Council.
Acknowledgements
We would like to thank Elizabeth Jones for insightful discussion on the origin of YS progenitors. We also thank Yan Zhang, Sophie Lutter, Lukas Stanczuk, and Ines Martinez-Corral for providing immunofluorescence images.
Conflict of interest: none declared.
References
- 1.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest 2014;124:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebischer D, Iolyeva M, Halin C. The inflammatory response of lymphatic endothelium. Angiogenesis 2014;17:383–393. [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest 2014;124:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon JB. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab 2010;21:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res 2016;118:515–530. [DOI] [PubMed] [Google Scholar]
- 6.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 2013;26:204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norrmen C, Vandevelde W, Ny A, Saharinen P, Gentile M, Haraldsen G, Puolakkainen P, Lukanidin E, Dewerchin M, Alitalo K, Petrova TV. Liprin (beta)1 is highly expressed in lymphatic vasculature and is important for lymphatic vessel integrity. Blood 2010;115:906–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med 2015;7:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004;5:74–80. [DOI] [PubMed] [Google Scholar]
- 10.Yao LC, Testini C, Tvorogov D, Anisimov A, Vargas SO, Baluk P, Pytowski B, Claesson-Welsh L, Alitalo K, McDonald DM. Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ Res 2014;114:806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saaristo A, Veikkola T, Enholm B, Hytonen M, Arola J, Pajusola K, Turunen P, Jeltsch M, Karkkainen MJ, Kerjaschki D, Bueler H, Yla-Herttuala S, Alitalo K. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J 2002;16:1041–1049. [DOI] [PubMed] [Google Scholar]
- 12.Enholm B, Karpanen T, Jeltsch M, Kubo H, Stenback F, Prevo R, Jackson DG, Yla-Herttuala S, Alitalo K. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res 2001;88:623–629. [DOI] [PubMed] [Google Scholar]
- 13.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 2015;522:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, John SW, Alitalo K, Ortega S, Makinen T. Nonvenous origin of dermal lymphatic vasculature. Circ Res 2015;116:1649–1654. [DOI] [PubMed] [Google Scholar]
- 15.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, Alitalo K, Graupera M, Makinen T. cKit Lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep 2015;10:1708–1721. [DOI] [PubMed] [Google Scholar]
- 16.Vittet D. Lymphatic collecting vessel maturation and valve morphogenesis. Microvasc Res 2014;96:31–37. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest 2014;124:888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koltowska K, Betterman KL, Harvey NL, Hogan BM. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development 2013;140:1857–1870. [DOI] [PubMed] [Google Scholar]
- 19.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007;204:2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leak LV, Burke JF. Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol 1968;36:129–149. [PMC free article] [PubMed] [Google Scholar]
- 21.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 2009;206:2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 2005;19:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wick N, Haluza D, Gurnhofer E, Raab I, Kasimir MT, Prinz M, Steiner CW, Reinisch C, Howorka A, Giovanoli P, Buchsbaum S, Krieger S, Tschachler E, Petzelbauer P, Kerjaschki D. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am J Pathol 2008;173:1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 2013;339:328–332. [DOI] [PubMed] [Google Scholar]
- 25.Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 2010;32:703–713. [DOI] [PubMed] [Google Scholar]
- 26.Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschke M, Santambrogio L, Kerjaschki D, Sixt M, Halin C. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep 2016;14:1723–1734. [DOI] [PubMed] [Google Scholar]
- 27.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol 2013;190:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, Magistrelli G, Masternak K, Chevailler A, Delneste Y, Jeannin P. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011;117:1196–1204. [DOI] [PubMed] [Google Scholar]
- 29.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002;21:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol 2007;27:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 2015;194:5200–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pond CM, Mattacks CA. The activation of the adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine 2002;17:131–139. [DOI] [PubMed] [Google Scholar]
- 33.Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med 2012;33:35–45. [DOI] [PubMed] [Google Scholar]
- 34.Benezech C, Mader E, Desanti G, Khan M, Nakamura K, White A, Ware CF, Anderson G, Caamano JH. Lymphotoxin-beta receptor signaling through NF-kappaB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity 2012;37:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell 2009;17:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bazigou E, Makinen T. Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci 2013;70:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, Augustin HG, Yla-Herttuala S, Alitalo K, Petrova TV. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol 2009;185:439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, Secker GA, Agalarov Y, Demir CS, Lawrence DM, Sutton DL, Tabruyn SP, Miura N, Salminen M, Petrova TV, Matthews JM, Hahn CN, Scott HS, Harvey NL. GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest 2015;125:2979–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes JM, Adams RH, Makinen T, Kiefer F, Kwak BR, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 2012;22:430–445. [DOI] [PubMed] [Google Scholar]
- 40.Sweet DT, Jimenez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, Kahn ML. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J Clin Invest 2015;125:2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, Kwak BR, Makinen T, Martinez-Corral I, Ortega S, Delorenzi M, Kiefer F, Davis MJ, Djonov V, Miura N, Petrova TV. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 2015;125:3861–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller MJ, McDole JR, Newberry RD. Microanatomy of the intestinal lymphatic system. Ann N Y Acad Sci 2010;1207(Suppl 1):E21–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe K, Jang JY, Park I, Kim Y, Ahn S, Park DY, Hong YK, Alitalo K, Koh GY, Kim P. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest 2015;125:4042–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernier-Latmani J, Cisarovsky C, Demir CS, Bruand M, Jaquet M, Davanture S, Ragusa S, Siegert S, Dormond O, Benedito R, Radtke F, Luther SA, Petrova TV. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J Clin Invest 2015;125:4572–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol 2012;5:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest 2013;123:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab 2013;17:671–684. [DOI] [PubMed] [Google Scholar]
- 48.Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K, Yla-Herttuala S. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464–472. [DOI] [PubMed] [Google Scholar]
- 50.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 2005;37:1072–1081. [DOI] [PubMed] [Google Scholar]
- 51.Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol 2010;176:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol 2012;49:32–43. [DOI] [PubMed] [Google Scholar]
- 53.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 2008;582:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner's guide. Neurochem Res 2015;40:2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015;212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andres KH, von During M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 1987;175:289–301. [DOI] [PubMed] [Google Scholar]
- 58.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015;36:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG, Chappell J, Wilting J, Weich HA, Yamagami S, Amano S, Mizuki N, Alexander JS, Peterson ML, Brekken RA, Hirashima M, Capoor S, Usui T, Ambati BK, Ambati J. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med 2009;15:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh N, Tiem M, Watkins R, Cho YK, Wang Y, Olsen T, Uehara H, Mamalis C, Luo L, Oakey Z, Ambati BK. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013;121:4242–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakao S, Hafezi-Moghadam A, Ishibashi T. Lymphatics and lymphangiogenesis in the eye. J Ophthalmol 2012;2012:783163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park DY, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong YK, Koh GY. Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity. J Clin Invest 2014;124:3960–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen VM, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, Immonen I, Alitalo K. The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest 2014;124:3975–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Truong TN, Li H, Hong YK, Chen L. Novel characterization and live imaging of Schlemm's canal expressing Prox-1. PLoS One 2014;9:e98245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong YK, Fawzi A, Liu X, Kume T, Quaggin SE. A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest 2014;124:4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol 2014;12:e1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev 2013;251:160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol 2012;33:271–280. [DOI] [PubMed] [Google Scholar]
- 69.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med 2000;192:1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 2005;22:19–29. [DOI] [PubMed] [Google Scholar]
- 71.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med 2001;194:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun 2012;4:424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rantakari P, Auvinen K, Jappinen N, Kapraali M, Valtonen J, Karikoski M, Gerke H, Iftakhar-E-Khuda I, Keuschnigg J, Umemoto E, Tohya K, Miyasaka M, Elima K, Jalkanen S, Salmi M. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat Immunol 2015; 16:386–396. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 74.Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol Med 2007;11:621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol 2008;181:6189–6200. [DOI] [PubMed] [Google Scholar]
- 76.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 2007;27:160–171. [DOI] [PubMed] [Google Scholar]
- 77.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 2009;10:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol 2011;12:879–887. [DOI] [PubMed] [Google Scholar]
- 79.Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, Chan L, Lucas B, Novitzky-Basso I, Nakamura K, Rulicke T, Nibbs RJ, Worbs T, Forster R, Rot A. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol 2014;15:623–630. [DOI] [PubMed] [Google Scholar]
- 80.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, Condeelis J, Skobe M. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med 2013;210:1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med 2004;200:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 2010;207:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007;316:295–298. [DOI] [PubMed] [Google Scholar]
- 84.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355–360. [DOI] [PubMed] [Google Scholar]
- 85.Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol 2009;10:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ito K, Morimoto J, Kihara A, Matsui Y, Kurotaki D, Kanayama M, Simmons S, Ishii M, Sheppard D, Takaoka A, Uede T. Integrin alpha9 on lymphatic endothelial cells regulates lymphocyte egress. Proc Natl Acad Sci U S A 2014;111:3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med 2010;207:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, Adler AJ, Chen L, Engelhard VH. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 2012;120:4772–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 2012;1:191–199. [DOI] [PubMed] [Google Scholar]
- 90.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol 2014;192:5002–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, LeibundGut-Landmann S, Garbi N, Reith W, Swartz MA, Hugues S. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med 2014;211:1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun 2014;5:3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, Liblau R, Makinen T, Engelhard VH. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun 2015;6:6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benezech C, White A, Mader E, Serre K, Parnell S, Pfeffer K, Ware CF, Anderson G, Caamano JH. Ontogeny of stromal organizer cells during lymph node development. J Immunol 2010;184:4521–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen JN, Tewalt EF, Rouhani SJ, Buonomo EL, Bruce AN, Xu X, Bekiranov S, Fu YX, Engelhard VH. Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PLoS One 2014;9:e87740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest 2014;124:953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo R, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT, Pytowski B, Zhu Z, Wang YJ, Schwarz EM, Xing L. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum 2009;60:2666–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jurisic G, Sundberg JP, Detmar M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm Bowel Dis 2013;19:1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol 2012;189:2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen J, Jiao S, Gao Y, Liu C, Duan Z, Li D, He Y, Wei B, Wang H. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-kappaB signaling and protects against endotoxin shock. Immunity 2014;40:501–514. [DOI] [PubMed] [Google Scholar]
- 101.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008;454:656–660. [DOI] [PubMed] [Google Scholar]
- 102.Alitalo AK, Proulx ST, Karaman S, Aebischer D, Martino S, Jost M, Schneider N, Bry M, Detmar M. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res 2013;73:4212–4221. [DOI] [PubMed] [Google Scholar]
- 103.Garrafa E, Imberti L, Tiberio G, Prandini A, Giulini SM, Caimi L. Heterogeneous expression of toll-like receptors in lymphatic endothelial cells derived from different tissues. Immunol Cell Biol 2011;89:475–481. [DOI] [PubMed] [Google Scholar]
- 104.Pegu A, Qin S, Fallert Junecko BA, Nisato RE, Pepper MS, Reinhart TA. Human lymphatic endothelial cells express multiple functional TLRs. J Immunol 2008;180:3399–3405. [DOI] [PubMed] [Google Scholar]
- 105.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol 2009;183:1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee KM, McKimmie CS, Gilchrist DS, Pallas KJ, Nibbs RJ, Garside P, McDonald V, Jenkins C, Ransohoff R, Liu L, Milling S, Cerovic V, Graham GJ. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood 2011;118:6220–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, Rot A. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol 2001;158:867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol 2005;6:403–411. [DOI] [PubMed] [Google Scholar]
- 109.Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011;118:205–215. [DOI] [PubMed] [Google Scholar]
- 110.Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, Vestweber D, Jackson DG. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res 2008;68:7293–7303. [DOI] [PubMed] [Google Scholar]
- 111.Sabin FR. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am J Anat 1902;1:367–389. [Google Scholar]
- 112.Huntington G, McClure C. The anatomy and development of the jugular lymph sac in the domestic cat (Felis domestica). Am J Anat 1910;10:177–312. [Google Scholar]
- 113.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 2007;21:2422–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol 2006;16:1244–1248. [DOI] [PubMed] [Google Scholar]
- 115.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med 2006;12:711–716. [DOI] [PubMed] [Google Scholar]
- 116.Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, Valasek P, Papoutsi M. Dual origin of avian lymphatics. Dev Biol 2006;292:165–173. [DOI] [PubMed] [Google Scholar]
- 117.Schneider M, Othman-Hassan K, Christ B, Wilting J. Lymphangioblasts in the avian wing bud. Dev Dyn 1999;216:311–319. [DOI] [PubMed] [Google Scholar]
- 118.Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, Terclavers S, Ciesiolka M, Kalin R, Man WY, Senn I, Wyns S, Lupu F, Brandli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med 2005;11:998–1004. [DOI] [PubMed] [Google Scholar]
- 119.Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn 2006;235:1554–1562. [DOI] [PubMed] [Google Scholar]
- 120.Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 2013;32:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 2012;120:2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koltowska K, Lagendijk AK, Pichol-Thievend C, Fischer JC, Francois M, Ober EA, Yap AS, Hogan BM. Vegfc regulates bipotential precursor division and Prox1 expression to promote lymphatic identity in zebrafish. Cell Rep 2015;13:1828–1841. [DOI] [PubMed] [Google Scholar]
- 123.Nicenboim J, Malkinson G, Lupo T, Asaf L, Sela Y, Mayseless O, Gibbs-Bar L, Senderovich N, Hashimshony T, Shin M, Jerafi-Vider A, Avraham-Davidi I, Krupalnik V, Hofi R, Almog G, Astin JW, Golani O, Ben-Dor S, Crosier PS, Herzog W, Lawson ND, Hanna JH, Yanai I, Yaniv K. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 2015;522:56–61. [DOI] [PubMed] [Google Scholar]
- 124.Ribatti D, Nico B, Crivellato E. The development of the vascular system: a historical overview. Methods Mol Biol 2015;1214:1–14. [DOI] [PubMed] [Google Scholar]
- 125.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med 2006;12:230–234. [DOI] [PubMed] [Google Scholar]
- 126.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 2005;106:4184–4190. [DOI] [PubMed] [Google Scholar]
- 127.Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, Yun SH, Yoon YS. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 2010;122:1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Buttler K, Lohrberg M, Gross G, Weich HA, Wilting J. Integration of CD45-positive leukocytes into newly forming lymphatics of adult mice. Histochem Cell Biol 2016;145:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 2010;116:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gritz E, Hirschi KK. Specification and function of hemogenic endothelium during embryogenesis. Cell Mol Life Sci 2016;73:1547–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahadevan A, Welsh IC, Sivakumar A, Gludish DW, Shilvock AR, Noden DM, Huss D, Lansford R, Kurpios NA. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev Cell 2014;31:690–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mouta-Bellum C, Kirov A, Miceli-Libby L, Mancini ML, Petrova TV, Liaw L, Prudovsky I, Thorpe PE, Miura N, Cantley LC, Alitalo K, Fruman DA, Vary CP. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85alpha, p55alpha, and p50alpha. Dev Dyn 2009;238:2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015;42:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015;518:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ulvmar MH, Martinez-Corral I, Stanczuk L, Makinen T. Pdgfrb-Cre targets lymphatic endothelial cells of both venous and non-venous origins. Genesis 2016; 54:350–358. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, Lindblom P, Norlin J, Betsholtz C, Heuchel R, Welsh M, Claesson-Welsh L. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood 2006;108:1877–1886. [DOI] [PubMed] [Google Scholar]
- 137.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]