Abstract
A photosensitive protein resembling the visual pigments of invertebrates enables phototactic archaebacteria to distinguish colour. This protein exists in two spectrally-distinct forms, one of which is a transient photoproduct of the other and each of which undergoes photochemical reactions controlling the cell’s swimming behaviour. Activation of a single pigment molecule in the cell is sufficient to signal the flagellar motor. This signal-transduction mechanism makes evident a colour-sensing capability inherent in the retinal/protein chromophore.
MANY bacterial species alter their swimming behaviour in response to environmental stimuli. Chemotactic bacteria, for example, sense particular nutritive and noxious substances and respond to them as attractants and repellents, respectively1. Halobacterium halobium, a flagellated archaebacterium which grows in saturated brine, exhibits Chemotaxis and is also phototactic; the cells are attracted to long wavelength visible light (the ‘red’ light response) and repelled by shorter wavelength light (the ‘blue’ light response)2. The first quantitative studies of these responses2 revealed both sensitivities present simultaneously. In the absence of photostimuli, the cells move in a three-dimensional random walk, swimming short stretches in an approximately straight line and periodically reversing direction through roughly 180°. Light intensity gradients bias the random walk by modulating the frequency of reversals3. Increases in blue or decreases in red light intensity are interpreted by the cell as movement in an unfavourable direction causing reversal of swimming direction. The opposite intensity changes suppress reversals. As in the case of bacterial Chemotaxis, the flagellar motor responses are transient; the cells adapt to the new light intensity and resume their prestimulus reversal frequency3.
Vitamin A aldehyde (retinal) forms the chromophore of visual pigments and is required also for photosensory reception in halobacterial phototaxis4,5. Two retinal-containing pigments, bacteriorhodopsin and halorhodopsin, function as light-driven ion transporters in H. halobium6. These pigments may be attractant receptors and their short wavelength photointermediates repellent receptors7. Mutants lacking both bacteriorhodopsin and halorhodopsin exhibit normal colour-discriminating phototaxis8, indicating that at least one additional retinal pigment must exist in this organism.
We have detected this third photoreactive retinal-containing membrane protein in H. halobium9. Like bacteriorhodopsin and halorhodopsin, photoexcitation of the third pigment is followed by a sequence of thermal steps returning the molecule to its original state. This cyclic process (or photocycle) has a half-time of ~0.8 s at 23 °C, in contrast to the faster (~ 10 ms) photocycles of bacteriorhodopsin and halorhodopsin. The slow-cycling rhodopsin does not appear to mediate active ion transport8,9 and is the only spectroscopically detected retinal pigment in phototactic strains which lack bacteriorhodopsin and halorhodopsin9,10. Addition of retinal to a mutant blocked in retinal synthesis generates both slow-cycling rhodopsin and phototaxis with similar kinetics and retinal concentration dependence10,11. These properties and its absorbance in the region of attractant (red) sensitivity10 make this protein a likely candidate for the attractant photoreceptor. The repellent photoreceptor, however, poses a problem because no separate pigment active in the region of repellent (blue) sensitivity is detectable by sensitive flash photolysis analysis (detection limit 50 molecules per cell, unpublished data). The work we report here shows that a solution to this dilemma can be found within the slow-cycling rhodopsin photoreactions. The repellent receptor is expected to absorb maximally near 370 nm according to phototaxis action spectra2,4; photoexcitation generates an intermediate species with maximal absorbance at 373 nm (S373) within a few microseconds9 which, in addition to its slow thermal relaxation, can more rapidly reconvert to the red-absorbing form of the molecule (sR587) by absorption of a second photon9.
We considered that slow-cycling rhodopsin in its S373 form might mediate the repellent responses. If this is correct, no repellent responses should occur in the absence of S373 and repellent sensitivity should appear when S373 is generated by illumination of sR587. The work presented here shows these predictions are borne out and reveal a mechanism for colour-sensing based on the dual role of slow-cycling rhodopsin as attractant and repellent photosensory receptor.
Swimming behaviour
The absorption spectrum of sR587 is so broad that any visible light will generate S373. Thus all published measurements of repellent responses have been made in the presence of S373 generated by the light used to observe the bacteria. To avoid exciting sR587, we here observe the cells with light of wavelenths >750 nm using an infrared-sensitive camera and track the cells on a video monitor. This light is beyond the absorption range of sR587 and therefore no S373 is generated. Under these conditions, strong UV light which has been found previously12 to be strongly repellent, causes no change in reversal probability (Fig. 1). Furthermore, addition of red background light which generates S373 sensitizes the cells to the repellent stimulus, causing nearly every cell to reverse swimming direction within 2 s (Fig. 1). We attribute this sensitization to the generation of a photostationary state containing the repellent receptor (S373) by red light.
Fig. 1.
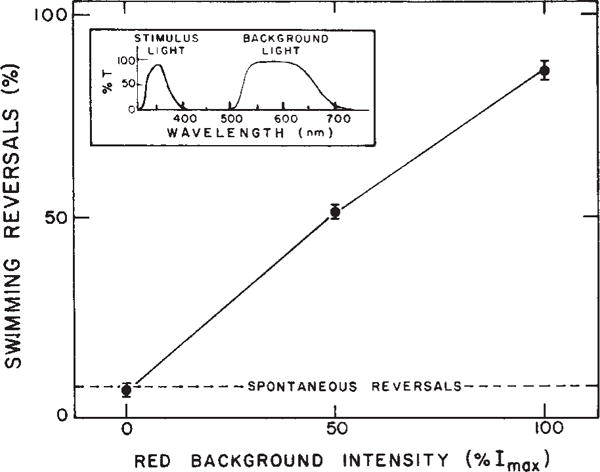
Dependence of near ultraviolet repellent response on visible background light. The figure shows the % of cells reversing within 2 s of the near UV stimulus as a function of red background intensity. Points are average values ± extreme values of 2 separate determinations, each based on >40 cells. The inset shows the transmittance spectra of the filter combinations used for stimulus and visible background irradiation. The stimulus and observation light from a 150 W mercury (Hg) lamp and 50 W tungsten Zeiss microscope illuminator, respectively, were combined through a glass beam splitter which allowed passage of 30% of the infrared observation light (Kodak 87A filter) and 30% of the Hg lamp radiation. The combined beam illuminated the cells through the microscope phase condensor. The red background light was provided by an optically collimated 50 W tungsten light source reflected by a quartz beam-splitter mounted below the condenser. Light fluxes at the sample were measured with a Kettering 65 Radiometer and intensities calculated using irradiation areas measured with a calibrated ocular Zeiss micrometer. Light intensity was varied with Kodak neutral density filters. Stimulus light intensity, 1,400 erg cm−2 s−1. Maximum red background intensity (Imax), 7.2 × 104 erg cm−2 s−1. Infrared radiation, >750 nm.
One might argue that the adaptation to the red stimulus lowered the threshold sensitivity to blue light because the background red light generated a transient attractant response when it was turned on. This possibility is ruled out because we show here that red light sensitizes the blue response even if it is delivered simultaneously with the blue stimulus.
Photochemical reactions
Because of its fast rise and slow decay, S373 accumulates in significant amounts in natural light absorbed by sR587, producing a photostationary state mixture of the two species sR587 and S373. The absorption spectra of these species, calculated from the photostationary state difference spectrum, are shown in Fig. 2. The spectra are sufficiently broad to permit laser photoexcitation of both species at 406 nm so that flash-induced absorbance changes at their regions of maximum absorption could be monitored with minimal interference from the intense actinic flash.
Fig. 2.
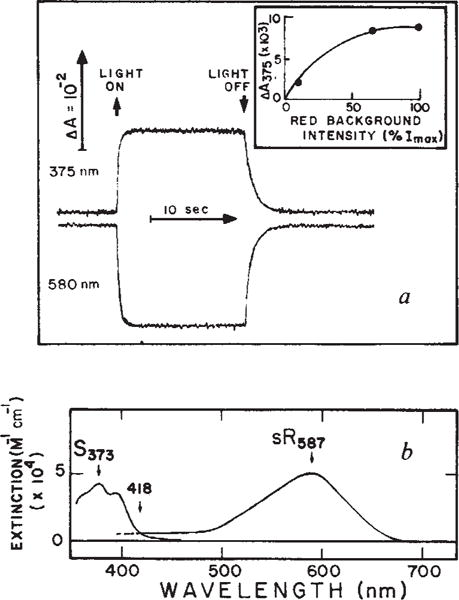
a, Absorbance changes of sensory rhodopsin (sR) at 375 and 580 nm induced by red background light. The sample was a suspension of Flx3R vesicles, prepared vesicles, prepared and regenerated in a 3 × 3 mm quartz cuvette clear on four sides with all-trans retinal as described10. The regeneration of sensory rhodopsin was monitored as described10, use of the regenerated system permitting precise quantitation of the sensory rhodopsin content. Flx3 vesicles containing native sensory rhodopsin gave the same phototransients as the regenerated protein in Flx3R shown here. Flash-induced absorbance changes were measured as described9. Background excitation was provided by a tungsten halogen lamp beam passed through a long pass Corning 2–61 (cutoff 610 nm) and Ditric Optics 700 nm short pass filter combination and applied to the sample at 90° with respect to the measuring beam. The maximum red light intensity (Imax) was 4.25 × 106 erg cm−2 s−1. The inset shows the light intensity-dependence of the absorbance changes at 375 nm (ΔA375). b, Spectrum of S373 and sR587. The latter is the spectrum obtained by regeneration of sensory rhodopsin apoprotein with all-trans retinal9 and the former was calculated by adding the sR587 spectrum to the light-induced difference spectrum obtained using the system described in a. The extinction coefficients are based on our measurements9,10 and have absolute uncertainty of ±15%.
In the absence of background illumination, excitation at 406 nm causes a transient depletion of sR587 (Fig. 3a). This is due to the sR587 to S373 photoconversion as shown previously9. In the presence of saturating background illumination, which depletes the sample of sR587 (Fig. 2a), excitation causes a transient regeneration of sR587 from S373 (Fig. 3b, d). Absorbance changes measured at 500 nm (Fig. 3c) reveal an intermediate state in the S373 to sR587 reaction which forms in less than 50 μs. Further analysis of flash kinetic data at other wavelengths (Fig. 3) shows that this intermediate state absorbs maximally near 510 nm and decays into sR587 with a half-time of about 80 ms. Absorption of red background light by sR587 regenerates S373 (Fig. 3d). Note that this establishes a cyclic reaction containing two photon absorption steps (Fig. 4).
Fig. 3.
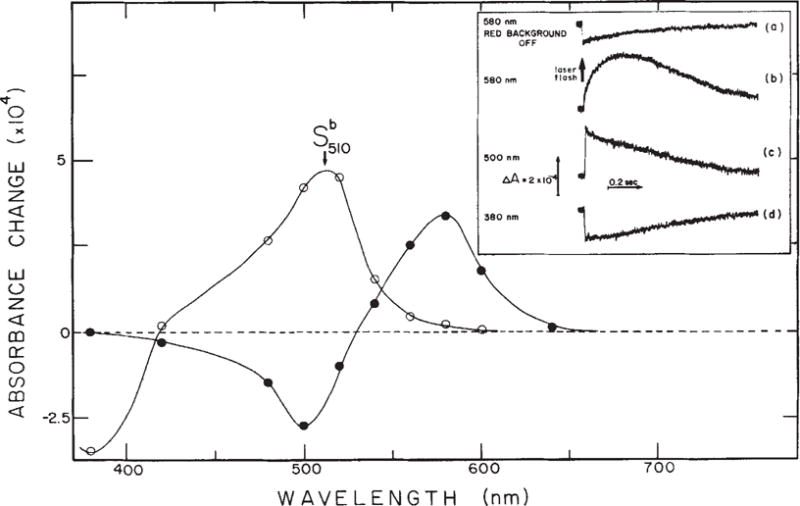
Flash-induced absorbance changes of S373. Inset, transient absorption changes of the sample shown in Fig. 2 in the absence (a) and in the presence (b–d) of 100% Imax red background illumination at three selected wavelengths. Actinic laser flash: 406 nm, 10 ns, 0.4 mJ. Main figure from traces shown in the inset and at other wavelengths the difference in the amplitude of absorbance change between 0 and 100 μs (○) and between 200 ms and 100 μs (●) were calculated and plotted as functions of wavelength. Note that at 580 nm the ratio of the forward to back reaction absorbance change amplitude is the same as the ratio of absolute absorbances of sR587 and S373 at 406 nm (Fig. 2). This indicates that the photochemical quantum yields of the two reactions are nearly equal.
Fig. 4.
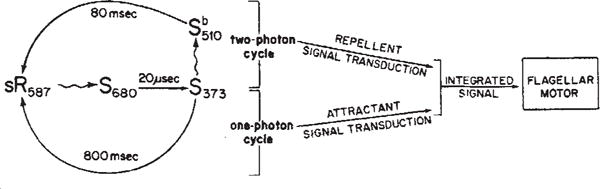
Mechanism of colour discrimination in H. halobium phototaxis. Photon absorption by sR587, the dark-adapted form of sensory rhodopsin, generates a short-lived species S680 which rapidly decays to S373. The S373 form can return to the sR587 form by either of two paths: a purely thermal relaxation or a photoinduced path. If S373 does not absorb a photon, it relaxes thermally to sR587 completing the ‘one-photon’ photocycle. During its transit through the one-photon cycle, the sensory rhodopsin molecule generates an attractant signal to the flagellar motor. If S373 absorbs a photon, it generates the intermediate . The relaxation of to sR587 completes a cycle containing two photon absorption steps. After absorption of the second photon in the ‘two-photon’ cycle, sensory rhodopsin generates a repellent signal to the flagellar motor. The attractant and repellent signals are integrated prior to or at their site of action on the flagellar motor3.
Colour-sensing mechanism
Spectral discrimination by slow-cycling rhodopsin can be understood as an interplay between the two-photon cycle discussed above and the one-photon cycle generated by photoexcitation of sR587 and its subsequent thermal steps (Fig. 4). We propose that the one-photon reaction generates an attractant signal, transmitted to the flagellar motor by an attractant signal transduction pathway. Increases (decreases) in this signal suppress (induce) flagellar reversals. These effects occur in the absence of any background illumination. In contrast, the two-photon cycle generates a repellent signal, transmitted and amplified separately from the attractant signal, but integrated eventually before or at the site of action on the flagellar motor3. Increases (decreases) in this signal induce (suppress) flagellar reversals.
Test of the mechanism
Previous studies have shown that the cells are at least an order of magnitude more sensitive to repellent (blue) light than to attractant (red) light intensity changes2,3. According to our interpretation this sensitivity difference must be conferred by amplification step(s) beyond the photoreceptor, because there can never be more repellent receptor molecules (S373 than the maximum number of molecules available for attractant reception (sR587) and their photochemical quantum yields are nearly equal (see Fig. 3 legend). Therefore the repellent signal from the two-photon cycle must be at least an order of magnitude more effective in inducing reversals than the one-photon cycle is in suppressing them. This leads to a seemingly paradoxical prediction which is a strong test of the mechanism: we should be able to construct conditions in which an increase in red light will generate a repellent rather than the usual attractant response.
According to the proposed mechanism (Fig. 4), the repellent signal produced by S373 photoexcitation should be generated by any of three illumination patterns. First, photoexciting S373 previously generated by background red light (as in Fig. 1). This is the usual stimulus which makes blue light a repellent under natural illumination.
Second, imposing an intense background of blue illumination which, in the absence of red light, will not influence the cells’ swimming behaviour because slow-cycling rhodopsin is almost completely in the sR587 form, followed by stimulation with red light. The S373 thereby generated will undergo the two-photon cycle, which should cause the repellent rather than the attractant response. This condition is realized in Fig. 5 where a given increase in red light was delivered in the absence and in the presence of blue background illumination. A clear suppression of spontaneous reversals occurs in the absence of background blue as expected from the one-photon cycle attractant signal. The dramatic result is that the same red light stimulus elicits a repellent (reversal-inducing) response in the presence of the highest blue light intensity.
Fig. 5.
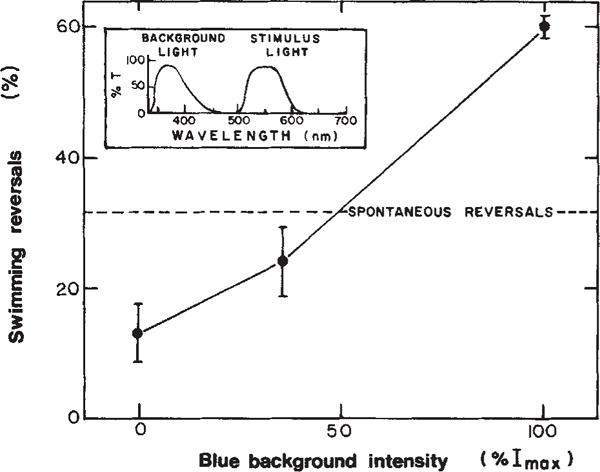
Effect of near ultraviolet background light on cell behavioural response to red light. Experimental conditions as described in Fig. 1 except that the response was assayed as the % reversals within 3 s of a red light stimulus. The range of spontaneous reversal frequencies of populations used in these studies was 14% ± 7% reversing within 2 s. All effects of background illumination on behaviour reported here and in Figs 2 and 6 have been confirmed in populations with both high and low spontaneous reversal frequencies.
Third, delivering both red and blue lights simultaneously at the same intensities as Fig. 5. Again the repellent response should result since it makes no difference whether the blue light was present prior to the generation of S373.
All the permutations of blue and red light stimuli are shown in Fig. 6. Our results can be interpreted in terms of the proposed model. Figure 6a gives the spontaneous reversal frequency of 22%, which is not affected significantly by blue alone. Figure 6b shows 16% reversals, because there is essentially no S373 (some S373 will be generated by the weak sR587 absorption in the blue, but calculations show the amount of S373 formed is so little that no two-photon cycling will occur in the time of the measurement). The three illumination patterns discussed above are shown by Fig. 6c, e and f respectively, all inducing an almost complete reversal response. Red light alone significantly suppresses reversals (Fig. 6d).
Fig. 6.
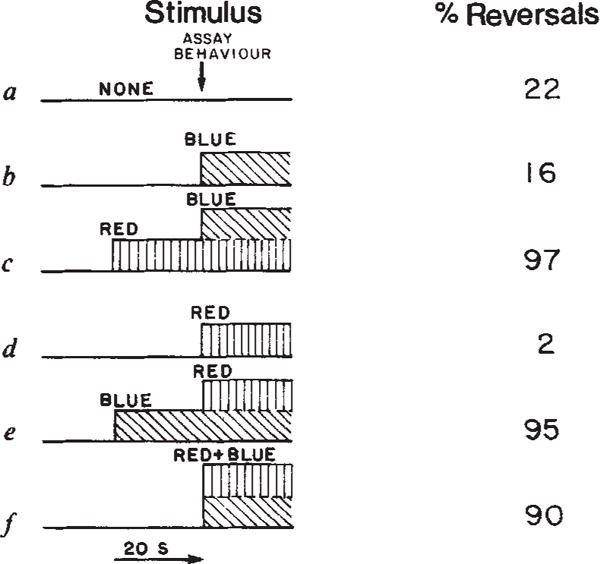
Behavioural response under various permutations of stimulus and background light. Values are averages of duplicate measurements which vary by less than 15%. Experimental conditions as described in Fig. 1 except that reversals were assayed within 3 s of the stimulus, blue and red light were delivered using a tungsten light source and wavelengths were selected by adding or removing filters from the single beam. The Hg light source was blocked in this experiment. ‘Blue’, 380–433 nm (Optics Technology 433 short pass filter+2 mm Plexiglass + two 1–75 Corning infrared absorbing glass), intensity 3.3 × 104 erg cm−2 s−1. ‘Red’, 530–700 nm (Corning 3–68 + two Corning 1–75), intensity 1.0 × 105 erg cm−2 s−1. ‘Red+Blue’, The tungsten beam filtered through two Corning 1–75 + 2 mm Plexiglass, intensity 1.5 × 105 erg cm−2 s−1.
Conclusions
The experiments we reported previously strongly implicated slow-cycling rhodopsin as the attractant receptor for H. halobium phototaxis9,10. The evidence presented here implicates this protein as the repellent receptor as well. We prove here that, like S373, the repellent receptor is a photoproduct of a slow-cycling, red-absorbing, retinal pigment with the properties of sR587.
From these experiments we propose a mechanism in which slow-cycling rhodopsin has two signal transducing forms, one transmitting an attractant signal and the other a repellent signal to the flagellar motor (Fig. 4). Increases in the repellent signal or decreases in the attractant signal induce flagellar reversals. Conversely, decreases in the repellent signal or increases in the attractant signal suppress flagellar reversals. Both induction and suppression of reversals are transient responses, demonstrating adaptation of the cell to the signals. The net effect is that the motility apparatus of the cell responds to changes in the numbers of slow-cycling rhodopsin molecules undergoing the one-photon and two-photon cycles. In view of these results, we suggest the original name9 ‘slow-cycling rhodopsinlike pigment’ be changed to ‘sensory rhodopsin’ of H. halobium, reflecting more clearly the physiological function of the molecule.
The absorption spectrum of S373 matches closely the ~370 nm action spectrum peak reported for phototaxis repulsion2,4,3. However, the absorption spectrum of sR587 matches some but not all of the published action spectra for phototaxis attraction. The attractant response was reported to have a single peak at ~565 nm in wild-type2,5 and two peaks, one at ~565 nm and the other at ~590 nm, near the sR587 absorption maximum in a bacteriorhodopsin-deficient strain12. The disagreement may be caused by the presence of bacteriorhodopsin and halorhodopsin which may influence the motility response directly or indirectly.
Dencher13 and Hildebrand and Schimz14 have proposed that two distinct receptors mediate the attractant and repellent sensitivities. Our data prove that the retinal-dependent repellent receptor is a photoproduct of a red absorbing pigment. Therefore their proposal requires the existence of at least one additional hypothetical red-absorbing retinal pigment. Our model requires only the single identified slow-cycling rhodopsin and, moreover, makes several specific quantitative predictions: (1) a delayed blue flash following a red flash will change from an attractant to a repellent stimulus with delay time predicted by the 20 μs rise time of S373; (2) mutants deficient in slow-cycling rhodopsin would be predicted to lose both attractant and repellent photosensitivities, and reversion to normal phototaxis behaviour should be accompanied by return of the pigment.
A remarkable finding from our measurements is that photoexcitation of a single molecule of S373 appears to be sufficient to generate a reversal-inducing repellent signal to the flagellar motor. From the measured intensity (3.6 × 104 erg cm−2 s−1) and spectrum of the background ‘red’ illumination (Fig. 1), the sR587 and S373 molar extinction spectra (Fig. 2b) and the parameters of the photocycle (Fig. 4), we calculate 2% of the sensory rhodopsin molecules will be in the S373 form in the photostationary state at 50% maximal intensity for red background (Fig. 1). From the regeneration of sensory rhodopsin absorption by retinal (see Fig. 2 legend) and the sensory rhodopsin extinction (54,000 M−1 cm−1) at that wavelength, we measure 5×103 molecules per cell, ~100 of which therefore will be in the S373 form. From the quantum flux of blue light used for the stimulus (Fig. 1) and the measured S373 extinction in the stimulus spectral range (Fig. 2b), we calculate the S373 population in the average cell will absorb only about two photons in the 2-s period over which reversals were assessed. Taking the quantum efficiency for photochemical reaction to be 0.5 ± 0.2, covering the range of all known retinal pigments, only ~0.7–~1.4 S373 molecules per cell on average would be activated photochemically by a stimulus which causes over 50% of the cells to respond. Therefore photoexcitation of a single S373 molecule appears to be sufficient to alter the cell’s swimming behaviour.
The cell is at least an order of magnitude less sensitive to attractant than repellent light2,3. In our interpretation this means the amplification of the S373 signal by the signal transduction machinery (Fig. 4) is at least 10× greater than that of sR587. The relative amplification in the two signalling pathways may depend on the specific conditions and stage of cell culture growth (unpublished observations).
The colour-sensing mechanism described here depends on the existence of two spectrally-distinct signalling forms of the same receptor. This could be a unique property of the sensory rhodopsin of halobacteria. Alternatively, the sensory rhodopsin mechanism may reflect an inherent potential of retinal/protein complexes—photoreversibility of photoconversion products of the retinal/opsin chromophore—important to the evolution of the rhodopsin family as photosensory receptors. Even retinal pigment photoproducts which are too short-lived to accumulate under physiological conditions (such as the K, L and M intermediates formed from bacteriorhodopsin6 and the early photo-products formed from mammalian rod rhodopsin14) are photoreversible. Invertebrate rhodopsins, like sensory rhodopsin and the non-retinal plant chromoprotein phytochrome, are photochromic; that is, they have photoreactions with thermal relaxation sufficiently slow to cause significant photostationary state accumulation of their photoreversible conformations16. The sensory rhodopsin mechanism, preserved in the archaebacterium H. halobium, may have provided a primitive colour-sensing capability. In the evolution of visual systems, this mechanism may have been maintained in some organisms but replaced in others by multiple receptor mechanisms with increased colour resolution.
Acknowledgments
We thank W. Stoeckenius, L. Stryer, E. Spudich and S. Sundberg for critical reading of the manuscript and J. Korenbrot for use of his infrared-sensitive video equipment. This work was supported by NIH grant GM27750 and an Irma T. Hirschl Trust Career Scientist Award (J.L.S.); NIH grant GM27057, NASA grant NSG-7151 and NSF grant PCM-8316139 (R.A.B.).
References
- 1.Koshland DE., Jr . Bacterial Chemotaxis as a Model Behavioral System. Vol. 2. Raven; New York: 1980. pp. 1–193. [Google Scholar]
- 2.Hildebrand E, Dencher N. Nature. 1975;257:46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- 3.Spudich JL, Stoeckenius W. J Photobiochem Photobiophys. 1979;1:43–53. [Google Scholar]
- 4.Dencher NA, Hildebrand E. Z Naturforsch. 1979;34:841–847. doi: 10.1515/znc-1979-9-1030. [DOI] [PubMed] [Google Scholar]
- 5.Sperling W, Schimz A. Biophys Struct Mech. 1980;6:165–169. doi: 10.1007/BF00535752. [DOI] [PubMed] [Google Scholar]
- 6.Stoeckenius W, Bogomolni RA. A Rev Biochem. 1982;52:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- 7.Wagner G. EMBO Workshop on Halophilic Microorganisms. Ischia, Italy: 1981. [Google Scholar]
- 8.Spudich EN, Spudich JL. Proc natn Acad Sci USA. 1982;79:4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogomolni RA, Spudich JL. Proc natn Acad Sci USA. 1982;79:6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spudich JL, Bogomolni RA. Biophys J. 1983;43:243–246. doi: 10.1016/S0006-3495(83)84345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrlich BE, Sehen CR, Spudich JL. J Membrane Biol. in the press. [Google Scholar]
- 12.Traulich B, Hildebrand E, Schimz A, Wagner G, Lanyi JK. Photochem Photobiol. 1983;37:577–579. [Google Scholar]
- 13.Dencher NA. Photochem Photobiol. 1983;38:753–767. [Google Scholar]
- 14.Hildebrand E, Schimz A. Photochem Photobiol. 1983;38:593–597. [Google Scholar]
- 15.Ottolenghi M. Adv Photochem. 1980;12:97–200. [Google Scholar]
- 16.Hamdorf K. In: Handbook of Sensory Physiology. Autrum H, editor. VII/6A. Springer; Berlin: 1979. [Google Scholar]


