Abstract
Background & Aims
Foxp3+ regulatory T cells (Tregs) in the intestine promote immune tolerance to enteric antigens. Previous studies have shown that C-C chemokine receptor 7 (CCR7)-dependent migration of intestinal dendritic cells to the mesenteric lymph nodes (mLN) is involved in peripheral Foxp3+ Treg accumulation in the intestine and the establishment of oral tolerance. However, the relative contribution of this CCR7+ dendritic cell–mLN–Treg axis to the total intestinal Foxp3+ Treg pool during the steady-state remains unclear. In this study, the contribution of CCR7, as well as the mLN and gut-associated lymphoid tissue (GALT), to the intestinal Foxp3+ Treg compartment in the small intestine (SI) and large intestine (LI) was assessed.
Methods
Intestinal Foxp3+ Tregs were quantitated in Ccr7-/- mice and in mice devoid of secondary lymphoid organs—including mLN and GALT—owing to a deficiency in lymphotoxin (LT) signaling. Specific analyses of Foxp3+Helios+ thymically derived (t)Tregs and Foxp3+Helios- peripherally derived (p)Tregs in the SI and LI, as well as the role for the mLN in supporting Foxp3+ pTreg development using the B6.Cg-Tg(TcraTcrb)425Cbn/J/ovalbumin (OVA) feeding system, were performed.
Results
Foxp3+ Tregs were enriched in the intestine relative to the mLN, independent of CCR7. In the absence of the mLN and GALT, normal frequency and numbers of Foxp3+ Tregs were observed in LTα-deficient (Lta-/-) mice. However, Foxp3+Helios- pTregs were decreased in the SI of Lta-/- mice, corresponding with defective Foxp3+ pTreg expansion to OVA. In the LI, however, the proportion of Foxp3+Helios- pTregs and Foxp3+ pTreg induction to OVA was comparable between Lta-/- and Lta+/+ mice, which coincided with preferential expression of Treg-inducing/immunoregulatory cytokines.
Conclusions
The overall size of the intestinal Foxp3+Treg pool is not impacted significantly by CCR7, mLN, or GALT during the steady-state. However, mLN/GALT appear to contribute to the Foxp3+ pTreg compartment in the SI, particularly in response to soluble oral antigen. These findings highlight important differences in the regulation of intestinal Tregs between the SI and LI, and suggest that enteric antigens may use mLN/GALT to induce Foxp3+ pTreg in the SI, while directly promoting Foxp3+ pTregs in the LI.
Keywords: Regulatory T Cell, Foxp3, Intestine
Abbreviations used in this paper: CCR, C-C chemokine receptor 7; DC, dendritic cell; GALT, gut-associated lymphoid tissue; IL, interleukin; LI, large intestine; LP, lamina propria; LT, lymphotoxin; mLN, mesenteric lymph nodes; mRNA, messenger RNA; OT-II, B6.Cg-Tg(TcraTcrb)425Cbn/J; OVA, ovalbumin; pTreg, peripherally derived Treg; SI, small intestine; SLO, secondary lymphoid organ; Spl, spleen; TCR, T-cell receptor; TGF, transforming growth factor; Treg, regulatory T cells; tTreg, thymically derived Treg
Summary.
This study showed that the absence of CCR7 or mesenteric lymph nodes/gut-associated lymphoid tissue did not appreciably impact total intestinal Foxp3+ regulatory T cell representation in the steady-state. However, mesenteric lymph nodes/GALT are required for normal peripherally induced Foxp3+ regulatory T cell differentiation in the small intestine, but not in the large intestine.
In the intestine, Foxp3+ regulatory T cells (Tregs) are present in the lamina propria (LP) where they help promote tolerance to enteric antigens deriving from food and commensal microbes.1, 2 Previous studies investigating the site of enteric antigen presentation and oral tolerance have shown the mesenteric lymph nodes (mLNs) to be involved in intestinal Foxp3+ Treg differentiation.3, 4 In particular, CCR7-expressing CD103+ dendritic cells (DCs) can carry enteric antigens from the intestine to the mLN, where they stimulate naive CD4+ T cells.4 Subsequently, a fraction of these activated CD4+ T cells differentiate into gut-tropic Foxp3+ peripherally derived Treg (pTregs) that home to the intestine where they expand in response to interleukin (IL)10 produced by LP CX3CR1+ macrophages.3, 5, 6, 7 However, the contribution of CCR7 and mLN/gut-associated lymphoid tissue (GALT) to the total Foxp3+ Treg pool in the small intestine (SI) and large intestine (LI) during the steady-state remains unclear.
In addition to Foxp3+ pTregs, a subset of CD4 single-positive thymocytes develops into Foxp3+ thymically derived Tregs (tTregs) and contributes to the total Foxp3+ Treg pool in peripheral tissues, such as the intestine. The Foxp3+ tTreg population is distinct from Foxp3+ pTregs in terms of development and T-cell receptor (TCR) repertoire.8, 9, 10, 11 The TCR reactivity of Foxp3+ tTregs is thought be directed toward self-antigens, and previous studies have suggested distinct functional niches for tTregs and pTregs based on these differences in TCR repertoire and antigen reactivity.8, 9, 12 However in the intestine, recent evidence has suggested both Foxp3+ tTregs and pTregs are capable of recognizing antigens derived from the commensal bacteria and may promote tolerance to the intestinal microbiota.13, 14 Nonetheless, the contribution of mLN/GALT to Foxp3+ tTregs and pTregs in healthy intestinal tissue along with differences between the SI and LI have yet to be investigated directly.
In this study, we investigated the requirement for the mLN/GALT in mediating intestinal Foxp3+ Treg development for both the SI and LI, and also assessed the contribution of tTregs and pTregs that comprise the total Foxp3+ Treg compartment. We show here that the enrichment of Foxp3+ Tregs in the SI LP and LI LP is independent of CCR7, and normal Foxp3+ Treg frequencies and cell numbers are observed in the intestine of mice devoid of all lymphotoxin (LT)-dependent secondary lymphoid organs (SLOs), including the mLN and GALT. Further evaluation of the intestinal Foxp3+ Treg compartment in the SI and LI showed that the majority (>60%) of the Foxp3+ Tregs expressed Helios, indicating that they may be of thymic origin. Nonetheless, there were distinct regional requirements for the mLN in Foxp3+ pTreg development whereby SLOs were required for Foxp3+ pTreg development in the SI, but were not required for the immunoregulatory cytokine-rich environment of the LI. Collectively, these findings highlight important differences in the composition and tissue site requirements between Foxp3+ Tregs in the SI and LI.
Materials and Methods
Mice
The following age- and sex-matched mice were purchased from The Jackson Laboratory (Bar Harbor, ME): C57BL/6 (B6), B6.129S2-Ltatm1Dch/J (Lta-/-), SPLx Lta-/- and B6.PL-Thy1a/CyJ (Thy1.1), and B6.Cg-Tg(TcraTcrb)425Cbn/J (OT-II) mice. Ltbr+/+ and Ltbr-/- mice are not commercially available and age- and sex-matched littermate controls were provided by one of the authors (R.D.N.). Experiments using Ltbr+/+ and Ltbr-/- mice were performed at Washington University-St. Louis. Germ-free mice were maintained as previously described.15 Mice were maintained under specific pathogen-free conditions and animal protocols were approved by the Institute Animal Care and Use Committee of Emory University and Georgia State University.
Antibodies and Reagents
The following antibodies were used from eBioscience (San Diego, CA) unless otherwise specified: Helios (22F6; Biolegend, San Diego, CA), CD90.1 (H1S51), TCRβ (H57-597; BD Biosciences, San Jose, CA), CD69 (H1.2F3), Vβ5 (MR9-4; BD Biosciences), Foxp3 (FJK16S), CD45RB (C363.16A), CD45.1 (A20), Vα2 (B20.1), CD3ε (eBio500A2), CD152 (UC10-4B9), folate receptor 4 (eBio12A5), CD73 (eBioTY/11.8), Vα2 (B20.1; BD Biosciences), CD25 (PC61.5), and CD4 (RM4-5; BD Biosciences).
Isolation of Intestinal LP Lymphocytes
Isolation of LP cells was performed as previously described.16, 17 For isolation of LP lymphocytes, cell suspensions were pelleted by centrifugation at 1500 rpm. The pellet was resuspended in 10 mL of 45% Percoll (GE Healthcare, Pittsburgh, PA) and underlaid with 70% Percoll. After centrifugation at 2000 rpm for 20 minutes at 20°C, the interface was harvested and washed.
Flow Cytometry
Isolated LP lymphocytes were resuspended in phosphate-buffered saline containing 5% fetal bovine serum. Live cells were identified using an Aqua Dead Cell Staining Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions, and Fc receptors were blocked with the antibody anti-FcγRIII/II (2.4G2) for 15 minutes at 4°C. After incubation, the cells were stained at 4°C for 30 minutes with fluorescence-labeled antibodies. Samples then were washed 2 times in phosphate-buffered saline containing 5% fetal bovine serum and intracellular staining was performed using a Foxp3 fixation/permeabilization kit (eBioscience). Flow cytometric analysis was performed on a LSR II (BD Biosciences).
In Vitro Suppression Assay
Isolated intestinal lamina propria lymphocytes were sorted for CD4+CD25+ cells and co-cultured with splenic DCs and carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+CD25-Thy1.1+ responder cells at a suppressor-to-responder ratio of 2:1. Lymphocytes were stimulated to proliferate with anti-CD3 antibody for 3 days in vitro. CFSE dilution was used to measure proliferation, and responders stimulated in the absence of suppressors were used to assess suppression.
In Vivo Foxp3+ Treg Cell Differentiation Studies
Naive CD4+CD25- T cells from spleen and peripheral lymph nodes were sorted (>99% purity) from ovalbumin (OVA; 323-339) peptide-specific TCR-transgenic (OT-II) Thy1.1 mice. Each Lta+/+ and Lta-/- recipient was adoptively transferred 3 × 106 OT-II Thy1.1 cells. Twenty-four hours after the transfer of cells, recipients were fed OVA via the drinking water at 20 mg/mL for 5 days, and switched to normal drinking water for 6 days. Lymphocytes from the intestinal LP and MLN were isolated and directly stained for Foxp3.
Statistics
Statistical analyses were performed with Prism software (GraphPad Software, La Jolla, CA) using the Student t test or a 1-way analysis of variance with Sidak’s post hoc test. Error bars represent SEM as indicated and P values of .05 or less were considered statistically significant, whereas P values greater than .05 were considered not statistically significant.
All authors had access to the study data and reviewed and approved the final manuscript.
Results and Discussion
Foxp3+ Tregs Are Enriched in the Intestinal LP Independent of CCR7
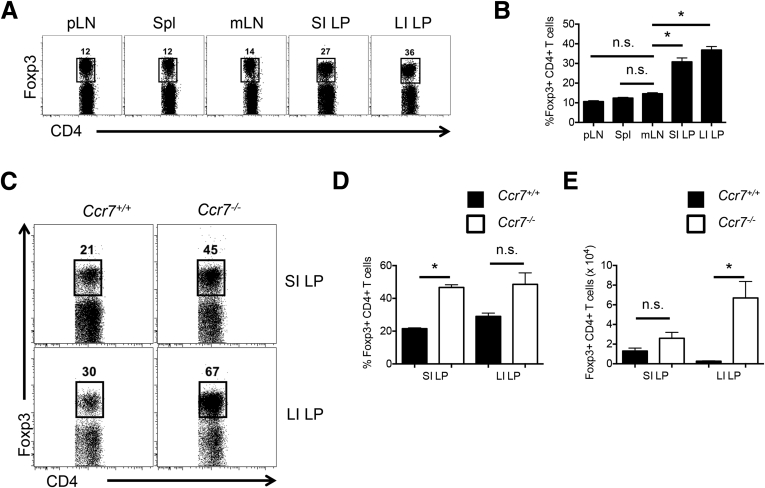
Because the mLN are implicated to be the site of intestinal Foxp3+ Treg development,3, 4, 18 an enrichment of Foxp3+ Tregs in the mLN would be expected relative to other SLOs that do not drain the lymphatics of the intestine and lack the intestinal antigen-presenting cells adept at inducing Foxp3+ Tregs. However, the frequency of Foxp3+ Tregs in the mLN (14.6% ± 0.50%) was observed to be comparable with frequencies in the peripheral lymph nodes (10.6% ± 0.46%) and spleen (Spl; 12.4% ± 0.37%) (Figure 1A and B). In contrast, Foxp3+ Tregs were enriched significantly in the SI LP (30.8% ± 2.00%) and the LI LP (36.8% ± 1.82%) relative to the mLN (Figure 1A and B). These results show that Foxp3+ Tregs are not preferentially enriched in the mLN relative to other SLOs that do not drain the intestine but are abundant in the intestine.
Figure 1.
Foxp3+ Tregs are enriched in the intestinal LP independent of CCR7. (A) Representative fluorescence-activated cell sorter plots pregated on TCRβ+ CD4+ cells, showing the proportion of Foxp3+ Tregs in the Spl, peripheral lymph node (pLN), mLN, SI LP, and LI LP of B6 mice. (B) Comparison of Foxp3+ Treg frequencies in the intestinal LP relative to the mLN and other SLOs. Data are representative of 4 independent experiments with n = 2–3 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using a 1-way analysis of variance with Sidak’s post hoc test. (C) Representative fluorescence-activated cell sorter plots of Foxp3+ Tregs pregated on TCRβ+ CD4+ cells in the SI LP and LI LP of Ccr7+/+ and Ccr7-/- mice. Comparison of Foxp3+ (D) Treg frequencies and (E) cell numbers between Ccr7+/+ and Ccr7-/- mice. Data are representative of 2 independent experiments with n = 4 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using the Student t test.
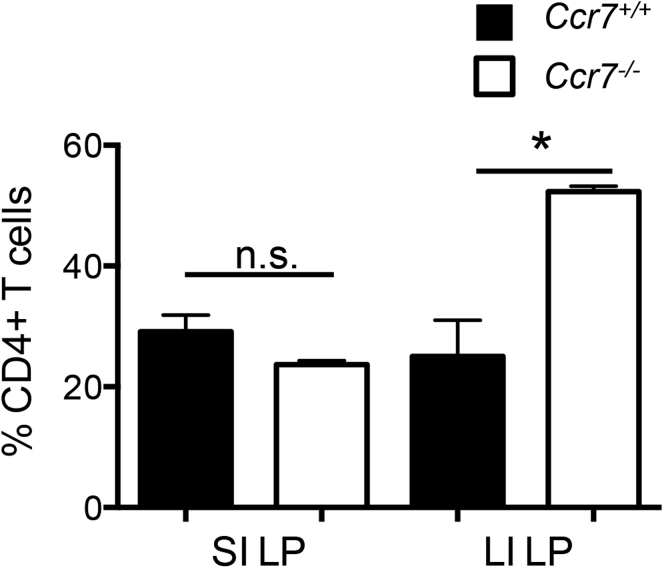
Because intestinal DCs are thought to carry enteric antigens to the mLN via CCR7 and provide antigenic stimulation to naive CD4+ T cells for Foxp3+ pTreg differentiation,4 we investigated whether the intestinal Foxp3+ Treg population was diminished in Ccr7-/- mice. On the contrary, Foxp3+ Treg frequencies (Figure 1C and D) and cell numbers (Figure 1E) within the SI LP and LI LP were similar or increased in the absence of CCR7. Total TCRβ+CD4+ T-cell frequencies are shown in Supplementary Figure 1. The reason for increased Foxp3+ Treg frequencies in Ccr7-/- mice may be explained by enhanced expression of peripheral tissue homing ligands, particularly CD103 and CCR2, on splenic Foxp3+ Tregs (data not shown).19 Taken together, these data suggest that CCR7-dependent migration of intestinal DCs to the mLN is dispensable for a normal representation of intestinal Foxp3+ Tregs.
Supplementary Figure 1.
CD4+ T cells accumulate in the intestinal LP independent of CCR7. Comparison of TCRβ+ CD4+ cell frequencies in the SI and LI LP between Ccr7+/+ and Ccr7-/- mice. Data are representative of 2 independent experiments with n = 4 mice per group. Error bars represent SEM. *P ≤ .05; NS, P > .05 using the Student t test.
mLN and Other LT-Dependent SLOs Are Not Required for a Normal Representation of Intestinal Foxp3+ Tregs
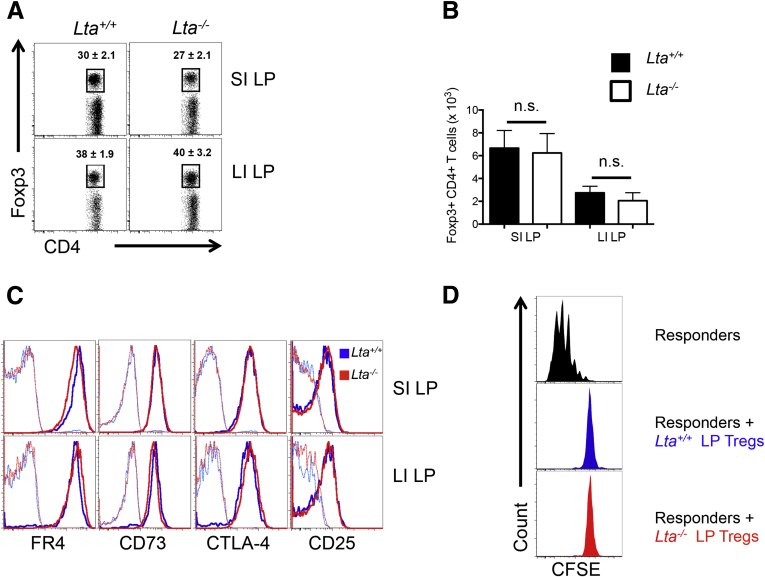
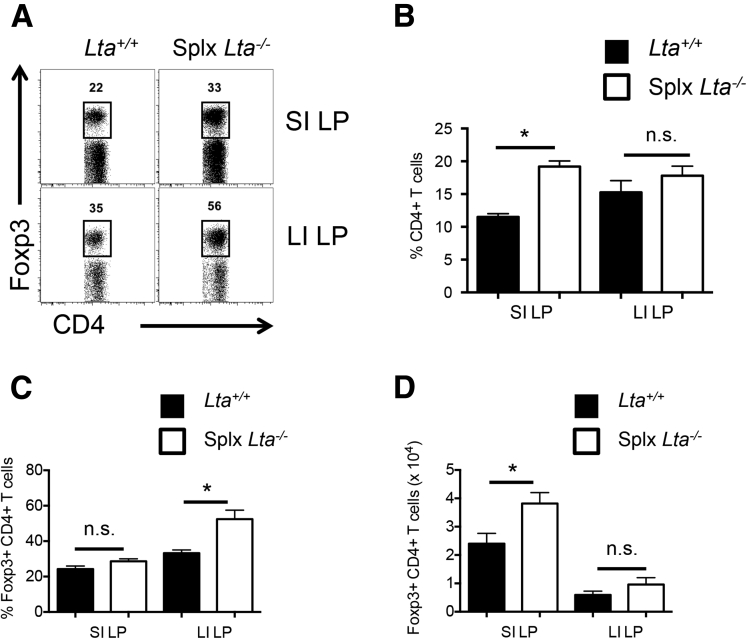
Next, we examined the contribution of mLN/GALT to the steady-state pool of intestinal Foxp3+ Tregs by analyzing Foxp3+ Tregs in the SI and LI of Lta-/- mice, which are void of the mLN, GALT, and other LT-dependent SLO. Interestingly, the frequency of Foxp3+ Tregs was similar between Lta+/+ and Lta-/- mice in the SI LP (30% ± 2.1% vs 27% ± 2.1%, respectively) (Figure 2A) and the LI LP (38% ± 1.9% vs 40% ± 3.2%, respectively) (Figure 2A). These data corresponded with comparable absolute numbers of Foxp3+ Tregs as well (Figure 2B). To verify that these were bona fide Foxp3+ Tregs, expression for the following Treg-associated markers was assessed: folate receptor 4, the ecto-5’-nucleotidase CD73, cytotoxic T-lymphocyte–associated protein 4, and CD25, the interleukin 2–receptor α-subunit (Figure 2C).20 Intestinal Foxp3+ Tregs from Lta-/- mice expressed these Treg-associated markers at levels indistinguishable from Lta+/+ mice (Figure 2C). Furthermore, intestinal Tregs from Lta+/+ and Lta-/- mice were confirmed to be similar in their capacity for functional suppression in vitro at a responder-to-suppressor ratio of 2:1 (Figure 2D). To investigate the Spl to be a potential site for intestinal Foxp3+ Treg expansion in the absence of the mLN and other LT-dependent SLOs, similar experiments were performed in splenectomized Lta-/- mice as well as lymphotoxin β–receptor–deficient (Ltbr-/-) mice, which served as an additional model of SLO deficiency. Both splenectomized Lta-/- and Ltbr-/- mice yielded similar results to Lta-/- mice (Supplementary Figures 2 and 3). Altogether, these findings indicate that intestinal Foxp3+ Treg development does not require the mLN or other LT-dependent SLO, although it should be noted that potential compensatory expansion of effector compartments may take place in Ccr7-/- and/or Lta-/- mice.
Figure 2.
Total intestinal Foxp3+ Treg compartment is independent of mLN and other LT-dependent SLOs. (A) Representative fluorescence-activated cell sorter plots, pregated on CD4+ TCRβ+ cells, with frequencies of Foxp3+ Tregs in the SI and LI LP of Lta+/+ and Lta-/- mice. (B) Absolute cell number of Foxp3+ Tregs in the SI and LI LP of Lta+/+ and Lta-/- mice. (C) Expression of Treg markers on Foxp3+CD4+ T cells in the SI and LI of Lta+/+ and Lta-/- mice. Unbolded histograms represent unstained controls. (D) In vitro suppression assay of intestinal LP Tregs from Lta+/+ and Lta-/- mice at a suppressor-to-responder ratio of 2:1. CFSE dilution was used to measure proliferation, and responders stimulated in the absence of suppressors (black histogram) were used to assess suppression. All panels are pregated on CD4+Thy1.1+cells. Data are representative of at least 3 independent experiments. Error bars represent SEM. n.s., P > .05 using the Student t test.
Supplementary Figure 2.
Intestinal Foxp3+ Treg development in splenectomized Lta-/-mice. (A) Representative fluorescence-activated cell sorter plots of intestinal Foxp3+ Tregs for Lta+/+ and splenectomized (Splx) Lta-/- mice. Comparison of (B) total TCRβ+ CD4+ T-cell frequencies, (C) intestinal Foxp3+ Treg frequencies, and (D) numbers for Lta+/+ and Splx Lta-/- mice. Data are representative of 2 independent experiments with n = 4 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using the Student t test.
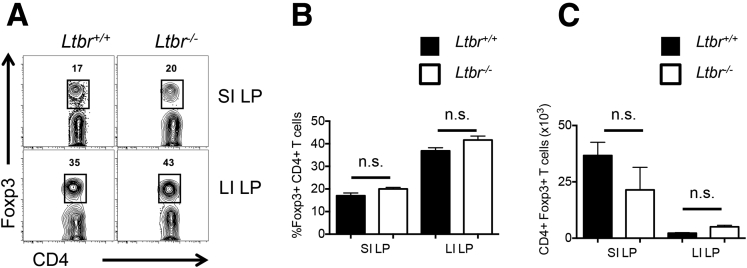
Supplementary Figure 3.
Intestinal Foxp3+ Treg development in Ltbr-/-mice. (A) Representative fluorescence-activated cell sorter plots of intestinal Foxp3+ Tregs in Ltbr+/+ and Ltbr-/- mice. Comparison of (B) intestinal Foxp3+ Treg frequencies and (C) numbers in Ltbr+/+ and Ltbr-/- mice. Data are representative of 2 independent experiments with n = 4 mice per group. Error bars represent SEM. n.s., P > .05 using the Student t test.
Foxp3+Helios- pTreg Representation in the SI, but Not LI, Requires SLO
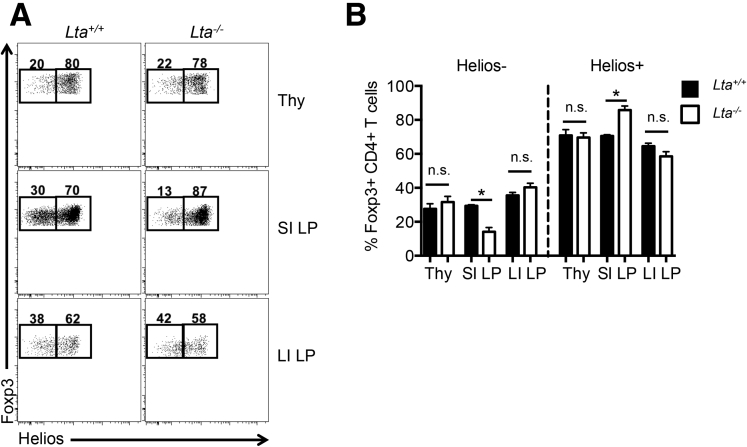
Furthermore, we investigated whether the absence of the mLN and other LT-dependent SLOs affected the proportion of Foxp3+ pTregs and tTregs in the intestine. Previous studies have shown that Helios, a member of the Ikaros transcription factor family, is expressed preferentially on Foxp3+ tTregs.21, 22 In contrast to neuropilin-1, another Foxp3+ tTreg marker,23, 24 Helios is suggested to be more reliable for discerning Foxp3+ tTregs from Foxp3+ pTregs,21, 25 however, Helios may be induced during T-cell activation and proliferation, thus limiting its ability to universally distinguish tTregs from pTregs.26 In defining Foxp3+Helios+ Tregs as tTregs and Foxp3+Helios- Tregs as pTregs in accordance with previous studies, Foxp3+ Tregs in the thymus predominantly expressed Helios for both Lta+/+ and Lta-/- mice as expected (∼80% of Foxp3+ Tregs) (Figure 3). In the intestine, the majority (>60%) of the Foxp3+ Tregs in the SI and LI expressed Helios as well. In the absence of the mLN, Foxp3+Helios- pTregs was decreased significantly in the SI LP of Lta-/- mice relative to Lta+/+ mice (14.1% ± 2.4% vs 29.3% ± 0.715%, respectively), which corresponded with proportionately more Foxp3+Helios+ tTregs in the SI LP of Lta-/- mice (Figure 3B). In contrast, the abundance of Foxp3+Helios- pTregs in the LI LP was similar between Lta+/+ and Lta-/- mice (Figure 3). Together, these results indicate that Foxp3+ Tregs in the intestine are derived predominantly from thymus, and Foxp3+ pTreg accumulation in the SI, but not LI, requires the mLN/GALT.
Figure 3.
mLN/GALT are involved in Foxp3+Helios- pTreg accumulation in the SI, but not the LI. (A) Representative fluorescence-activated cell sorter plots of Helios expression by Foxp3+CD4+ T cells in the thymus (Thy), SI LP, and LI LP of Lta+/+ and Lta-/- mice. (B) Comparison of Foxp3+Helios- pTregs and Foxp3+Helios+ tTregs between Lta+/+ and Lta-/- mice. Data are representative of 3 independent experiments with n = 3 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using the Student t test.
Distinct Requirements for the mLN/GALT Between Foxp3+ pTreg Differentiation in the SI and LI
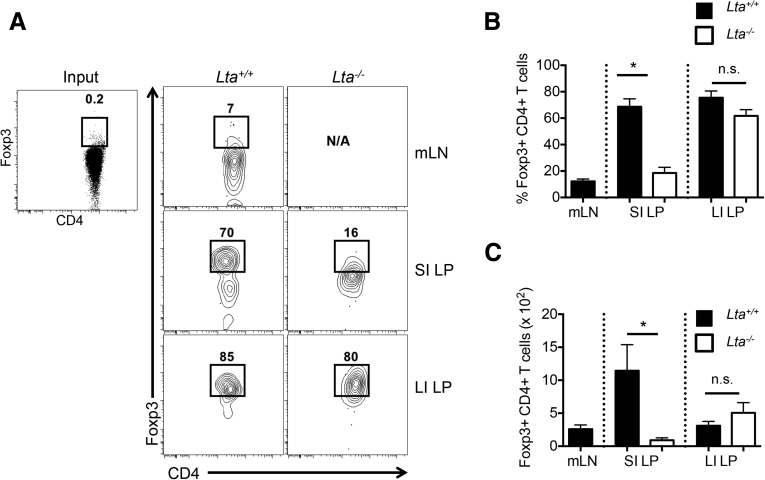
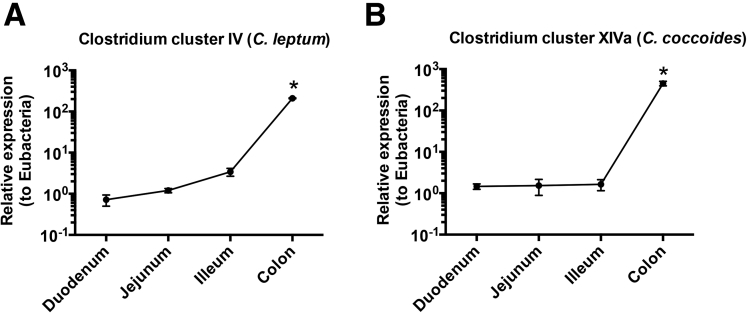
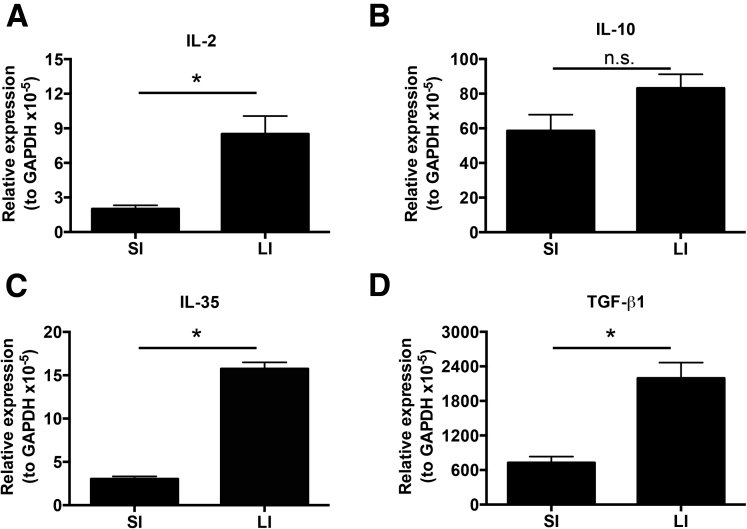
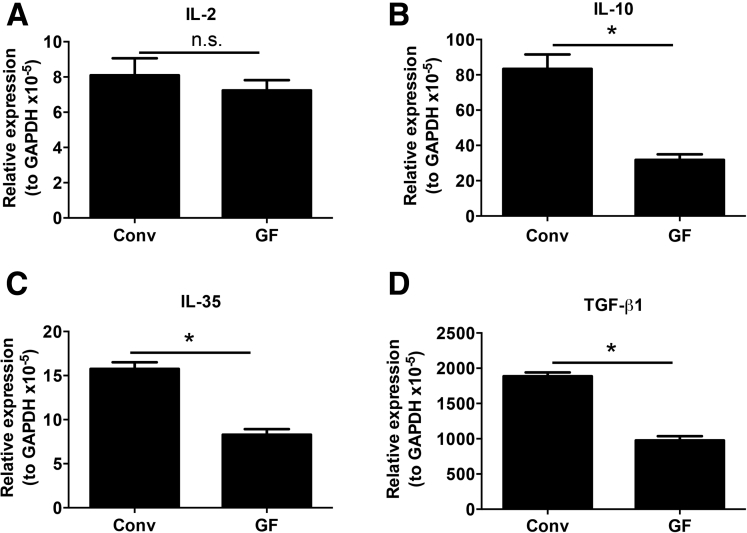
To confirm our finding that Foxp3+ pTreg representation in the SI LP, but not the LI LP, is dependent on the mLN, we used the OT-II transfer OVA feeding model to study antigen-specific Foxp3+ pTreg development.3, 6, 7 Intestinal Foxp3+ Treg differentiation was assessed among donor Thy1.1+CD4+ T cells expressing the transgenic TCR Vβ5+Vα2+ after 5 days of OVA water and 6 days of normal drinking water. Foxp3+ Treg differentiation in Lta+/+ mice was observed to be robust in the SI LP (67.1% ± 5.6%) and LI LP (85.7% ± 4.6%), but relatively poor in the mLN (11.1% ± 2.2%) (Figure 4A and B). Interestingly, Foxp3+ Treg differentiation in Lta-/- mice was impaired in the SI LP (24.6% ± 5.6%) but not in the LI LP (82.0% ± 3.3%) (Figure 4A and B). The absolute cell number corresponded with these frequencies (Figure 4C). Together, these data further support the distinct requirements between the SI and LI for the mLN in Foxp3+ pTreg development. Interestingly, clusters IV and XIVa of the genus Clostridium preferentially reside in the large intestine (Supplementary Figure 4),27 and are known to induce Foxp3+ Tregs.27 We also observed significantly higher messenger RNA (mRNA) expression of the Treg-inducing factors IL2, IL35, and transforming growth factor (TGF)-β mRNA in the LI compared with the SI (Supplementary Figure 5). This augmented expression of immunoregulatory cytokines in the LI was dependent on the microbiota because germ-free mice showed significantly reduced levels of IL10, IL35, and TGF-β mRNA when compared with LI from conventionally housed mice (Supplementary Figure 6). These data are consistent with the reduced abundance of Foxp3+ Tregs in the LI of germ-free mice.27
Figure 4.
Distinct requirements for mLN in Foxp3+ Treg differentiation in the SI and LI. Foxp3+ Treg differentiation of naive CD4+CD25- OT-II Thy.1.1 cells after adoptive transfer into congenic Thy1.2 Lta+/+ and Lta-/- mice and fed OVA water (20 mg/mL) for 5 days followed by 6 days of normal drinking water. Data are pregated on Thy1.1+CD4+ T cells expressing the transgenic TCR Vα2 and Vβ5 (OT-II cells). (A) Representative fluorescence-activated cell sorter plots of Foxp3 induction comparing Lta+/+ and Lta-/- mice. (B) Frequencies and (C) cell numbers of Foxp3+ Tregs induced from Thy1.1+ OT-II cells. Data are representative of 3 independent experiments with n = 3–5 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using the Student t test.
Supplementary Figure 4.
Analyses of Clostridium clusters IV and XIVa in the SI and LI. DNA was isolated from the upper (duodenum), middle (jejunum), and lower (ileum) small intestine, and colon then was analyzed for 16S ribosomal DNA for Clostridium IV and XIVa groups. Quantitation of bacteria is shown relative to Eubacteria. Data are representative of 2 independent experiments with n = 4 mice per group. Error bars represent SEM. *P ≤ .05 using the Student t test.
Supplementary Figure 5.
Treg-inducing cytokines are expressed preferentially in the LI. (A) IL2, (B) IL10, (C) IL35, and TGF-β1 mRNA expression in SI and LI. Data are representative of 2 independent experiments with n = 3 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using the Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Supplementary Figure 6.
Influence of the microbiota on Treg-inducing cytokine expression in the LI. (A) IL2, (B) IL10, (C) IL35, and TGF-β1 mRNA expression in the LI of WT C57BL/6 mice housed under conventional (Conv) or germ-free (GF) conditions. Data are representative of 2 independent experiments with n = 2 mice per group. Error bars represent SEM. *P ≤ .05; n.s., P > .05 using the Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The intestinal LP maintains the largest reservoir of Foxp3+ Tregs, and the mLNs have been show to be involved in peripheral Foxp3+ Treg accumulation in the intestine and the establishment of oral tolerance.3, 4, 18 In this study, we show that the total intestinal Foxp3+ Tregs compartment was not influenced significantly by the absence of mLN and other LT-dependent SLOs. However, the absence of the mLN was associated with a specific reduction in Foxp3+Helios- pTregs in the SI, corresponding with an increase in Foxp3+Helios+ tTregs, whereas their abundance in the LI remained unaffected. This requirement for the mLN in regulating Foxp3+ pTreg development in the SI, but not the LI, indeed was confirmed using the OT-II–OVA feeding system to assess antigen-specific intestinal Foxp3+ Treg differentiation. We must emphasize that our conclusions are based predominantly on data obtained using lta-/- and ltbr-/- mice. Because these mice lack secondary lymphoid organs and GALT, compensatory changes may take place in this context that alter physiological lymphocyte migration and differentiation processes. Thus, future studies using complementary models of SLO deficiency and/or CCR7 deficiency are warranted to more thoroughly define the contribution of lymphoid structures and chemokine receptors to the intestinal Treg compartment. Another important technical caveat to our study is that we used OT-II T-cell receptor transgenic T cells on a RAG-sufficient background, which may have allowed for the expression of non-OVA T-cell receptors. Although we did not observe Foxp3 Treg differentiation in the absence of OVA, we cannot exclude the possibility that non-OVA antigen(s) induced intestinal Foxp3+ Treg differentiation followed by OVA-driven expansion of these cells. Further studies using OT-II T-cell–receptor transgenic T cells on a RAG-deficient background should clarify the potential contribution of non-OVA antigens to intestinal Foxp3+ Treg differentiation.
Our data also provide evidence that the intriguing requirement for the mLN in regulating Foxp3+ pTreg development in the SI, but not the LI, may be attributed at least partially to the microbiota, and preferential pTreg-inducing factors, such as TGF-β, IL35, and IL10, in the LI. However, it remains unclear how the mLN/GALT positively contributes to the Foxp3+ pTreg pool in the SI. It is possible that pTreg differentiation in the SI is inefficient compared with the LI and that the mLN/GALT provide a site for increased CD4+ T-cell expansion and homing to the SI to compensate for inefficient Foxp3+ pTreg differentiation. Overall, these findings highlight important differences in the regulation of intestinal Tregs between the SI and LI, and suggest that enteric antigens may use mLN/GALT to induce Foxp3+ pTreg in the SI, although they may promote Foxp3+ pTregs directly in the LI.28 It should be noted, however, that the use of Helios as a marker for tTregs within the intestine may have limitations. In particular, Helios may not be useful as a marker for a particular line of Tregs under all conditions, particularly those involving T-cell activation.26, 29 Neuropilin-1 also has been purported to distinguish tTregs from pTregs,23, 24 and may complement Helios in certain contexts.21 Without definitive cell surface markers for tTregs and pTregs, additional studies are needed to further define the overall contribution of mLN and GALT to these specific subsets of T cells.
Acknowledgments
The authors thank Ifor R. Williams (Emory University School of Medicine), Charles A. Parkos, and Asma Nusrat (University of Michigan Medical School) for critical discussions, and Aaron Rae (Emory University Department of Pediatrics and Children’s Healthcare of Atlanta Flow Core) for cell sorting.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by grants from the Emory and Children’s Pediatric Center Seed Grant Program (T.L.D) and by 1R00AA01787001 and 1R01DK097256 (T.L.D.) and 1F30DK097904-03 (D.G.) from the National Institutes of Health.
Supplementary Material
References
- 1.Pabst O., Mowat A.M. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilate A.M., Lafaille J.J. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 3.Hadis U., Wahl B., Schulz O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Worbs T., Bode U., Yan S. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coombes J.L., Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun C.M., Hall J.A., Blank R.B. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curotto de Lafaille M.A., Lafaille J.J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Haribhai D., Williams J.B., Jia S. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschenbrenner K., D'Cruz L.M., Vollmann E.H. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 11.Jordan M.S., Boesteanu A., Reed A.J. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 12.Haribhai D., Lin W., Edwards B. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cebula A., Seweryn M., Rempala G.A. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lathrop S.K., Bloom S.M., Rao S.M. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chassaing B., Koren O., Goodrich J.K. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina-Contreras O., Geem D., Laur O. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geem D., Medina-Contreras O., Kim W. Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp. 2012;63:e4040. doi: 10.3791/4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz O., Jaensson E., Persson E.K. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider M.A., Meingassner J.G., Lipp M. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S., Miyara M., Costantino C.M. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 21.Singh K., Hjort M., Thorvaldson L. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naive mice. Sci Rep. 2015;5:7767. doi: 10.1038/srep07767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton A.M., Korty P.E., Tran D.Q. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav M., Louvet C., Davini D. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss J.M., Bilate A.M., Gobert M. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milpied P., Renand A., Bruneau J. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol. 2009;39:1466–1471. doi: 10.1002/eji.200839040. [DOI] [PubMed] [Google Scholar]
- 26.Akimova T., Beier U.H., Wang L. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atarashi K., Tanoue T., Shima T. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obata Y., Furusawa Y., Endo T.A. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat Immunol. 2014;15:571–579. doi: 10.1038/ni.2886. [DOI] [PubMed] [Google Scholar]
- 29.Gottschalk R.A., Corse E., Allison J.P. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]