Abstract
Microglia are derived from myelogenous cells and contribute to immunological and inflammatory responses in central nervous system. They play important roles not only in infectious diseases and inflammation after stroke, but also in psychiatric diseases such as schizophrenia. While recent studies suggest the significances of serum- and glucocorticoid-inducible kinases (SGKs) in other immune cells such as macrophages, T cells and dendritic cells, their role in microglia remains unknown. Here we, for the first time, report that SGK1 and SGK3 are expressed in multiple microglial cell lines. An SGK inhibitor, gsk650394, inhibits cell viability. In addition, lipopolysaccharide-induced expression of inflammatory regulators iNOS and TNFα was enhanced by gsk650394. Furthermore, translocation of NF-κB was enhanced by gsk650394. Taken together, these findings suggest that SGKs may play an important role in regulating microglial viability and inflammatory responses.
Keywords: SGK1, microglia, inflammation, iNOS, NF-κB
1. Introduction
Brain inflammation has been suggested to be associated with various neurological disorders. The inflammatory responses are mediated mainly by non-neuronal cells such as microglia and astrocytes. Microglial cells are derived from myelogenous cells and they have many characters of immune cells [1,2]. They work to maintain brain homeostasis, for example, by removing dead cells under resting condition. When brain receives severe stresses such as bacterial infection, stroke and tramatic injury, microglial cells are activated. Once activated, they release inflammatory cytokines such as tumor necrosis factor α (TNFα) and produce reactive oxygen species via inducible nitric oxide synthase (iNOS) [3,4]. As these events promote neuronal dysfunction, anti-inflammatory reagents appear to be beneficial for those disorders mentioned above [5,6].
In addition to disorders that have apparent acute stressful causes, anti-inflammation therapy can also be applied to neuropsychiatric disorders such as schizophrenia and chronic neurodegenerative disorders such as amyotrophic lateral sclerosis [7,8]. Therefore, further studies of microglial inflammatory responses and their modulators sheds light on comprehensive understanding and future therapeutic strategy of neurological disorders.
Serum- and glucocorticoid-inducible kinase 1 (SGK1) is a member of SGK family. Its expression is rapidly induced by serum and glucocorticoid [9]. Increasing evidence suggests that SGKs including SGK1 contribute to various physiological and pathophysiological processes [9,10]. In particular, SGKs, especially SGK1, have recently been shown to play a role in immune cells. For example, pathogenic IL-17-producing T cells are induced under high salt condition in an SGK1-dependent manner, and the induction is anticipated to exacerbate autoimmune encephalomyelitis [11]. Also, inhibition of SGK1 affects neutrophil chemotaxis [12]. In addition, recent studies have found SGK1 to be implicated in NF-κB activity, and that the regulated NF-κB signaling is associated with immunological significances such as inflammatory responses in monocytes and differentiation of dendritic cells [13,14].
Although all of the SGK family members are expressed in brain [15], the detailed distribution and function are not clear. There have been reports that describe apparent existence of SGK1 in neurons, astrocytes and oligodendrocytes [16,17,18]. In addition, the existence of SGK1 in a minor proportion of microglia in brain has been demonstrated by Wärntges et al [18]. However, the presence of other SGKs and the roles of SGKs in microglial cells remain to be elucidated. This study examines the expression of SGK isoforms and explores the effects of SGK inhibition in multiple microglial cell lines.
2. Materials and Methods
2.1 Reagents and antibodies
The following reagents and antibodies were used: Isogen (Nippon Gene); lipopolysaccharide (LPS Escherichia coli 111:B4, Sigma); gsk650394 (Selleckchem); protease inhibitor cocktail (Sigma); 2,3-diaminonaphthalene, tetracycline (Dojindo); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT, Wako); fluorescein diacetate (FDA, Wako); propidium iodide (PI, Wako); Cytoplasmic & Nuclear Protein Extraction Kit (101Bio); rabbit polyclonal antibody against actin (Sigma); mouse monoclonal antibody against iNOS (BD Biosciences); rabbit polyclonal antibody against NF-κB p65 (Cell Signaling), rabbit polyclonal antibody against lamin B1 (MBL).
2.2 Cell culture
A microglial cell line BV-2 was grown in Dulbecco's modified eagle medium (DMEM) with 5% fetal bovine serum (FBS) and antibiotics. Another microglial cell line N9 was grown in DMEM with 10% FBS and antibiotics.
2.3 RT-PCR
Total RNAs of BV-2, N9 and mouse brain cortex (9 months old male BL57/C6) were extracted with Isogen. cDNAs were synthesized from 500 ng total RNA in 20-μl reactions using oligo(dT)15 and reverse transcriptase (Funakoshi).
PCR reactions were performed using 1 μl of cDNAs as templates in 1 x PCR reaction buffer, 1 mM each dNTP, 0.04 μl Taq DNA polymerase, and 1 μM each primer in 20-μl reactions. The PCR amplification cycles consisted of denaturation at 94°C for 2 min, 35 cycles of denaturation at 94°C for 45 sec, annealing at 61°C to for 45 sec, and extension at 72°C for 1 min. PCR products were separated by electrophoresis on a 2% agarose gel, detected using ethidium bromide.
The primers used for PCR were described in Supplementary Table 1. Primer pairs were designed to bracket at least an intron for each gene to rule out amplification of genomic DNA.
2.4 Quantitative Real-time PCR
Quantitative real-time PCR was performed to validate the expression changes of selected genes using SYBR® Premix Ex Taq (TaKaRa) and the Thermal Cycler Dice Real Time System (Takara) in accordance with the manufacturer’s protocols. The PCR amplification cycles consisted of denaturation at 95°C for 30 sec, 40 cycles of denaturation at 95°C for 5 sec, and annealing/extension at 60°C to for 30 sec, followed by the detection of melt curve, 65°C to 95°C. Real-time PCR reactions were carried out in duplicate for each sample and the average values were applied to the ΔΔCt method for data analysis. Primer sets were described in the “Supplementary Table 2”.
2.5 MTT assay
MTT solution was solved in phosphate-buffered saline (PBS) at a concentration of 5 mg/ml. Cells were seeded at 5 x 104 in 24-well plates. On the following day cells were incubated with media (450 μl) in the absence or presence of gsk650394. Twenty four h later, 50 μl of MTT solution was added into each well and incubated for 3 h. Media was then removed and 200 μl of 0.04 N HCl/2-propanol was added. They were transferred into 96-well plates and absorbance was measured at 570 nm with 690 nm as reference using a microplate reader (Molecular Devices).
2.6 FDA and PI staining
Cells were seeded at 5 x 104 per well in 24-well plates. On the following day cells were incubated with media in the absence or presence of gsk650394. For staining of live and dead neurons, cultures were incubated with PBS containing FDA (3 μg/ml) and PI (5 μg/ml) for 5 min, followed by washing with PBS. Live (FDA-positive) and dead (PI-positive) cells were viewed and counted with a fluorescent microscope (Axio Observer Z1, Zeiss) at excitation/emission wavelengths of 470 nm/505-535 nm for FDA and 585 nm/615 nm for PI.
2.7 Immunoblotting
Immunoblotting was performed as described [19,20]. Cells cultured on 35 mm dishes or 6-well plates were lysed in lysis buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1% Triton X-100, and protease inhibitor). After centrifugation at 15,000 × g at 4°C for 30 min, the lysates were collected. For the extraction of nuclear protein, Cytoplasmic & Nuclear Protein Extraction Kit was used according to the manufacturer’s instruction. The aliquots were then mixed with Laemmli sample buffer and boiled at 95°C for 10 min. The samples were resolved by 7.5% SDS-PAGE, followed by electrotransfer to polyvinylidene difluoride membranes. For visualization, blots were probed with antibodies against iNOS (1:2000), NF-κB p65 (1:1000), lamin B1 (1:1000) or actin (1:2000), and detected using horseradish peroxidase-conjugated secondary antibodies (1:2000; Promega) and an ECL kit (Bio-RAD).
2.8 Measurement of nitric oxide (NO) metabolite
The production of NO was determined by measurement of nitrite, a stable product of NO, using fluorometric reagent 2,3-diaminonaphthalene [21,22]. 100 μl of samples were transferred to 96-well plate, and incubated with 10 μl fresh 2,3-diaminonaphthalene solution (50 μg/ml in 0.62 N HCl) for 10 min at room temperature. The reactions were terminated with 5 μl of 2.8 N NaOH. Formation of 2,3-diaminonaphthotriazole was measured using fluorescent multi-well plate reader (SpectraMax Gemini, Molecular Devices) with excitation/emission at 365/450 nm. The fluorescence signal was digitized and analyzed using SoftMax Pro software.
2.9 Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde in PBS, followed by permeabilization in PBS containing 0.2% Triton X-100. The cells were first incubated with NF-κB p65 antibodies (1:100), and then with the Alexa488-conjugated secondary antibodies (Invitrogen). For DNA staining, coverslips were incubated with DAPI. Fluorescent images were analyzed using an Axio Observer Z1 (Zeiss).
2.10 Statistical Analysis
Data are presented as means ± SEM. Differences between groups were compared using one-way ANOVA, or unpaired Student’s t-test as appropriate. Bonferroni’s test was used to compensate for multiple experimental procedures. p < 0.05 was regarded as statistically significant.
3. Results
3.1 Expression of SGK isoforms in microglial cells
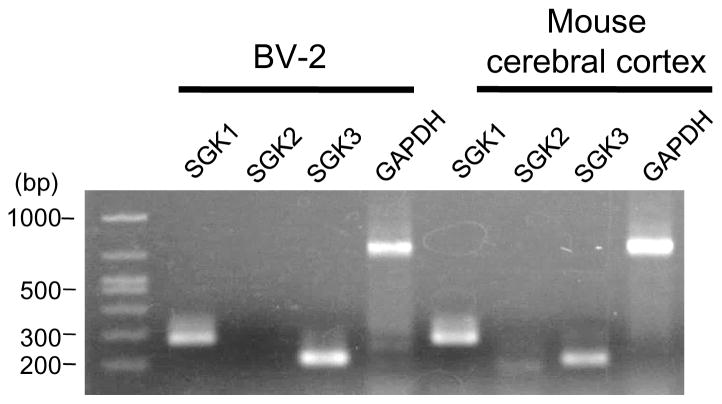
SGK family members, including SGK1, SGK2, and SGK3, are expressed in brain [15]. Recent studies have disclosed their potential functions [9,23]. For example, their roles in neuronal cells and oligodendrocytes have been documented [17,24,25,26]. A study by Wärntges et al. also reported the presence of SGK1 in microglia in a minor proportion in brain [18]. However, their function in microglial cells remains unknown. In this study, we first examined the presence of SGK isoforms in a microglial cell line, BV-2, with RT-PCR. As shown in Fig. 1, bands of the expected size for SGK1 and SGK3, but not SGK2, were detected in BV-2 cells. In contrast, all subunits were expressed in brain, as shown in a previous report [15]. Similar result was also observed from another microglial cell line, N9 (Supplementary Fig. 1).
Figure 1. SGK1 and SGK3 are expressed in BV-2 cells.
Total RNAs were isolated from the indicated tissues. Equal amounts of total RNA were reverse-transcribed and PCR-amplified using specific primers for each SGK isoform or GAPDH. Expected sizes of the fragments are: 279-bp (SGK1), 184-bp (SGK2), 204-bp (SGK3) and 723-bp (GAPDH).
3.2 The effect of an SGK inhibitor on cell viability in microglial cells
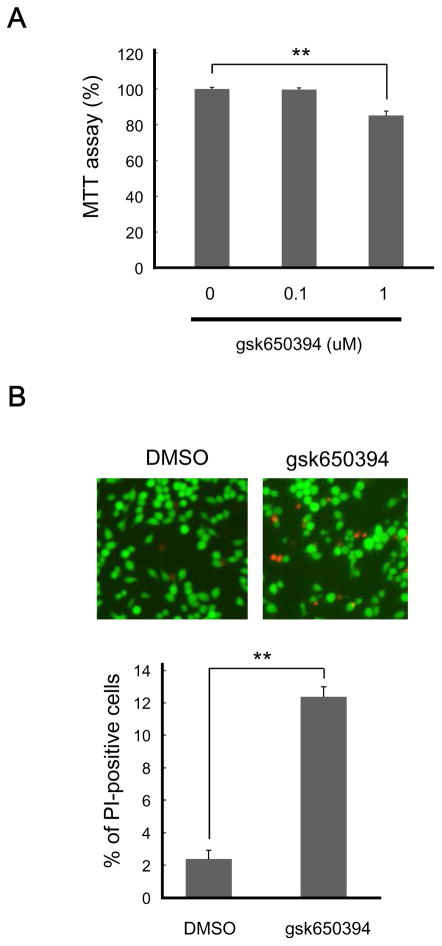
Next we assessed the effect of SGKs on the basal viability of microglial cells by MTT assay, a colorimetric assay for cell numbers. An SGK inhibitor, gsk650394, diminished MTT values in BV-2 cells at 1 μM, but not at 0.1 μM. Interestingly, a higher dose of gsk650394 was needed to exhibit the effect in N9 cells; a treatment of cells with 10 μM gsk650394 decreased MTT values, while 1 μM gsk650394 did not (Fig. 2A and Supplementary Fig. 2A). In any cases, SGK activity influences viability of microglia.
Figure 2. SGK inhibition impairs cell viability.
BV-2 cells were incubated with the indicated concentration of SGK inhibitor gsk650394 for 24 h. (A) Cell viability was measured by MTT assay. n = 4-8; ** p < 0.01 vs 0 μM, ANOVA followed by Bonferroni’s test. (B) Cells were stained with FDA for live cells and PI for dead cells. n = 3; ** p < 0.01 vs DMSO, Student’s t-test.
To determine whether this reduction of viability is due to cellular injury or delayed proliferation, we stained cells with FDA and PI since proportion of PI-positive cells represents cellular toxicity [27,28]. As shown in Fig. 2B and Supplementary Fig. 2B, PI-positive cells were increased in the presence of gsk650394 for both BV-2 and N9 cells. Together, these results suggest that SGK activity plays a role in maintaining the viability of microglia.
3.3 The effect of an SGK inhibitor on inflammatory responses
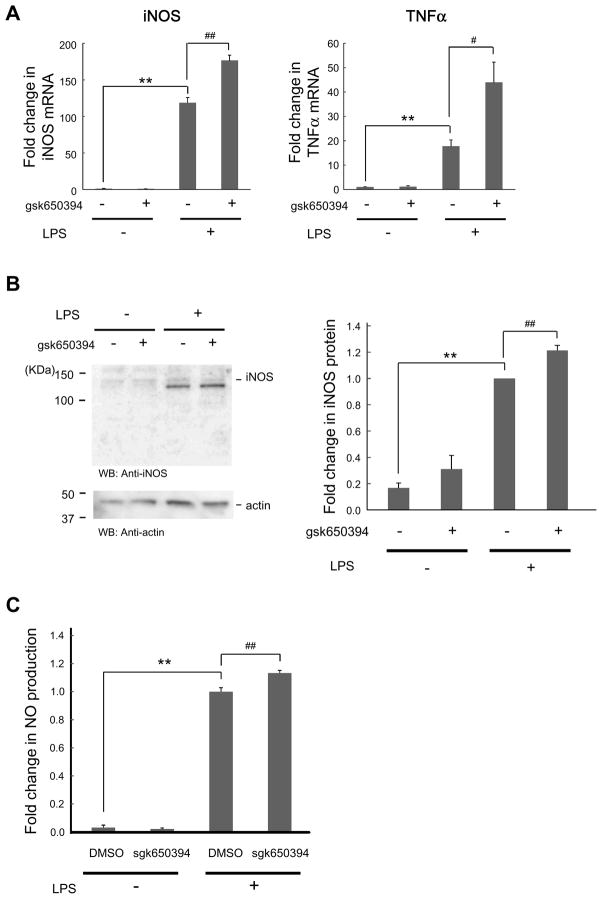
Since SGKs are implicated in inflammatory responses in some immune cells [13,14], we tested whether they play a role in microglial activation and inflammatory responses. In BV-2 cells stimulated with LPS, we found that the levels of gene expression of inflammatory mediators, iNOS and TNFα, were upregulated (Fig. 3A). The level of SGK1 was not altered (data not shown). Correspondingly, iNOS protein and the product of iNOS, NO, were recognized (Figs. 3B and C). When gsk650394 was applied 30 min before LPS treatment, those increases were enhanced (Figs. 3A–C). Consistent with the findings in BV-2 cells, enhanced NO production was also found by administration of gsk650394 in N9 cells (Supplementary Fig. 3). Together, these results are consistent with an enhancing effect of SGK inhibition on microglial inflammation. Thus, SGK activity is suggested to suppress inflammatory responses in microglial cells.
Figure 3. SGK inhibition increases LPS-induced inflammatory responses.
Cells were incubated in the absence or presence of gsk650394 (1 μM) for 30 min, followed by LPS (500 ng/ml) administration for 4 h (A), 8 h (B), and 24 h (C). (A) After RNA extraction and reverse transcription to synthesize cDNA, quantitative real-time PCR was performed to monitor mRNA levels of iNOS and TNFα in cells treated with indicated reagents. n = 3. (B) BV-2 cell lysates were analyzed by immunoblotting with the indicated antibodies. n = 4. (C) Media were taken and NO was measured by assessing nitrite, a natural metabolic product of NO. n = 6–8. ** p < 0.01 vs LPS, # p<0.05, ## p < 0.01 vs DMSO (0 μM gsk650394), Student’s t-test.
3.4 Enhancement of NF-κB signaling by SGK inhibitor
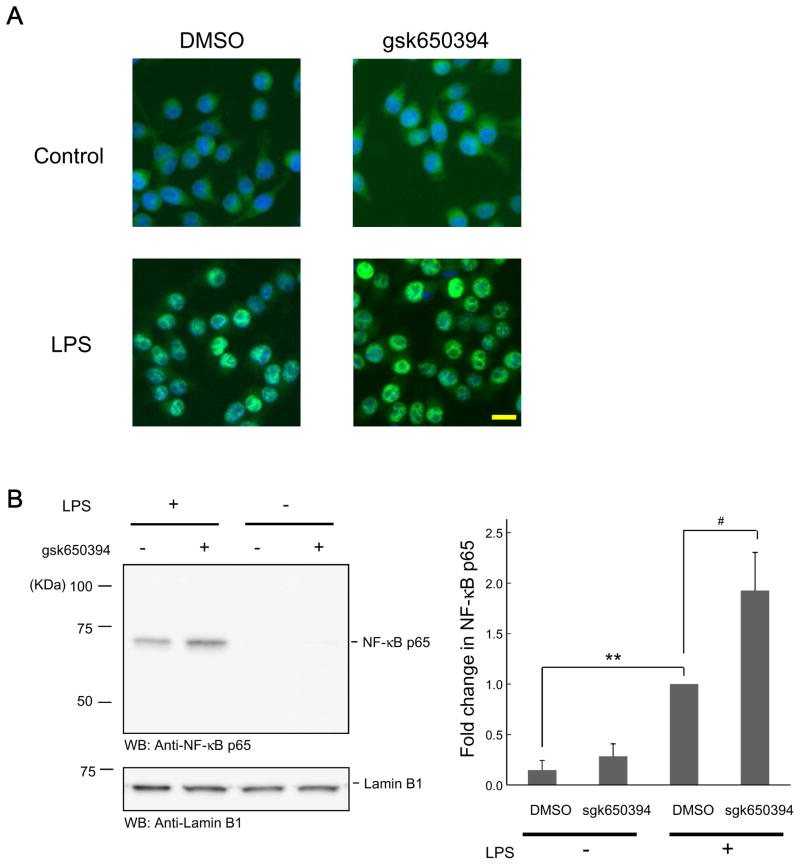
Given the fact that inflammatory factors are facilitated by SGK inhibition and that NF-κB signaling is regulated by SGK1 in other immune cells [13,14], we inspected the effect of SGK inhibition on NF-κB signaling. First, we probed nuclear translocation of NF-κB by LPS application and found that fluorescent signals of NF-κB seemed to be more intensive in nuclei in the presence of gsk650394 (Fig. 4A). To provide a quantitative analysis, nuclear components were extracted and immunoblotting was carried out. As a result, higher amount of NF-κB was detected in nuclei in the presence of gsk650394, suggesting an enhanced translocation of NF-κB (Fig. 4B). Taken together, these findings support the view that SGKs are fundamental regulators for microglial viability and inflammatory activity.
Figure 4. SGK inhibition enhances NF-κB signaling activity.
(A) BV-2 cells were incubated in the absence or presence of gsk650394 (1 μM) for 30 min, followed by LPS (500 ng/ml) administration for 30 min, and then fixed. NF-κB p65 protein was visualized by indirect immunofluorescence staining using an antibody for NF-κB p65. For nuclear staining, the cells were stained with DAPI. Bar = 20 μm. (B) Cells were treated as described in (A) and nuclear samples were extracted, followed by Western blotting using anti- NF-κB p65 antibody. Protein loading was monitored by anti-lamin B1 antibody. n = 4. ** p < 0.01 vs LPS, # p<0.05 vs DMSO, Student’s t-test.
4. Discussion
Early studies have revealed the pivotal function of SGK1 in renal salt reabsorption [29]. Since then, significances of SGKs in other systems such as reproduction and immunity have been demonstrated [11,30]. Similarly, there have been some reports which propose the relationship between SGKs and neurological diseases such as psychiatric and neurodegenerative disorders. In the case of depression, physical stresses increase SGK1 in neuronal progenitor cells, which plays a role in depression [31]. In support of this, samples of human depressive patients have SGK1 expression at higher levels compared to the control [31]. On the other hand, the latest study shows decreased expression of all of SGK isoforms in the prefrontal cortex of patients with posttraumatic stress disorder [32]. Experimental inhibition of SGK1 leads to abnormal behaviors in rats and compensatory expression of SGK1 [32]. Based on these findings, SGK activity could either deteriorate or improve brain disorders under different situations. Apart from psychiatric disorders, Zhang et al. reported that neuronal SGK1 overexpression decreases brain injury induced by experimental ischemic stress [26]. In another report, SGK1 in microvascular endothelial cells of brain seemed to aggravate ischemic brain injury [25]. In line with this, we have also reported that SGK inhibition by specific inhibitors is beneficial to ischemic brain injury [33]. Collectively, the functional role of SGKs is complicated presumably due to multiple reasons such as specific distribution in distinct cell types, diverse levels of expression, and presence of different splicing variants. As the roles of SGKs may be highly diverged among different types of cells in brain, the studies of SGKs in each type of cells are required. In this regard, study of SGKs in microglia is valuable and will help for comprehensive understanding of the significance of SGKs in brain.
In the present study, we have demonstrated that SGK1 and 3 are expressed in microglial cells and that inhibition of SGK activity enhances microglial inflammatory responses. Thus, the basal SGK activity is likely to suppress the activation of microglial cells. In addition, clear influence of SGKs on cell viability was also observed in the present study. This pro-survival effect is conceivable due to their effect on Akt/protein kinase B signaling, which could prevent apoptosis [9,34]. We found that doses of gsk650394 necessary for cell viability and inflammatory response are different between two microglial cell lines. These results suggest that an extent of their contribution to cellular processes may be subject to microglial appearances. Nonetheless, the trend of the effect is likely to be same; SGK inhibition promotes inflammatory responses. We have to be cautious in considering SGK inhibition for therapeutic intervention as the net effect of inflammation on brain function can be either beneficial or detrimental [35,36]. However, if SGK inhibition preferentially affects the microglia than other types of cells in brain such as neurons, the regulation of SGK activity in microglial cells may provide a novel therapeutic approach for neuronal disorders in which inflammatory processes are implicated.
Supplementary Material
SGK1 and SGK3 are expressed in multiple microglial cell lines.
Inhibiting SGK activity reduces microglial viability.
SGK inhibition enhances microglial inflammatory responses via NF-κB signaling.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research No. 16K09520 from the Japan Society for the Promotion of Science, Japan (K.I.), Grant-in-Aid for Scientific Research on Innovative Areas “Glial Assembly” from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (T.U.), and NIH R01NS066027 and NIHMD S21MD000101 (Z.-G.X).
Abbreviations
- SGK
serum- and glucocorticoid-inducible kinase
- TNFα
tumor necrosis factor α
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- FDA
fluorescein diacetate
- PI
propidium iodide
- DMEM
Dulbecco's modified eagle medium
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- NO
nitric oxide
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 3.Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA. Retinoic acid inhibits expression of TNF-α and iNOS in activated rat microglia. Glia. 2005;50:21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 5.Breitner JC. The role of anti-inflammatory drugs in the prevention and treatment of Alzheimer's disease. Annu Rev Med. 1996;47:401–411. doi: 10.1146/annurev.med.47.1.401. [DOI] [PubMed] [Google Scholar]
- 6.Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- 7.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lang F, Gorlach A, Vallon V. Targeting SGK1 in diabetes. Expert Opin Ther Targets. 2009;13:1303–1311. doi: 10.1517/14728220903260807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgon J, Robertson AL, Sadiku P, Wang X, Hooper-Greenhill E, Prince LR, Walker P, Hoggett EE, Ward JR, Farrow SN, Zuercher WJ, Jeffrey P, Savage CO, Ingham PW, Hurlstone AF, Whyte MK, Renshaw SA. Serum and glucocorticoid-regulated kinase 1 regulates neutrophil clearance during inflammation resolution. J Immunol. 2014;192:1796–1805. doi: 10.4049/jimmunol.1300087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Gao S, Duan X, Liang S, Scott DA, Lamont RJ, Wang H. Inhibition of serum- and glucocorticoid-inducible kinase 1 enhances TLR-mediated inflammation and promotes endotoxin-driven organ failure. FASEB J. 2015;29:3737–3749. doi: 10.1096/fj.15-270462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid E, Xuan NT, Zahir N, Russo A, Yang W, Kuhl D, Faggio C, Shumilina E, Lang F. Serum- and glucocorticoid-inducible kinase 1 sensitive NF-κB signaling in dendritic cells. Cell Physiol Biochem. 2014;34:943–954. doi: 10.1159/000366311. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- 16.Slezak M, Korostynski M, Gieryk A, Golda S, Dzbek J, Piechota M, Wlazlo E, Bilecki W, Przewlocki R. Astrocytes are a neural target of morphine action via glucocorticoid receptor-dependent signaling. Glia. 2013;61:623–635. doi: 10.1002/glia.22460. [DOI] [PubMed] [Google Scholar]
- 17.Miyata S, Koyama Y, Takemoto K, Yoshikawa K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T, Tohyama M. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS One. 2011;6:e19859. doi: 10.1371/journal.pone.0019859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wärntges S, Friedrich B, Henke G, Duranton C, Lang PA, Waldegger S, Meyermann R, Kuhl D, Speckmann EJ, Obermuller N, Witzgall R, Mack AF, Wagner HJ, Wagner A, Broer S, Lang F. Cerebral localization and regulation of the cell volume-sensitive serum- and glucocorticoid-dependent kinase SGK1. Pflugers Arch. 2002;443:617–624. doi: 10.1007/s00424-001-0737-1. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue K, Furukawa T, Kumada T, Yamada J, Wang T, Inoue R, Fukuda A. Taurine inhibits K+-Cl− cotransporter KCC2 to regulate embryonic Cl− homeostasis via with-no-lysine (WNK) protein kinase signaling pathway. J Biol Chem. 2012;287:20839–20850. doi: 10.1074/jbc.M111.319418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Xiong ZG. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc Res. 2009;83:547–557. doi: 10.1093/cvr/cvp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588:3349–3354. doi: 10.1113/jphysiol.2010.190926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata S, Hattori T, Shimizu S, Ito A, Tohyama M. Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed Res Int. 2015;2015:492367. doi: 10.1155/2015/492367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Fang S, Wan C, Kong Q, Wang G, Wang S, Zhang H, Zou H, Sun B, Sun W, Zhang Y, Mu L, Wang J, Wang D, Li H. Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci Rep. 2015;5:16548. doi: 10.1038/srep16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Qian C, Li SQ. Protective effect of SGK1 in rat hippocampal neurons subjected to ischemia reperfusion. Cell Physiol Biochem. 2014;34:299–312. doi: 10.1159/000363000. [DOI] [PubMed] [Google Scholar]
- 27.Leng TD, Lin J, Sun HW, Zeng Z, O'Bryant Z, Inoue K, Xiong ZG. Local anesthetic lidocaine inhibits TRPM7 current and TRPM7-mediated zinc toxicity. CNS Neurosci Ther. 2015;21:32–39. doi: 10.1111/cns.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- 29.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salker MS, Christian M, Steel JH, Nautiyal J, Lavery S, Trew G, Webster Z, Al-Sabbagh M, Puchchakayala G, Foller M, Landles C, Sharkey AM, Quenby S, Aplin JD, Regan L, Lang F, Brosens JJ. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17:1509–1513. doi: 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- 31.Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, Thuret S, Price J, Uher R, Riva MA, Pariante CM. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2013;110:8708–8713. doi: 10.1073/pnas.1300886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licznerski P, Duric V, Banasr M, Alavian KN, Ota KT, Kang HJ, Jonas EA, Ursano R, Krystal JH, Duman RS. Decreased SGK1 expression and function contributes to behavioral deficits induced by traumatic stress. PLoS Biol. 2015;13:e1002282. doi: 10.1371/journal.pbio.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue K, Leng T, Yang T, Zeng Z, Ueki T, Xiong ZG. Role of serum- and glucocorticoid-inducible kinases in stroke. J Neurochem. 2016;138:354–361. doi: 10.1111/jnc.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.