Abstract
Objective
To analyze kidney cancer patients referred for evaluation at a high-volume genetics service at a comprehensive cancer center, and identify factors associated with positive tests for hereditary cancer syndromes.
Methods
A retrospective review of patients referred to the Clinical Genetics Service at Memorial Sloan-Kettering Cancer Center was performed, and patients with a personal history of kidney cancer were identified. Patient and disease characteristics were reviewed. Four variables including age at diagnosis of kidney tumor, presence of syndromic manifestations, family history of kidney cancer, and number of primary malignancies were evaluated for association with positive test results in two groups: patients tested for renal cell carcinoma syndromes and Lynch syndrome. Guidance for genetic testing strategy in kidney cancer patients is provided.
Results
Between 1999 and 2012, 120 patients with a history of kidney cancer were evaluated by the Clinical Genetics Service. The mean age at kidney cancer diagnosis was 52 years (IQR: 42–63), with 57% being female. A family history of kidney cancer was reported by 39 patients (33%). Time between diagnosis of first cancer and genetic consultation was ≤1 year in 54%, 2–5 years in 23%, and >5 years in the remaining 23%. Overall, 95 patients were tested for genetic abnormalities with 27 (28%) testing positive. Testing for renal cell cancer (RCC)- related syndromes was performed on 43 patients, with 13 testing positive (30%). Lynch syndrome testing was positive in 9 patients (32%) after 28 were tested. In RCC-associated syndromes, young age of diagnosis was associated with positive test results. Conversely, syndromic manifestations and increasing number of primary malignancies were associated with positive Lynch testing.
Conclusions
The discovery of inherited kidney cancer syndromes has provided a unique opportunity to identify patients at increased risk for cancer. Factors associated with positive genetic testing are unique to different syndromes. This data suggests that in kidney cancer patients evaluated for hereditary cancer syndromes, young age is associated with diagnosis of RCC syndromes, while syndromic manifestations and multiple primaries are found in Lynch syndrome. These results along with clinical awareness may be useful for practicing urologists to select kidney cancer patients to refer for genetic counseling.
Keywords: Kidney Cancer, inherited cancer syndromes, genetic testing
INTRODUCTION
According to the American Cancer Society, it is estimated that about 65,150 new cases of kidney cancer (40,430 in men and 24,720 in women) will be diagnosed in the United States in 2013(1). The majority of primary renal malignancies are renal cell carcinomas (RCC) with most of the remaining comprised of upper tract urothelial carcinoma (UTUC)(2, 3). Several risk factors have been identified for both RCC and UTUC, including a shared link between smoking and cancer development. Additionally, both have been associated with hereditary cancer syndromes. The most extensively characterized RCC syndrome is von Hippel-Lindau Syndrome (VHL), with others including Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC), Hereditary Papillary Renal Cell Carcinoma (HPRCC), Birt-Hogg-Dubé Syndrome (BHD), Tuberous Sclerosis (TSC), and SDH-associated Renal Cell Carcinoma. Lynch syndrome is a DNA mismatch repair disorder associated with development of UTUC.
The central tenet for extending survival for patients with hereditary cancer syndromes has been early identification of at-risk patients. These patients are offered genetic testing and syndrome specific screening to identify early manifestations that can be treated prior to development of morbidity or mortality. For several RCC syndromes, studies have found that conservative management appears to preserve renal function without increased mortality (4–6). In other syndromes, such as HLRCC, early and aggressive treatment may be preferable (7). Decreased colorectal cancer mortality has been demonstrated in patients with Lynch syndrome enrolled in colorectal cancer surveillance programs (8). Patients can consider assisted reproductive technology to avoid transmitting a syndrome to offspring.
The benefits of testing are not without risk, however. Testing can be costly for patients and research indicates that insurance companies vary in reimbursement of genetic counseling and testing (9). Increased levels of anxiety and psychological stress have been associated with genetic testing in some cases. In patients evaluated for Lynch syndrome, anxiety at the time of test disclosure was increased for patients testing positive although this declined over time (10). In families with VHL syndrome, clinically relevant distress has been identified in mutation carriers and those who tested negative (11). Increased anxiety levels have also been documented in partners of VHL patients who do not share the genetic susceptibility (12). Because of the complexities associated with genetic testing for cancer susceptibility, the American Society of Clinical Oncology recommends pre- and post- test counseling for patients undergoing genetic testing (13).
Identification of patients with hereditary cancer syndromes requires a multidisciplinary team. Syndromic manifestations may be identified by a variety of care providers, including family physicians, dermatologists, gynecologists, urologists and medical oncologists. Additionally, pathologists and radiologists may suspect cancer syndromes while reviewing tumor specimen or imaging studies. At MSKCC, all groups have been instrumental in identifying patients who may require clinical genetics counseling. In this study we identified 120 kidney cancer patients referred to the Clinical Genetics Service between 1999 and 2012. We evaluated commonly associated factors and have made recommendations for referring patients who may benefit from genetic counseling.
METHODS
In an institutional review board-approved study, we performed a retrospective review of patients with a history of kidney cancer referred to Clinical Genetics Service for evaluation of potential hereditary cancer syndrome. Patients were included regardless of the potential syndrome considered. All recommended clinical testing was performed in a CLIA-certified laboratory and confirmed by our institution’s clinical genetics laboratory. Patient and disease characteristics including age, gender, ancestry/ethnicity, family history, tumor size, histology, nodal status, metastases, genetic testing results, time between first cancer diagnosis and genetic consultation, associated manifestations, and history of previous malignancy were recorded.
Genetic testing was grouped by association with renal cell carcinoma syndromes (VHL, FH, FLCN, MET, TSC, and SDH), or Lynch syndrome (MSH2, MSH6, MLH1, and PMS2) which is associated with upper tract urothelial carcinoma. Four variables were evaluated for association with positive test results: age at diagnosis of kidney tumor, family history of kidney tumors, presence of manifestations associated with tumor syndromes, and total number of primary malignancies. Examples of syndromic manifestations include uterine fibroids, hemangioblastoma, pneumothorax, lung blebs, and cutaneous changes such as characteristic rash, leiomyomata, or fibrofolliculomas. Fisher’s exact test and t-test were used to test association with positive mutation status, with p<0.05 considered statistically significant. Statistical analysis was performed using STATA version 12.0 (StataCorp, College Station, TX).
RESULTS
In total, 120 patients with a history of kidney cancer were evaluated between 1999 and 2012 (Table 1). Patients were referred for genetic counselling based on the clinical judgment of the managing clinical provider. The median age at diagnosis was 52 years (IQR: 42–63), with 57% being females and 43% males. One hundred patients (83%) were Caucasian, of which 44 reported Ashkenazi Jewish heritage and 7 were non-Ashkenazi. The remaining included 6 African Americans, 6 Hispanics, 5 Asians, and 3 unknown/other. Thirty-nine patients had a family history of kidney cancer (33%), and 103 (86%) had a family history of other cancers. Time between diagnosis of first cancer and genetic consultation was 1 year or less for 54% of the cohort, 2–5 years for 23%, and ≥5 years for the remaining 24%.
Table 1.
Patient characteristics
| All Patients (n=120) | RCC Syndrome Testing (n=43) | Lynch Syndrome Testing (n=28) | |
|---|---|---|---|
| Gender | |||
| Male | 52 (43%) | 22 (51%) | 12 (43%) |
| Female | 68 (57%) | 21 (49%) | 16(57%) |
| Age of Kidney Cancer | |||
| <40 | 25 (21%) | 15 (35%) | 2 (7%) |
| 40–49 | 28 (23%) | 13 (30%) | 4 (14%) |
| 50–59 | 26 (22%) | 6 (14%) | 9 (32%) |
| 60–69 | 29 (24%) | 8 (19%) | 7 (25%) |
| 70+ | 12 (10%) | 1(2%) | 6 (21%) |
| Race | |||
| Caucasian | 101 (84%) | 30 (70%) | 26 (93%) |
| Non-Caucasian | 19 (16%) | 13 (30%) | 2 (7%) |
| Heritage | |||
| Ashkenazi Jewish | 44 (37%) | 9 (21%) | 11 (39%) |
| Non-Ashkenazi | 76 (63%) | 34 (79%) | 17 (61%) |
| Family History Kidney Cancer | |||
| Present | 39 (33%) | 23 (55%)* | 8 (29%) |
| Absent | 79 (67%) | 19 (45%) | 20 (71%) |
| Syndromic Manifestations | |||
| Present | 46 (38%) | 21 (49%) | 10 (36%) |
| Absent | 76 (62%) | 22 (51%) | 18 (64%) |
| Primary Malignancies | |||
| 1–2 | 86 (72%) | 41 (95%) | 15 (54%) |
| >2 | 34 (28%) | 2 (5%) | 13 (46%) |
One patient without data
Kidney cancer was the first cancer diagnosis for 66 (55%) patients, with the majority being clear cell renal cell carcinoma, followed by papillary and unclassified type both identified in 6 patients (9%) respectively. Tumor size was reported in 88 (73%) patients, with a median tumor size of 4.1 cm (interquartile range 2.5–7.8 cm) (Table 2). Of the patients with renal cell carcinoma, 77% had T1 disease, 10% had T2 disease, and 13% T3 and higher. Of the 51 patients with data on disease grade, 23 (45%) patients had high-grade disease (10 patients with transitional cell carcinoma) (Fuhrman grade III or IV or high-grade transitional cell carcinoma). Fifteen (13%) patients had multiple tumors, 12 of whom presented with synchronous tumors at diagnosis. Fourteen patients had metastasis at the time of evaluation, and 6 of them died due to their disease within a median 1.5 years after cancer diagnosis.
Table 2.
Pathologic Characteristics
| All Patients (n=120) | RCC Syndrome Testing (n=43) | Lynch Syndrome Testing (n=28) | ||||
|---|---|---|---|---|---|---|
| Median Tumor Size cm (IQR) | N=88 | 4.1 (2.5–7.8) | N=36 | 6.0 (3.2–12.0) | N=14 | 3.2 (1.8–4.0) |
| Tumor Grade | N= 51 | N=18 | N=10 | |||
| Low (I–II) | 28 (23%) | 10 (23%) | 5 (18%) | |||
| High (III, IV, High Grade) | 23 (19%) | 8 (19%) | 5 (18%) | |||
| Lymph Nodes Positive (%) | N= 106 | 17 (14%) | N=41 | 12 (28%) | N=22 | 3 (11%) |
| Metastatic Disease (%) | N= 107 | 14 (12%) | N=42 | 10 (23%) | N=22 | 0 (0%) |
Of the 120 patients referred for genetic evaluation, 95 underwent genetic testing and 27 (28%) tested positive for a genetic condition (Table 3). Testing performed was based on discussing the individual cases by members of the Clinical Genetics Service. Lynch syndrome represented the most common syndrome suspected, with 28 patients tested and nine (32%) patients found to harbor mutations, three of which were associated with the Muir-Torré subtype of the syndrome. Another five patients were suspected to have Lynch syndrome based on clinical criteria, without confirmatory testing. BRCA1/BRCA2 testing was recommended for 26 patients, of whom five (19%) were found to have mutations. Sixteen patients were tested for VHL syndrome, with three (19%) mutations identified. Fumarate hydratase (FH) mutations were evaluated in 14 patients with 5 (36%) identified. Folliculin (FLCN) testing in five patients was negative, while one patient tested positive at an outside hospital. The remaining testing identified oneTSC1 mutation, one SDHB mutation, one RET mutation, and one p53 mutation (Table 2). Of the patients who tested positive for a genetic defect, the median age was 48.5 years (range 1–71). Kidney cancer was the presenting cancer in 14/27, multiple tumors in 6/27, and was metastatic in 5/27. Kidney cancer was the cause of death in 3/27 patients over a median time of 4 years after cancer diagnosis. Twenty-two of the 27 patients (81%) had a family history of cancer, with 11 (41%) patients having a family history of kidney cancer. Tissue analysis was performed when needed to make final diagnosis.
Table 3.
Patients with positive genetic testing
| Sex | Age | Race | Ashkenazi | Mutation | Syndrome | Syndromic Manifestations | FH Kidney Cancer | Kidney Tumor |
|---|---|---|---|---|---|---|---|---|
| M | 34 | Caucasian | + | VHL | VHL | Retinal angiomas, epididymal cysts | + | ccRCC |
| F | 56 | Caucasian | − | VHL | VHL | Retinal angiomas | + | ccRCC |
| M | 46 | Hispanic | − | VHL | VHL | Cerebellar hemangioblastoma, Pancreatic Cyst | − | ccRCC |
| M | 24 | Caucasian | − | FH | HLRCC | None | + | Mixed RCC with papillary features |
| F | 32 | Hispanic | − | FH | HLRCC | Fibroids, Skin Lesions | − | Papillary RCC, Type II |
| M | 61 | Caucasian | − | FH | HLRCC | None | + | Collecting Duct RCC |
| M | 42 | Hispanic | − | FH | HLRCC | None | − | Collecting Duct RCC |
| M | 34 | Asian | − | FH | HLRCC | Tongue Papules | + | Unclassified RCC |
| F | 62 | African American | − | BHD | BHD | Lung Cysts | + | Chromophobe RCC |
| M | 18 | Caucasian | − | SDHB | Paraganglioma | None | + | ccRCC, Paraganglioma |
| F | 24 | Other | − | TSC1 | Tuberous Sclerosis | Cephalgia | + | Papillary RCC, Unclassified RCC, AML |
| F | 71 | Caucasian | − | MLH1 | Lynch Syndrome | None | − | UTUC |
| M | 71 | Caucasian | + | MSH2 | Lynch Syndrome | Colonic polyps | + | UTUC |
| M | 62 | Asian | − | MSH2 | Lynch Syndrome | None | − | UTUC |
| F | 65 | Caucasian | − | MSH6 | Lynch Syndrome | None | − | ccRCC |
| F | 57 | Asian | − | MSH2 | Lynch Syndrome | Fibroids | + | UTUC |
| F | 45 | Caucasian | − | MSH2 | Lynch Syndrome | Colon Polyps | − | UTUC |
| F | 44 | Caucasian | − | MSH2 | Lynch (Muir-Torre) | Sebaceous adenoma | − | UTUC |
| M | 55 | Caucasian | − | MSH2 | Lynch (Muir-Torre) | Sebaceous adenoma | + | UTUC |
| F | 64 | Caucasian | − | MSH2 | Lynch (Muir-Torre) | Keratoacanthoma, Sebaceous Adenoma | − | UTUC |
| F | 64 | Caucasian | − | RET | MEN2a | None | − | ccRCC |
| M | 1 | Caucasian | − | p53 | Li-Fraumeni | None | − | Wilms, ccRCC |
| F | 62 | Caucasian | + | BRCA1 | Breast Ovarian | None | − | RCC |
| M | 49 | Caucasian | + | BRCA2 | Breast Ovarian | None | − | ccRCC |
| F | 59 | Caucasian | + | BRCA1 | Breast Ovarian | None | − | ccRCC |
| F | 36 | Caucasian | + | BRCA1 | Breast Ovarian | Infertility | − | ccRCC |
| M | 48 | Caucasian | + | BRCA2 | Breast Ovarian | None | − | ccRCC |
In bivariate analysis, we evaluated age at diagnosis of kidney cancer (continuous), family history of kidney cancer, presence of syndrome manifestations, and multiple primary cancers (>2) with positive genetic testing for either RCC-associated syndromes or Lynch syndrome (Table 4). Positive genetic testing for RCC-associated syndromes was associated with early age of kidney cancer diagnosis (Mean age: 37.1 vs 47.7, p=0.025), but not presence of syndromic manifestations, family history of kidney cancer, or number of primary malignancies. Positive testing for Lynch syndrome was associated with presence of syndromic manifestations (p=0.035) and increasing number of primary malignancies (p=0.042), but not age of kidney cancer diagnosis or family history of kidney cancer.
Table 4.
Statistical Analysis
| RCC Syndrome Testing (n=43) | Lynch Syndrome Testing (n=28) | All Testing (n=120) | |
|---|---|---|---|
| Age | 0.03 | 0.6 | 0.3 |
| Family History Kidney Cancer | 0.7 | 1.0 | 0.4 |
| Syndromic Manifestations | 0.7 | 0.04 | 0.1 |
| Multiple Primary Cancers (>2) | 0.5 | 0.04 | 0.3 |
| Caucasian Ethnicity | 0.2 | 0.095 | 0.036 |
| Ashkenazi Jewish Heritage | 0.7 | 0.049 | 0.3 |
Grey Shading: Exploratory Analysis
In an exploratory analysis, we evaluated for self-reported ancestry by separately using Caucasian and Ashkenazi Jewish as variables. For RCC syndromes, ethnicity did not predict genetic test results. For Lynch syndrome, Ashkenazi Jewish ethnicity appeared to be associated with positive test results (p=0.049). Using the four clinical variables along both ancestry groups, we evaluated for an association with positive testing in the entire cohort of 120 patients. In this, self-reported Caucasian race may be associated with positive testing (p=0.036) but the remaining were not.
DISCUSSION
The occurrence of hereditary cancer syndromes in kidney cancer patients is not well-defined; however, studies have suggested nearly 4% of kidney cancers are attributable to inherited susceptibility (14–16). Genetic registry data suggests that many patients with hereditary syndromes remain unrecognized (17). Tumor surveillance after confirmatory testing has been shown to improve outcomes, and is the objective following positive testing. For this reason, several factors have been suggested to clinicians for consideration of genetic testing in patients with kidney cancer. Among patients with RCC, young age at diagnosis, positive family history of kidney cancer, and presence of syndromic features along with histologic subtype have been used in testing schema(18), although with variable amounts of supportive evidence. In Lynch syndrome patients, studies have found an association with multiple primary malignancies and younger age of diagnosis (19–21). In this study we confirmed that patients with positive testing for RCC syndromes were more likely to present at a younger age, while patients with Lynch syndrome were more likely to have syndromic manifestations and multiple primary malignancies.
Several authors have proposed young age at onset as a criterion for considering genetic testing, with cutoffs varying between 40 and 46(18, 22). Patients with VHL syndrome have a mean age at diagnosis of RCC between 38.9–44 years (17, 23). In patients with BHD syndrome, median age at diagnosis is 50.7 years (24). While reporting on upper urinary tract carcinoma in Lynch syndrome patients, Crockett et al. found the median age at tumor diagnosis to be 62; this was significantly younger than patients without Lynch syndrome(21). In our study, young age at diagnosis was significant, but only in patients testing positive for RCC syndromes (37.1 vs. 44.7). Since we focused only on patients undergoing testing, the older median age of onset in Lynch syndrome may have contributed to the disparity in the association between younger age of onset and positive testing.
A family history of kidney cancer may raise suspicion of a hereditary syndrome. In our study, we were unable to find an association between a family history of kidney cancer and positive testing for RCC syndromes or for Lynch syndrome. Conversely, it is known that having a family member with kidney cancer empirically results in an increased likelihood of developing kidney cancer(25). However, a portion of this risk may be imparted by high frequency low-penetrance alleles (26) or as yet unidentified genetic factors. Family members also share known environmental risk factors, such as tobacco exposure. Furthermore, de novo mutation rates vary across cancer syndromes and may explain the absence of family history in some positive cases. For instance, the majority of Tuberous Sclerosis cases are the result of de novo mutations(27), while about 20% of VHL cases are thought to be de novo(28).
Syndromic manifestations are unique and easily identifiable signs that raise a clinician’s attention to the possibility of an underlying hereditary cause; such manifestations range from benign tumors such as lipomas, uterine fibroids or skin fibroids, to rare cutaneous lesions such as Shagreen patches. In our study, only Lynch syndrome testing was found to be associated with syndromic manifestations. In this case, syndromic manifestations included the skin tumors associated with the Muir-Torré Lynch syndrome variant such as sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Manifestations that occur frequently in the general population may be coincidental and unrelated to a patient’s kidney cancer. For instance, uterine fibroids are associated with HLRCC although the vast majority of fibroids are not due to a cancer syndrome. Conversely, due to clinical variability, some patients with syndromes do not display visible manifestations. As an example, our series did include patients with HLRCC but without the oft-associated leiomyomata of the skin.
In a study of families meeting Amsterdam criteria for Lynch syndrome testing, mutations in MLH1 and MSH2 conferred a 2.4 times increased risk of syndromic malignancies. This became significant when limiting a founder mutation known to lack extracolonic tumors (29). Subsequent case reports have demonstrated families with multiple malignancies and Lynch syndrome mutations (30, 31). In this study, we confirmed that multiple primary malignancies are associated with positive Lynch syndrome testing. This result is expected, given the multi-organ cancer risks in Lynch syndrome. One would also expect Lynch syndrome mutations to be associated with a family history of other associated cancer types, including colon, uterine and upper gastrointestinal, although this hypothesis was not specifically tested. For RCC syndromes, an association with multiple primaries was not identified. This may be attributable to variations in mutation frequencies, penetrance, or phenotypic variation. Unlike Lynch syndrome, some of the RCC syndromes are not associated with carcinomas in multiple organs.
In an exploratory analysis, we found that self-reported ancestry was not associated with positive RCC syndrome testing. However, Ashkenazi Jewish ethnicity may be associated with Lynch Syndrome positivity. The association is likely due to the large regional Jewish population and three founder mutations for Lynch syndrome identified in the Ashkenazi Jewish population(32, 33). Evaluating all patients tested for any syndrome, Caucasian ethnicity appeared to have a potential relationship with positive test results.
BRCA is a tumor suppressor gene that was associated with multiple malignancies including breast, ovarian, and prostate tumors. Using the model of VHL and BAP1, it is plausible that BRCA mutations could be a risk factor for kidney cancer, or associated with adverse pathologic characteristics. In fact, previous reports did show germline mutations of BRCA1 in kidney cancer patients (34, 35). We therefore included those patients who were suspected of having inherited form of kidney cancer, and who tested positive for BRCA, in this paper as a hypothesis generating group of cases.
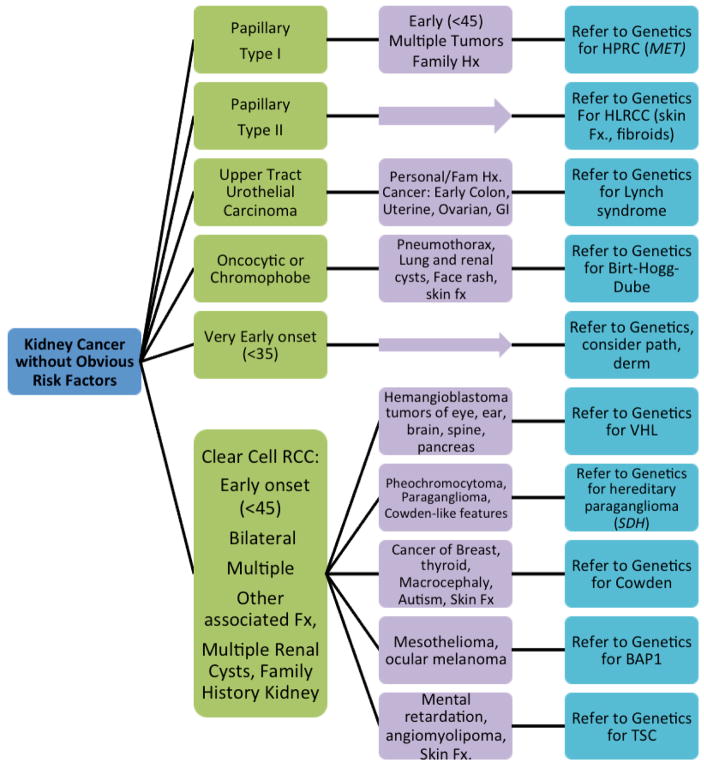
Because of the complexity of hereditary kidney cancer syndromes and the subtlety in clinical presentation, clinicians may feel uncertain as to who would benefit from genetic counseling. Figure 1 represents factors to consider in evaluating patients for referral to the Clinical Genetics Service. Tumors that occur at an early age (<45 or especially under age 40), are bilateral or multifocal, or unexplained by traditional risk factors (smoking, hypertension, obesity, diabetes, occupational, childhood radiation or chemotherapy, analgesic abuse, etc) are suspicious for hereditary risk. Relevant tumor histological subtypes and specific benign features in the patient or family members can raise suspicion. As noted above, many kidney cancer syndromes display specific skin features such as leiomyomata in HLRCC and fibroangiomas in TSC. Referring a patient for dermatology workup, and requesting skin biopsy reports, may be helpful in diagnosis. Attention should be paid to the collection of multiple different features in an individual, and features that are otherwise rare in the general population. Attention should also be paid to the fact that under-screening patients for kidney cancer is common, and could be easily remedied by standardizing management of kidney cancer patients through pathways similar to the one suggest in this paper.
Figure 1.
Guidance for Referral of Kidney Cancer Patients for Genetics Evaluation, based on histologic subtype. Standard referral criteria (such as those published by the National Comprehensive Cancer Network (www.nccn.org)) for Lynch syndrome, breast and ovarian cancer and other syndromes are not shown here, but should also be used.
It is important to realize that our study group represents a highly selected group of patients suspicious for a hereditary predisposition, by virtue of the fact that they have already been referred to clinical genetics. Thus, the rates observed would not apply to a standard oncology practice. Still our findings are significant in that a large percentage of the referred population were found to carry germline mutations. Other limitations to this study include a small sample size, the high representation of Ashkenazi Jewish (44/120) patients in our sample, incomplete clinical data in some patients with remote cancer histories or treatments at other centers, and the burden of multiple testing. It is unknown how many patients were referred for genetic counseling but did not follow through. Further, some low-risk patients may have self-referred out of concern for hereditary risk. These, however, would only have been offered testing when medically appropriate. Some patients may have been referred for reasons unrelated to their kidney cancer, such as a family history of breast and/or ovarian cancer. Finally, it is difficult to isolate the effect of early-age cancer diagnosis on the referral pattern from an actual association between young age and positive testing.
CONCLUSION
As understanding of hereditary cancer syndromes improves, kidney cancer provides a unique example with readily available clinical testing. We identified several factors that may predict positive test results in kidney cancer patients undergoing evaluation in a clinical genetics clinic. Although these risk factors have previously been associated with cancer syndromes, a need for studies of testing practices remains. Barriers to genetic testing include cost, anxiety, and concerns of genetic discrimination. Concurrently, missed carriers lose the opportunity for early screening, syndrome-specific treatment options, and assisted reproductive technology. Our study identified a younger age of cancer diagnosis in patients with RCC syndromes, while syndrome-positive patients were more likely to have syndromic manifestations and multiple primary tumors. Although family history of kidney cancer was not an indicator, we feel that familial kidney cancer clusters are a valid reason for referral. It is possible that the sample size was not large enough to see this effect. Also, as medical science advances, additional hereditary factors may be elucidated to further assist these families. The guidelines presented here will hopefully assist practicing urologists to identify patients at-risk for hereditary cancer syndromes.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Silverman DT, McLaughlin JK, Brown CC, Connelly RR, Fraumeni JF., Jr Comparison of the descriptive epidemiology of urinary tract cancers. Cancer causes & control: CCC. 1990;1(2):133–41. doi: 10.1007/BF00053164. [DOI] [PubMed] [Google Scholar]
- 3.Guinan P, Vogelzang NJ, Randazzo R, Sener S, Chmiel J, Fremgen A, et al. Renal pelvic cancer: a review of 611 patients treated in Illinois 1975–1985. Cancer Incidence and End Results Committee. Urology. 1992;40(5):393–9. doi: 10.1016/0090-4295(92)90450-b. [DOI] [PubMed] [Google Scholar]
- 4.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. The Journal of urology. 2004;172(1):63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 5.Pavlovich CP, Grubb RL, 3rd, Hurley K, Glenn GM, Toro J, Schmidt LS, et al. Evaluation and management of renal tumors in the Birt-Hogg-Dube syndrome. The Journal of urology. 2005;173(5):1482–6. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 6.Coleman JA, Russo P. Hereditary and familial kidney cancer. Current opinion in urology. 2009;19(5):478–85. doi: 10.1097/MOU.0b013e32832f0d40. [DOI] [PubMed] [Google Scholar]
- 7.Grubb RL, 3rd, Franks ME, Toro J, Middelton L, Choyke L, Fowler S, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. The Journal of urology. 2007;177(6):2074–9. doi: 10.1016/j.juro.2007.01.155. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 8.de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130(3):665–71. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Latchaw M, Ormond K, Smith M, Richardson J, Wicklund C. Health insurance coverage of genetic services in Illinois. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(8):525–31. doi: 10.1097/GIM.0b013e3181e3916d. [DOI] [PubMed] [Google Scholar]
- 10.Aktan-Collan K, Kaariainen H, Jarvinen H, Peltomaki P, Pylvanainen K, Mecklin JP, et al. Psychosocial consequences of predictive genetic testing for lynch syndrome and associations to surveillance behaviour in a 7-year follow-up study. Familial cancer. 2013 doi: 10.1007/s10689-013-9628-9. [DOI] [PubMed] [Google Scholar]
- 11.Lammens CR, Bleiker EM, Verhoef S, Hes FJ, Ausems MG, Majoor-Krakauer D, et al. Psychosocial impact of Von Hippel-Lindau disease: levels and sources of distress. Clinical genetics. 2010;77(5):483–91. doi: 10.1111/j.1399-0004.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 12.Lammens CR, Bleiker EM, Verhoef S, Ausems MG, Majoor-Krakauer D, Sijmons RH, et al. Distress in partners of individuals diagnosed with or at high risk of developing tumors due to rare hereditary cancer syndromes. Psycho-oncology. 2011;20(6):631–8. doi: 10.1002/pon.1951. [DOI] [PubMed] [Google Scholar]
- 13.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K American Society of Clinical O. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(5):893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 14.Coleman JA. Familial and hereditary renal cancer syndromes. The Urologic clinics of North America. 2008;35(4):563–72. v. doi: 10.1016/j.ucl.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Choyke PL, Glenn GM, Walther MM, Zbar B, Linehan WM. Hereditary renal cancers. Radiology. 2003;226(1):33–46. doi: 10.1148/radiol.2261011296. [DOI] [PubMed] [Google Scholar]
- 16.Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. Family history and risk of renal cell carcinoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10(9):1001–4. [PubMed] [Google Scholar]
- 17.Maddock IR, Moran A, Maher ER, Teare MD, Norman A, Payne SJ, et al. A genetic register for von Hippel-Lindau disease. Journal of medical genetics. 1996;33(2):120–7. doi: 10.1136/jmg.33.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan-Smutko G. Genetic testing by cancer site: urinary tract. Cancer journal. 2012;18(4):343–9. doi: 10.1097/PPO.0b013e31826246ac. [DOI] [PubMed] [Google Scholar]
- 19.Lin KM, Shashidharan M, Ternent CA, Thorson AG, Blatchford GJ, Christensen MA, et al. Colorectal and extracolonic cancer variations in MLH1/MSH2 hereditary nonpolyposis colorectal cancer kindreds and the general population. Diseases of the colon and rectum. 1998;41(4):428–33. doi: 10.1007/BF02235755. [DOI] [PubMed] [Google Scholar]
- 20.Win AK, Young JP, Lindor NM, Tucker KM, Ahnen DJ, Young GP, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(9):958–64. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crockett DG, Wagner DG, Holmang S, Johansson SL, Lynch HT. Upper urinary tract carcinoma in Lynch syndrome cases. The Journal of urology. 2011;185(5):1627–30. doi: 10.1016/j.juro.2010.12.102. [DOI] [PubMed] [Google Scholar]
- 22.Brian Shuch SV, Lindsay Middleton W, Linehan Marston. Defining Early-onset Kidney Cancer: Implications For Genetic Counseling. Journal of Clinical Oncology. 2013;31(6_suppl) doi: 10.1200/JCO.2013.50.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. The Quarterly journal of medicine. 1990;77(283):1151–63. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 24.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dube syndrome. The American journal of surgical pathology. 2002;26(12):1542–52. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Zbar B, Glenn G, Merino M, Middelton L, Peterson J, Toro J, et al. Familial renal carcinoma: clinical evaluation, clinical subtypes and risk of renal carcinoma development. The Journal of urology. 2007;177(2):461–5. doi: 10.1016/j.juro.2006.09.037. discussion 5. [DOI] [PubMed] [Google Scholar]
- 26.Stadler ZK, Thom P, Robson ME, Weitzel JN, Kauff ND, Hurley KE, et al. Genome-wide association studies of cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(27):4255–67. doi: 10.1200/JCO.2009.25.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genetics in medicine: official journal of the American College of Medical Genetics. 2007;9(2):88–100. doi: 10.1097/gim.0b013e31803068c7. [DOI] [PubMed] [Google Scholar]
- 28.Richards FM, Payne SJ, Zbar B, Affara NA, Ferguson-Smith MA, Maher ER. Molecular analysis of de novo germline mutations in the von Hippel-Lindau disease gene. Human molecular genetics. 1995;4(11):2139–43. doi: 10.1093/hmg/4.11.2139. [DOI] [PubMed] [Google Scholar]
- 29.Bisgaard ML, Jager AC, Myrhoj T, Bernstein I, Nielsen FC. Hereditary non-polyposis colorectal cancer (HNPCC): phenotype-genotype correlation between patients with and without identified mutation. Human mutation. 2002;20(1):20–7. doi: 10.1002/humu.10083. [DOI] [PubMed] [Google Scholar]
- 30.Lynch HT, Taylor RJ, Lynch JF, Knezetic JA, Barrows A, Fodde R, et al. Multiple primary cancer, including transitional cell carcinoma of the upper uroepithelial tract in a multigeneration HNPCC family: molecular genetic, diagnostic, and management implications. The American journal of gastroenterology. 2003;98(3):664–70. doi: 10.1111/j.1572-0241.2003.07329.x. [DOI] [PubMed] [Google Scholar]
- 31.Tavakkol Z, Keller JJ, Furmanczyk PS, Bennett RL, Chien AJ. Germline mutation in MSH6 associated with multiple malignant neoplasms in a patient With Muir-Torre syndrome. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(22):e195–8. doi: 10.1200/JCO.2011.41.5562. [DOI] [PubMed] [Google Scholar]
- 32.Raskin L, Schwenter F, Freytsis M, Tischkowitz M, Wong N, Chong G, et al. Characterization of two Ashkenazi Jewish founder mutations in MSH6 gene causing Lynch syndrome. Clinical genetics. 2011;79(6):512–22. doi: 10.1111/j.1399-0004.2010.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee B, Rennert G, Ahn J, Dishon S, Lejbkowicz F, Rennert HS, et al. High risk of colorectal and endometrial cancer in Ashkenazi families with the MSH2 A636P founder mutation. Gastroenterology. 2011;140(7):1919–26. doi: 10.1053/j.gastro.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rashid MU, Gull S, Faisal S, Khaliq S, Asghar K, Siddiqui N, et al. Identification of the deleterious 2080insA BRCA1 mutation in a male renal cell carcinoma patient from a family with multiple cancer diagnoses from Pakistan. Familial cancer. 2011;10(4):709–12. doi: 10.1007/s10689-011-9467-5. Epub 2011/07/14. [DOI] [PubMed] [Google Scholar]
- 35.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. Journal of the National Cancer Institute. 2002;94(18):1358–65. doi: 10.1093/jnci/94.18.1358. Epub 2002/09/19. [DOI] [PubMed] [Google Scholar]