Summary
RuBPCase activase (RCA), an abundant photosynthetic protein is strongly down-regulated in response to Manduca sexta’s oral secretion (OS) in Nicotiana attenuata. RCA-silenced plants are impaired not only in photosynthetic capacity and growth, but also in jasmonic acid (JA)-isoleucine (Ile) signaling, and herbivore resistance mediated by JA-Ile dependent defense traits. These responses are consistent with a resource-based growth-defense trade-off.
Since JA+Ile-supplementation of OS restored WT levels of JA-Ile, defenses and resistance to M. sexta, but OS supplemented individually with JA- or Ile did not, the JA-Ile deficiency of RCA-silenced plants could not be attributed to lower JA or Ile pools or JAR4/6 conjugating activity. Similar levels of JA-Ile derivatives after OS elicitation indicated unaltered JA-Ile turnover and lower levels of other JA-conjugates ruled out competition from other conjugation reactions. RCA-silenced plants accumulated more methyl jasmonate (MeJA) after OS elicitation, which corresponded with increased jasmonate methyltransferase (JMT) activity.
RCA-silencing phenocopies JMT over-expression, wherein elevated JMT activity redirects OS-elicited JA flux towards inactive MeJA, creating a JA sink which depletes JA-Ile and its associated defense responses.
Hence RCA plays an additional non-photosynthetic role in attenuating JA-mediated defenses and their associated costs potentially allowing plants to anticipate resource-based constraints on growth before they actually occur.
Keywords: Herbivory, jasmonate methyl transferase, jasmonate signaling, Manduca sexta, methyl jasmonate, Nicotiana attenuata, plant defense, RuBPCase activase
Introduction
Plants are attacked by a variety of herbivores and in response, plants activate defenses which can directly or indirectly affect the attacking herbivores (Kessler & Baldwin, 2001; Steppuhn et al., 2004; Zavala et al., 2004). Plants are thought to deploy two alternative strategies against herbivores: (1) resistance and (2) tolerance. These strategies are well studied and are explained by different theories. Among these, the optimal defense theory (OD) (Mckey, 1974; Mckey, 1979; Rhoades, 1979) enjoys the most empirical support. This theory proposes that the distribution of defenses within a plant reflects the fitness value of the tissue for the plant, with higher value tissues being better defended than the less valuable tissues. Moreover, this theory assumes that defenses are costly and a trade-off exists between defense and growth, which in turn explains the prevalence of inducible defenses (Coley et al., 1985; Heil & Baldwin, 2002). Hence during herbivore attack, plants re-adjust their resource investment strategies to reoptimize their allocation of resources to resistance and tolerance mechanisms, growth and reproduction. Under these circumstances, a rapid reallocation of resources to tolerance rather than defense response could maximally reduce the negative fitness consequences of herbivore attack (Schwachtje & Baldwin, 2008). However, very little is known about the molecular mechanisms that plants use to optimize their resource allocation after herbivore attack. For example, while tolerating herbivory, plants allocate newly assimilated carbon to their roots to be used for post-herbivory re-growth, rather than transporting it to the young leaves (Schwachtje et al., 2006).
When Nicotiana attenuata is attacked by its specialist lepidopteran herbivore, Manduca sexta, fatty acid amino acid conjugates (FACs), present in larval oral secretions (OS) activate early defense responses by activating the jasmonic acid (JA) signaling network (Halitschke et al., 2001). JA, a linolenic acid-derived compound, is rapidly and transiently accumulated after herbivory (Creelman et al., 1992; Farmer & Ryan, 1992; Baldwin et al., 1994). JA biosynthesis begins in chloroplasts after lipase activation, which release fatty acids from the membrane lipids. Free linolenic acid is converted to 13S hydroperoxyoctadecatrienoic acid (HPOT) by a specific lipoxygenase which is subsequently converted to 12-oxo-phytodienoic acid (OPDA) by allene oxide synthase (AOS) and allene oxide cyclase (AOC). OPDA is transported to the peroxisome and after reduction and three cycles of β-oxidation by the acyl CoA oxidase 1 enzymes, multifunctional protein, and L-3-ketoacyl CoA-thiolase, is transformed to JA (Schaller & Stintzi, 2009). JA is then exported to the cytosol through the peroxisomal membrane by membrane proteins (Arai et al., 2008), where it is further metabolized. The accumulation of JA is regulated not only by JA biosynthetic genes and their associated transcription factors but also by the availability of fatty acid precursor (Howe & Schilmiller, 2002; Chung et al., 2008; Paschold et al., 2008; Skibbe et al., 2008; Kallenbach et al., 2010). A portion of JA is conjugated to different amino acids of which the isoleucine conjugate (JA-Ile) associates with Coronatine insensitive 1 (COI1) to promote the degradation of Jasmonate ZIM domain (JAZ) repressors by the 26S proteasome (Thines et al., 2007). The degradation of the JAZ repressor releases the MYC 2 transcription factor from repression and activates JA-responsive genes involved in plant defense (Fonseca et al., 2009; Memelink, 2009). JA-Ile is the active molecule triggering downstream defense responses and therefore, the magnitude of JA-Ile is directly correlated with the magnitude of a plant’s defense response. In N. attenuata, silencing the expression of JAR4/6, the enzyme conjugating JA and Ile, attenuates JA-Ile production and resistance against attack from M. sexta larvae (Wang et al., 2007).
A comparative proteomic-transcriptomic study revealed that while defense-related genes are up-regulated after herbivory, photosynthesis-related genes are down-regulated (Giri et al., 2006; Bilgin et al., 2010). Remarkably, herbivore attack causes a greater reduction in a plant’s photosynthetic capacity than would be predicted based on the canopy area removed by the herbivore (Zangerl et al., 2002). RuBPCase activase (RCA), an abundant photosynthetic protein is strongly down-regulated after herbivore attack or simulated herbivory (Giri et al., 2006). RCA modulates the activity of RuBPCase, the major photosynthetic protein involved in carbon fixation, by removing inhibitory sugar phosphates from the active site of enzyme (Portis, 2003). RCA’s role in photosynthesis and growth is well studied and RCA-deficient plants have reduced photosynthetic rates, growth, and accumulate less biomass (He et al., 1997; Ilyin et al., 2005). Reduced growth limits the food available for herbivores; therefore, decreasing growth could be a part of plant’s defense strategy (Hermsmeier et al., 2001; Hahlbrock et al., 2003). In addition, a decrease in carbon (C) supply could alter the expression of genes of enzymes involved in C-utilization and storage (Koch, 1996).
Previously, we observed that in addition to impaired photosynthetic capacity and growth, RCA-silenced N. attenuata plants were impaired in JA-Ile signaling, herbivore resistance and many defense traits that mediate resistance (Mitra & Baldwin, 2008) (Fig. 1). The reduction in photosynthesis and growth associated with RCA-silencing was congruent with RCA’s biochemical function as revealed from work with Arabidopsis, tobacco, and rice (He et al., 1997; Ilyin et al., 2005). However, the decrease in JA-Ile accumulation after RCA-silencing was novel. Prior experimentation had ruled out limitations in the Ile pool at the wound site or activity of the conjugating enzyme as being responsible for the attenuated JA-Ile levels of RCA-silenced plants (Mitra & Baldwin, 2008). As a member of the AAA+ (for ATPases associated with a variety of cellular activities) protein family, RCA may also be involved in other cellular processes (Ogura & Wilkinson, 2001). In different plant systems, the regulation of RCA in response to UV-B light, ozone, drought, and heat stress (Pelloux et al., 2001; Liu et al., 2002; Bota et al., 2004; Demirevska-Kepova et al., 2005) suggests that RCA is involved in diverse stress-related functions. Recently, RCA in Arabidopsis was found to be down-regulated at both transcript and protein levels in a COI1-dependent manner, after elicitation with JA (Shan et al., 2011). The increased susceptibility of RCA-deficient N. attenuata plants to herbivore attack (Mitra & Baldwin, 2008) suggested an additional, defense-related role for RCA other than in RuBPCase activation.
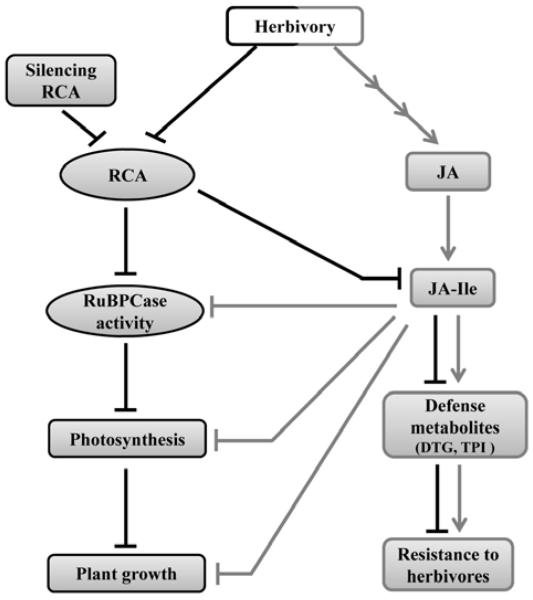
Figure 1. An overview of the consequences of herbivory and RCA-silencing in N. attenuata plants.
In Nicotiana attenuata, attack from Manduca sexta larvae results in a jasmonic acid (JA)-isoleucine (Ile) burst, which increases a suit of JA-induced defense compounds [diterpene glycosides (DTGs) and trypsin protease inhibitors (TPI)], and herbivore resistance and reduces the levels of major photosynthetic proteins RuBPCase and RuBPCase activase, plants’ photosynthetic rate, and growth. RCA-silenced plants are impaired in their RuBPCase activity, photosynthetic capacity, growth, JA-Ile signaling, JA-induced defense compounds DTGs and TPI and consequently herbivore resistance. Black lines depict the consequences of RCA-silencing and gray arrows, the interaction between herbivory-induced JA-mediated defense pathway and photosynthesis and growth. The research presented here reveals how RCA down-regulation also down-regulates JA-signaling and its associated defenses.
Previously, we characterized two independently transformed RCA-silenced lines (line 1 and line 2) with similar degrees of reductions in photosynthetic rate, JA-Ile levels, and resistance against M. sexta larvae (Mitra & Baldwin, 2008). Here we used a single RCA-silenced line (line 2) to elucidate the mechanisms responsible for its attenuated JA-Ile accumulation and herbivore resistance. We examine its JA metabolism and JA-signaling after simulated herbivory with JA or Ile or both (JA+Ile) supplementations. Since adenylation of JA initiates its conjugation to amino acids and adenylation is an energy-demanding process (Staswick et al., 2002), a decrease in photosynthetic capacity may reduce the ATP supply required for JA adenylation. By extending the dark period in wild type (WT) plants, we examined the effect of reduced net carbon gain on JA-adenylation and consequently on JA-Ile accumulation. Lastly, we examine the growth of RCA-silenced plants after simulated herbivory and methyl jasmonate (MeJA) treatment.
Materials and Methods
Plant material and growth conditions
Previously characterized RCA-silenced homozygous line was used in all experiments (Mitra & Baldwin, 2008). The plants of 31st inbred generation of N. attenuata (originally collected from Utah, USA and the same accession used to create the transformed plants) were used as WT plants. Previously, we have shown that the growth and herbivory induced responses of empty vector transformed plants (that can be used as transgenic controls) were similar to those of WT plants (Mitra & Baldwin, 2008; Schwachtje et al., 2008); therefore we used WT plants as controls in all experiments. Seeds were smoke-germinated on Gamborg’s B5-medium (Kruegel et al., 2002). Plants were grown in 1L pots containing a peat-based substrate (Klasmann Tonsubstrat, Geeste-Groß Hesepe, Germany), in the glasshouse of the Max Planck Institute for Chemical Ecology (Jena, Germany) at 24-26°C, 16h light (supplemental lighting by Philips Sun-T Agro 400 and 600 W sodium lights), and 55% humidity. Four- to five-week-old rosette plants were used for all experiments.
Supplementation experiments
Fully expanded (+1) rosette leaves were punctured with a pattern wheel and the wounds were immediately treated with 20 μL of OS that contained either 0.625 μmol JA or 0.625 μmol [13C6] Ile or both 0.625 μmol [13C6] JA and 0.625 μmol Ile (dissolved in 30% (v/v) ethanol/water), while control plants were wounded and treated with similarly diluted OS (diluted in 30% (v/v) ethanol/water). These concentrations of JA and Ile have been shown in previous research to restore the deficiencies of either JA or Ile required to activate JA-Ile signaling at WT levels in either JA- or Ile-deficient plants (Kang et al., 2006; Paschold et al., 2007). The levels of JA and JA-Ile in WT N. attenuata plants reached their maxima after 60 min of W+OS elicitation and returned to basal levels 180 min after W+OS elicitation. Therefore, leaf tissue was harvested 45, 60, and 180min after the treatments.
Analysis of JA and JA-conjugates
About 200mg of harvested leaf tissue from each genotype was extracted and analyzed for JA and JA-Ile levels by an LC/MS/MS system configured with an electro-spray ionization source (1200L Varian, Palo Alto, CA, USA), as described previously (Wang et al., 2007). Negative or positive ionization mode was used depending on the jasmonate structure, as described in Stitz et al. (2011). The 13C6-JA-Ile was used as an internal standard for the relative quantification of hydroxylated (OH)-JA, OH-JA-Ile, carboxylated (COOH)-JA-Ile, JA-Valine (JA-Val) and JA-Glucose (JA-Glc).
Extended night experiment
In tobacco, photosynthetic carbon assimilates, the sugars, play an important role in the regulation of N-metabolism. Under a short-day condition, tobacco plants become C- and N- limited compared to plants grown under long days, which fix more C and accumulate more N (Matt et al., 1998). Since RCA-silenced plants are likely C-limited, we evaluated if the C-limitations could be responsible for shortages in the levels of N-containing Ile and consequently, the impaired JA-Ile accumulations observed in RCA-silenced plants. In addition, the C-limitation may limit the ATP available for JA-adenylation (Statwick, 2002). To test these hypotheses, we grew rosette-stage WT plants, normally grown under 16h: 8h (light: dark) regimes, under three different light: dark periods, namely, 16h: 8h, 12h: 12h, and 8h: 16h for one day and the levels of starch were determined as a measure of net C-gain as described previously (Smith & Zeeman, 2006; Machado et al., 2013). We considered 16h:8h, 12h:12h, and 8h:16h light regimes as providing normal, moderate and severely depleted C regimes, respectively. Fully expanded (+1) rosette leaves were wounded with a pattern wheel and the wounds were treated with M. sexta OS (1:1 diluted with water). Tissues were harvested after 1h of elicitation which corresponded to the end of the light period [at 22.00h (10.00pm), 18.00h (6.00pm), and 14.00h (2.00pm)] or the end of dark period [06.00h (6.00am). Harvested tissues were analyzed for starch, JA, JA-Ile, and MeJA contents.
M. sexta larval performance
Rosette (+1) leaves were wounded and treated with OS or OS containing JA or Ile or JA+Ile. To evaluate the effects of supplementation with JA, Ile, and JA+Ile on M. sexta larval mass, freshly hatched larvae were placed on the treated leaves of 15 replicate plants of each genotype, 24h after elicitation and larval mass was recorded after 12 days of feeding on these elicited plants.
In vivo and in vitro enzyme assays
Fully expanded (+1) rosette leaves were punctured with a pattern wheel and immediately treated with 20 μL OS that contained 0.625 μmol JA (dissolved in 30% (v/v) ethanol/water) or lanolin that contained 150 μg MeJA, while control plants were wounded and treated with similarly diluted OS (diluted in 30% (v/v) ethanol/water) or pure lanolin respectively. Tissues were harvested 60 minutes after elicitation and levels of MeJA or JA were quantified.
For the in vitro enzyme assays, fully expanded (+1) rosette leaves were punctured with a pattern wheel and the puncture wounds were immediately treated with 20 μL OS (1:1 diluted with water). Leaf tissue was harvested 60 minutes after elicitation. Untreated samples served as controls. Total protein was extracted from 200 mg leaf tissue in a buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM KCl, 5 mM EDTA, and 10 mM β-mercaptoethanol. Protein concentration was determined by 2D quant kit (Amersham) with BSA as a standard.
Jasmonate methyl esterase activity (JMT) was determined by measuring the production of MeJA from 1 mM JA and 1 mM S-adenosyl methionine (SAM). The 50μL assay buffer contained 50 mM Tris-HCl (pH 7.5), 100 mM KCl, and 10 mM β-mercaptoethanol. The reaction mixture was incubated at 20°C for 30 min, and MeJA was extracted with 100 μL of ethyl acetate (Seo et al., 2001). Amounts of MeJA produced were determined by LC/MS/MS as previously described (Stitz et al., 2011b). In vitro MeJA esterase (JME) activity was estimated by measuring the amount of JA released from de-esterified MeJA as previously described (Wu et al., 2008).
Plant growth
Growth performance was estimated by repeated measures of stalk lengths during the rosette-to-flowering transition. Fully expanded rosette leaves (+1) of WT and RCA-silenced plants were wounded with a pattern wheel and treated with M. sexta OS (diluted with water 1:1) or lanolin contained 150μg MeJA. Stalk lengths were determined seven days after treatments. Untreated plants served as controls.
Statistical analysis
Data were analyzed with Stat View (Abacus Concepts, Inc., Berkeley, CA, USA) in all the experiments, data were subjected to one way ANOVA and the statistical significance was determined using Fisher’s least significant difference (LSD) post hoc test.
Results
Consequences of herbivory and RCA-silencing in N. attenuata plants
Previously we described the consequences of herbivory and RCA-silencing on N. attenuata plants. Here we summarize the previous results (Fig. 1) to facilitate the understanding of the relation between ‘responses to herbivory’ and ‘RCA-silencing’ in N. attenuata plants. When N. attenuata is attacked by its native herbivore M. sexta, JA and JA-Ile levels accumulate rapidly and transiently. JA-Ile is the main signaling molecule which increases a suit of JA-dependent defense compounds [namely, nicotine, diterpene glycosides (DTGs) and trypsin protease inhibitors (TPIs)] and herbivore resistance. At the same time, the levels of the major photosynthetic proteins RuBPCase and RuBPCase activase, and the plant’s photosynthetic capacity and growth are reduced. RCA-silenced plants, on the other hand have reduced RuBPCase activase activity, photosynthetic rate, growth and JA-Ile signaling, JA-induced defense compounds (DTGs and TPI) and consequently, herbivore resistance (Fig. 1). Therefore, RCA silencing affects not only a plant’s photosynthetic rate and growth but also impairs its defense responses. These results suggest that RCA plays a direct role in optimizing growth and defense in N. attenuata.
Impaired JA-Ile signaling in RCA-silenced plants does not result from lower JA pools, reduced JA-adenylation or -conjugation activity at the wound site
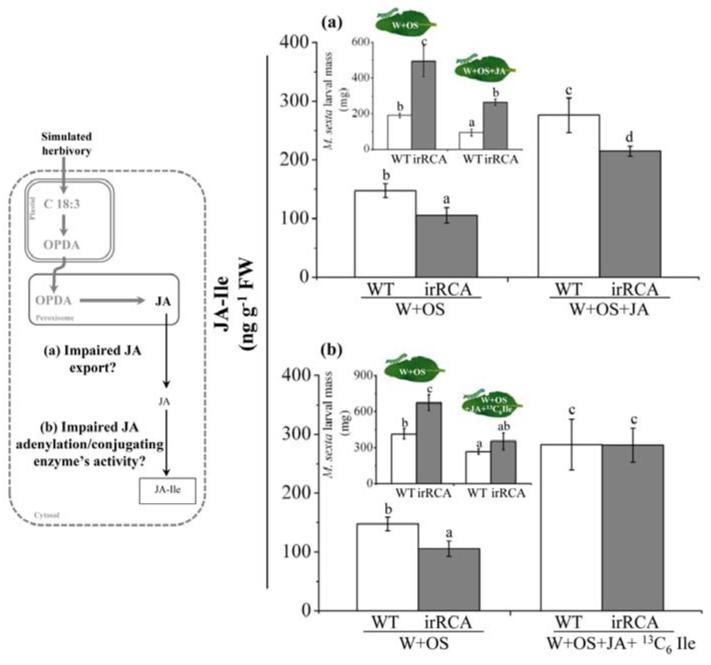
Previously, we reported that the attenuated JA-Ile levels observed in RCA-silenced plants do not result from decreased Ile pools at the wound site or lower transcript levels of JAR4/6 (Mitra & Baldwin, 2008). However, compartmentalization of wound-induced JA might restrict the conjugation of JA with Ile and influence the accumulation of JA-Ile after wound (W) and spit (OS)-elicitation. To evaluate if deficiencies in the availability of JA for conjugation was responsible, we measured JA-Ile accumulation in RCA-silenced plants treated with JA-supplemented OS (W+OS+JA). W+OS+JA treatment increased the basal levels but did not restore JA-Ile to the levels found in OS-elicited WT plants (ANOVA; F3,12 = 17.56; P= 0.005) (Fig. 2a). From these results we infer that lower JA pools at the wound site are not responsible for the attenuated JA-Ile burst in RCA-silenced plants.
Figure 2. Attenuated JA pools or JA-Ile conjugating activity at the wound site do not account for the attenuated JA-Ile burst and herbivore resistance in RCA-silenced plants.
Left panel depicts Jasmonic acid (JA) and JA-isoleucine (Ile) biosynthesis which takes place in three different cellular compartments: OPDA is synthesized in chloroplasts; OPDA is converted to JA in peroxisomes and subsequently conjugated with Ile in the cytosol. The thickness of the arrow and font size indicates relative metabolite accumulations. Right panels show results. OS-elicited RCA-silenced plants accumulated (a) significantly less JA-Ile compared to WT plants with or without JA supplementation to the OS, but when the OS was supplemented with both JA and 13C6 Ile, the elicited JA-Ile levels of RCA-silenced plants were fully restored to WT levels, as was herbivore resistance. (b). Values are means (± SE) of four replicate plants from each genotype and treatment. M. sexta larvae reared on W+OS or W+OS+JA elicited RCA-silenced plants gained significantly more body mass than did larvae on similarly elicited WT plants (Fig a, inset). However, resistance was fully restored when larvae were reared on W +OS+JA+ 13C6 Ile elicited RCA-silenced plants. These larvae attained similar body mass as those on WT plants (Fig b, inset). Values are means of 15 (± SE) replicate larvae per genotype and treatment. Different letters indicate significant differences at P ≤ 0.05 by one-way ANOVA. irRCA= inverted repeat RCA; W+OS = wound + M. sexta oral secretion.
Adenylation of JA initiates its conjugation to amino acids (Staswick et al., 2002) and adenylation is known to be an energy-demanding process; therefore, to test whether decreases in JA-Ile levels in RCA-silenced plants are due to impaired JA adenylation, we measured JA-Ile accumulation in WT and RCA-silenced plants treated with W+OS+JA+13C6 Ile. If RCA-silenced plants had attenuated conjugating enzyme activity or JA adenylation, they would not have been able to make JA-Ile at WT levels even after JA and 13C6Ile supplementation. However, this treatment restored JA-Ile levels of RCA-silenced plants to those of WT plants (ANOVA; F3, 12 = 6.98; P= 0.19) (Fig. 2b), demonstrating that neither the activity of the conjugating enzyme nor the efficiency of JA-adenylation is altered after RCA-silencing.
In addition, to examine the influence of net C-gain on JA and JA-Ile accumulation, WT N. attenuata plants were grown under three different light regimes for one day and their starch, JA and JA-Ile levels were measured. The levels of starch accumulated at the end of the light period reflect the net C-gain. We found that the extended night depleted the net C-gain by 24% and 41% in 12h:12h and 8h:16h (light: dark) regimes, respectively compared to the normal i.e. 16h:8h (light: dark) regime (ANOVA; F2,6 = 4.5; P12h: 12h = 0.09; P8h: 16h = 0.02; Fig. S1a). JA accumulation was significantly decreased (ANOVA; F5, 12 = 6.7; P = 0.003) in all samples collected at the end of the dark period compared to the samples collected at the end of light period; however, JA-Ile accumulation was only lower in samples collected at the end of 16h dark period (ANOVA; F5, 12 = 3.18; P = 0.02; Fig. S1b). JA and JA-Ile levels in the samples collected at the end of 12h and 8h dark period were similar (ANOVA; F5,12= 6.7; PJA> 0.05; ANOVA; F5,12 = 3.18; PJA-Ile > 0.05), but significantly lower for JA-Ile levels in the samples collected at the end of 16h dark period, compared to the samples collected at the end of 8h dark period (ANOVA; F5,12 = 6.7; P = 0.008; ANOVA; F5,12 = 3.18; PJA-Ile = 0.005; Fig. S1b). From these results we infer that a severe decrease in net C-gain reduces JA-Ile bursts but not JA bursts after OS elicitation.
Resistance to M. sexta attack in RCA-silenced plants is restored by treatment with both JA and Ile, but not JA or Ile alone
To examine the impact of JA, Ile or JA+Ile supplementation on the performance of herbivore, we compared the growth of M. sexta larvae fed on WT and RCA-silenced plants treated with W+OS or W+OS+JA or W+OS+13C6 Ile or W+OS+JA+13C6 Ile. We found that the larvae fed on W+OS (ANOVA; F1, 28= 11.97; P= 0.002), W+OS+JA (ANOVA; F3, 56 = 12.92; P= 0.01) (Fig. 2a inset), and W+OS+13C6 Ile (ANOVA; F3, 56= 13.15; P < 0.0001; Fig. S2) treated RCA-silenced plants gained significantly more mass than did larvae on comparably treated WT plants. Larvae fed on W+OS+JA+13C6 Ile treated plants did not differ in mass gain between WT and RCA-silenced plants (ANOVA; F3, 56 = 9.8; P= 0.24) (Fig. 2b inset). These results demonstrate that JA-Ile accumulation determines larval performance, from which we infer that the attenuated JA-Ile levels of RCA-silenced plants are responsible for the impaired herbivore resistance of these plants.
JA-Ile turnover is unaltered after RCA-silencing
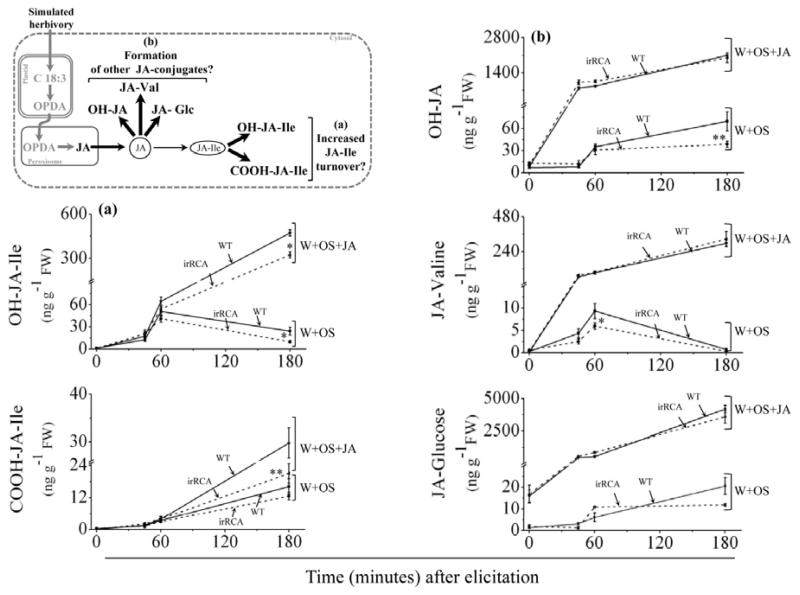
To evaluate if increased conversion of JA-Ile to its inactive derivatives namely, hydroxy- (OH) and carboxy- (COOH) JA-Ile could explain the lower levels of JA-Ile in RCA-silenced plants, we measured the levels of OH-JA-Ile and COOH-JA-Ile after W+OS elicitation in WT and RCA-silenced plants. Additionally, WT and RCA-silenced plants were treated with W+OS+JA to increase the levels of JA-Ile derivatives and their accumulations were measured over a 3h period. W+OS and W+OS+JA treated WT and RCA-silenced plants showed similar levels of OH-JA-Ile after 45 and 60 minutes of induction (ANOVA(W+OS); F7,24 = 21.29; P > 0.05; ANOVA(W+OS+JA) F7,24 = 272.64; P> 0.05; Fig. 3a). However, after 180min, RCA-silenced plants showed a significant decrease in OH-JA-Ile levels compared to WT plant (Fig. 3a) in both W+OS (ANOVA (W+OS); F7,24 = 21.29; P= 0.01) and W+OS+JA (ANOVA (W+OS+JA); F7,24 = 272.64; P = 0.02) treated plants. The level of COOH-JA-Ile in W+OS treated RCA-silenced plants were similar to those of WT plants throughout the time course (ANOVA (W+OS); F7,24 = 25.59; P> 0.05; Fig. 3a). However, W+OS+JA treated RCA-silenced plants showed a significant decrease in COOH-JA-Ile levels only 180min after elicitation (ANOVA (W+OS+JA); F7,24 = 39.01; P= 0.002). From these results, we infer that the JA-Ile turnover remains unaltered after RCA-silencing.
Figure 3. Attenuated JA-Ile bursts in RCA-silenced plants is not due to either increased JA-Ile turnover or competition from other known conjugation reactions for JA.
The accumulation of (a) hydroxy-(OH) JA-Ile and carboxy- (COOH) JA-Ile in RCA-silenced plants was significantly decreased after W+OS elicitation. After W+OS+JA elicitation the level of OH-JA-Ile in RCA-silenced plants was similar to that of WT plants but the level of COOH-JA-Ile was significantly lower compared to those of WT plants. The accumulation of (b) OH-JA, JA-Valine (JA-Val), and JA-glucose (JA-Glc) was significantly lower in RCA-silenced plants than in WT plants after W+OS elicitation; however, after W+OS+JA elicitation, the levels of OH-JA, JA-Val, and JA-Glc accumulation were similar to that of WT plant. Values are means (± SE) of 4 replicate plants from each genotype and treatment. Asterisks indicate significant differences at P ≤ 0.05 (*) and P ≤ 0.005 (**) by one-way ANOVA. W+OS = wound + M. sexta oral secretion.
JA flux is not redirected from Ile to other known JA-derivatives in RCA-silenced plants
JA metabolism is controlled by multiple competing enzymes and the lower JA-Ile levels could result from increased flux to other conjugates. To evaluate this possibility we measured the accumulation of all other major JA-conjugates known to occur in N. attenuata, namely OH-JA, JA-Val, JA-Glc after W+OS and W+OS+JA elicitations. The levels of OH-JA (ANOVA; F7, 24 = 14.13; POH-JA= 0.0009) and JA-Val (ANOVA; F7, 24 = 55.5; PJA-Val= 0.05) were significantly lower in RCA-silenced plants (compared to WT plants), after 180 and 60 min of W+OS elicitation, respectively (Fig. 3b). The levels of JA-Glc were similar in W+OS treated WT and RCA-silenced plants (ANOVA; F7, 24 = 7.58; PJA-Glc= 0.055). JA supplementation substantially increased levels of OH-JA (23 fold), JA-Val (25 fold), and JA-Glc (125 fold), compared to the OS treatment. However, the accumulation of OH-JA (ANOVA; F7, 24 = 79.35; POH-JA >0.05), JA-Val (ANOVA; F7, 24 = 36.85; PJA-Val >0.05), and JA-Glc (ANOVA; F7, 24 = 67.12; PJA-Glc >0.05) were similar in WT and RCA-silenced plants (Fig. 3b).
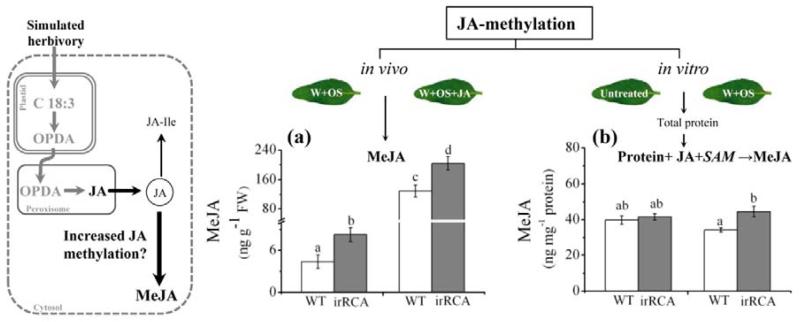
RCA-silenced plants have elevated MeJA levels and JA-methylation activity
A proportion of herbivory-elicited JA is can be esterified to its volatile form MeJA, a reaction mediated jasmonate methyl transferase (JMT) (Seo et al., 2001). However, in WT N. attenuata the amounts of MeJA produced are very low (von Dahl & Baldwin, 2004). To determine whether JA methylation activity contributes to the decreased JA-Ile level in RCA-silenced plants, we measured JA methylation (in vivo and in vitro) ability of both RCA-silenced and WT plants. In vivo methylation ability was determined by measuring the formation of MeJA in RCA-silenced plants after treating with W+OS or W+OS+JA. We found that RCA-silenced plants synthesized 40% more MeJA than did WT plants (ANOVA; F3, 12 = 37.84; PW+OS = 0.03; PW+OS+JA = 0.007) (Fig. 4a). In vitro JA methylation activity was determined using the total protein extracts of untreated and W+OS treated leaves. We found that methylation activity was similar in untreated WT and RCA-silenced plants but after W+OS elicitation the methylation activity increased by 36% in RCA-silenced plants compared to WT plants (ANOVA; F3,15 = 1.65; PUntreated = 0.59; PW+OS = 0.049) (Fig. 4b).
Figure 4. RCA-silenced plants accumulate more methyl jasmonate (MeJA) than do WT plants which correlates with increased methylation activity of free JA.
The accumulation of (a) MeJA (in vivo) increased after W+OS and W+OS+JA elicitation and basal levels were higher in RCA-silenced plants. To measure the JA-methylation activity in vitro, total protein was extracted from W+OS treated rosette leaves (+1) of RCA-silenced and WT plants. Untreated plants served as controls. Methyltransferase activity was determined by measuring the production of MeJA from JA and S-adenosyl- methionine (SAM) (b) Protein extracts of RCA-silenced plants produced significantly more MeJA when supplemented with JA and SAM. Values are means (± SE) of 4-5 replicate plants from each genotype and treatment. Different letters indicate the significant difference at P ≤ 0.05 by one-way ANOVA. W+OS = wound + M. sexta oral secretion.
It is known that JA and MeJA are inter-convertible and MeJA is hydrolyzed to JA by Jasmonate methylesterase (JME) (Stuhlfelder et al., 2002; Stuhlfelder et al., 2004). Therefore, a decrease in MeJA demethylation activity might also have contributed to the increased MeJA levels in RCA-silenced plants. In vivo demethylation ability was determined by measuring the formation of JA in RCA-silenced plants after treating with lanolin containing MeJA or pure lanolin. JA was not detected in lanolin treated control samples and no significant difference was found in the levels of the cleavage product of MeJA in WT and RCA-silenced plants (ANOVA; F1, 6 = 0.52; P = 0.49) (Fig S3a). Similarly in an in vitro assay, protein extracts of WT and RCA-silenced leaves hydrolyzed similar amounts MeJA to form JA (ANOVA; F3,16 = 2.98; PUntreated = 0.07; PW+OS = 0.1) (Fig S3b). From these results we infer that the increase in MeJA levels in RCA-silenced plants could be attributed to an increase in JA methylation activity and not to a decrease in demethylation activity.
RCA-silenced plants phenocopy the JA metabolism and signaling behavior of JMT-over expressing plants
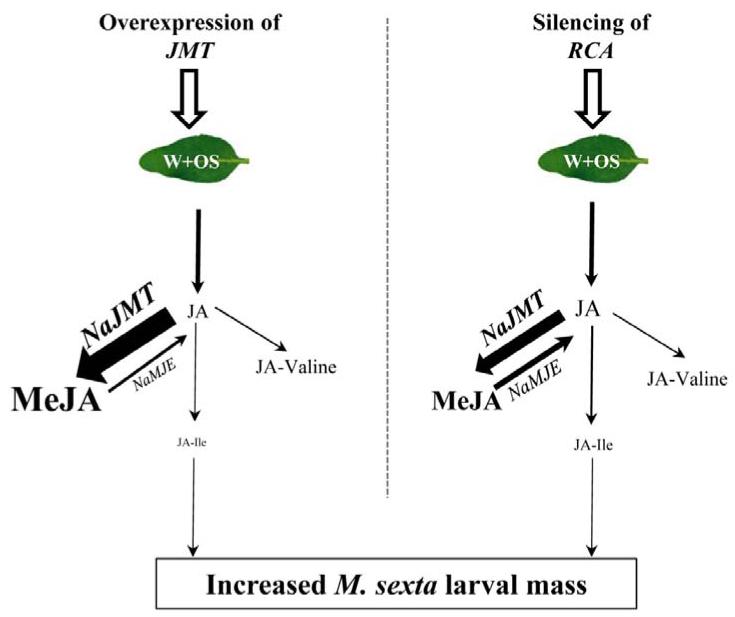
Ectopic expression of Arabidopsis thaliana (At) - JMT in N. attenuata reduced the accumulation of JA-Ile by 95% and that of the other AA-JA-derivatives by 30% (Table 1). The resulting increase in the JA methylation activity (93%) redirected the flux of JA to MeJA and compromised the plants’ defenses (Stitz et al., 2011a; Stitz et al., 2011b). As such, ectopic overexpression of JMT creates a JA sink, diverting JA to inactive MeJA without influencing the JA pathway prior to the formation of JA, and hence phenocopies RCA-silenced plants (Fig. 5): JA-Ile and other AA-JA-derivatives decreased by 28% and 36%, respectively and the JA methylation activity and MeJA accumulation increased by 40% and 36% respectively. Interestingly, the decrease in JA-Ile corresponded with a stoichiometric increase in MeJA levels (Fig S4).
Table 1. JA metabolism and signaling in RCA-silenced and JMT-overexpressed plants are similarly regulated after simulated herbivory.
Table shows the percent increase or decrease in accumulation of JA, JA-Ile, and MeJA, activity of methyltransferase and methylesterase, and mass gain of M. sexta larvae in JMT-overexpressed and RCA-silenced plants as compared to WT plants. Up-arrows (↑) and down arrows (↓) signify increases and decreases, respectively. The values of JMT-overexpressed plants are from Stitz et al. (2011) and in this study, M. sexta larvae were fed untreated JMT-overexpressed plants and hence their larval mass gain cannot directly be compared to the larval mass gain of larvae fed on OS-elicited RCA-silenced plants (††).
| Traits | Regulation after simulated herbivory ( with respect to WT plants) | |
|---|---|---|
| Overexpressed JMT | Silenced RCA | |
| JA | 27% ↓ | No change |
| JA-Ile | 95% ↓ | 28% ↓ |
| JA-Valine | 31% ↓ | 36% ↓ |
| MeJA | 96% ↑ | 40% ↑ |
| JME activity | 30% ↓ | No change |
| JMT activity | 93% ↑ | 36% ↑ |
| M. sexta larval mass | ††61% ↑ | 61% ↑ |
Figure 5. RCA- silencing phenocopies jasmonate methyltransferase (JMT) over-expression in N. attenuata plants.
A model of JA metabolism and signaling in OS-induced RCA-silenced plants, in which the elevated methyltransferase activity redirects OS-elicited JA flux to the inactive MeJA than to active signaling molecule, JA-Ile, or to other less active JA-derivatives thereby compromising elicited defense responses. Font size and arrow thickness are proportional to the intensity of metabolite flux, enzyme activity, and M. sexta larval mass. W+OS = wound + M. sexta oral secretion.
RCA-silencing attenuates the OS and MeJA-elicited growth reductions
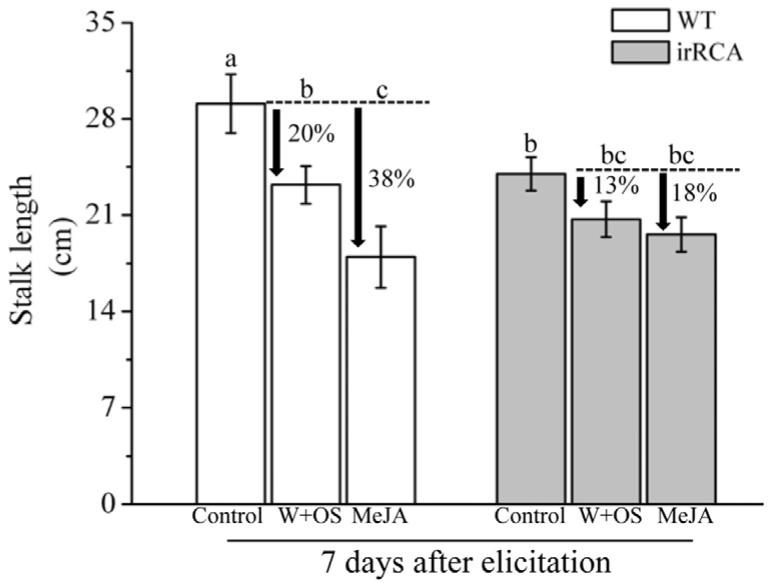
OS-elicitation and MeJA treatment of WT plants are both known to decrease N. attenuata growth, particularly under competitive growth in both the field and glasshouse (Baldwin, 1998; Halitschke et al., 2001; Zavala et al., 2004). To evaluate the growth performance of RCA-silenced plants after OS and MeJA treatments, we recorded the stalk length of W+OS and MeJA treated RCA-silenced plants and compared those with the similarly treated WT plants. Plants were grown in individual pots to provide a conservative measure of growth effects. After treating with OS and MeJA, the growth of WT plants was reduced by 20% (ANOVA; F5,24= 5.7, PW+OS= 0.01) and 38% (ANOVA; F5,24= 5.7 =, PMeJA< 0.0001), respectively, whereas the growth of RCA-silenced plants was only reduced by 13% (ANOVA; F5,24= 5.7, PW+OS= 0.17) and 18% (ANOVA; F5,24= 5.7, PMeJA= 0.07), respectively (Fig.6); these reductions were significantly different from each other in WT, whereas in RCA, they were not. From these results, we infer that the attenuated induced defenses of RCA-silenced plants may contribute to sustained growth of these plants after elicitation by herbivores.
Figure 6. Growth of RCA-silenced plants is less affected by simulated herbivory and MeJA treatment compared to WT plants.
Fully expanded (+1) leaves of WT and RCA-silenced plants were wounded (W) with a pattern wheel and treated with M. sexta oral secretion (OS) or MeJA and stalk length was recorded seven days after elicitation. Untreated plants served as controls. W+OS or MeJA treatments significantly delayed stalk elongation in WT plants, but the effects in RCA-silenced plants were not significant from the respective controls. Values are means (± SE) of five replicate plants from each genotype and treatment. Different letters indicate the significant difference at P ≤ 0.05 by one way ANOVA. WT = white bars; irRCA = grey bars
Discussion
Since the herbivory-elicited accumulation of JA in RCA-silenced plants was indistinguishable from that of WT plants, the attenuated JA-Ile signaling of RCA-silenced plants could result from: (1) reduced JA pool at the wound site, (2) impaired JA adenylation, (3) impaired JA-Ile conjugating enzyme activity, or (4) altered JA metabolism. The results from the JA and Ile supplementation experiments ruled out the first hypothesis and demonstrated that reduced JA pool at the wound site could not account for the impaired JA-Ile accumulation. Moreover, the results from supplementation experiments with JA + 13C6 Ile and the extended night experiments allowed us to rule out deficiencies in JA-adenylation or conjugating enzyme activity. Attack-elicited JA and JA-Ile is metabolized to its OH or COOH forms or JA is conjugated with other molecules (Miersch et al., 2008; Wang et al., 2008; Koo et al., 2011). Conversion of JA or JA-Ile to its hydroxy- or carboxy- derivatives deactivates JA signaling (Miersch et al., 2008; Koo et al., 2011) and the conjugation of JA with molecules other than Ile also disables defense signaling (Wang et al., 2008). Therefore, RCA-silenced plants could have an increased JA-Ile turnover or JA could be conjugated to amino acids other than Ile. However, the level of JA-Ile- and the levels of the major JA-derivatives in RCA-silenced plants suggested that JA-Ile accumulation is neither influenced by increased JA-Ile turnover nor outcompeted by conjugation reactions involving other amino acids or glucose.
JA can also be methylated to MeJA, a semi-volatile organic compound involved in plant defense and many other developmental pathways (Creelman & Mullet, 1997; Wasternack & Hause, 2002; Wasternack & Hause, 2013). In Arabidopsis, the methylation of JA is catalyzed by the enzyme jasmonic acid-O-methyl transferase (JMT), an enzyme whose corresponding transcripts are up-regulated in response to wounding or JA application (Seo et al., 2001). Ectopic expression of A. thaliana JMT (AtJMT) in N. attenuata creates a metabolic sink in the JA pathway which redirects the flux of JA towards MeJA and strongly reduces the accumulation of herbivory-induced JA and JA-Ile and the JA-associated defense responses. RCA-silenced plants, like the JMT-over-expressing N. attenuata plants (ovAtJMT), have high MeJA accumulations and JMT activity both in vivo and in vitro. ovAtJMT plants have JMT activity that is elevated by 93%, which corresponds to a 96% increase in MeJA levels and 27% and 95% decreases in JA and JA-Ile levels, respectively. Similarly, RCA-silenced plants have 36% increases in JMT activity, corresponding to a 40% increase in MeJA level and a 28% decrease in JA-Ile levels, all without any changes in JA levels. RCA-silenced plants therefore phenocopy ovAtJMT plants in all aspects, with the exception of their unaltered JA levels. In this regard, RCA-silenced plants are more similar to Arabidopsis JMT over-expressing plants in which MeJA level is increased without altering the normal JA burst (Seo et al., 2001). The difference may simply reflect differences in the strength of the JA sink. In both RCA-silenced N. attenuata plants and JMT overexpressing Arabidopsis the JA-sink strengths are substantially less (~50%) than that of the JMT overexpressing N. attenuata plants. The involvement of the abundant and important photosynthetic protein, RCA, in regulating JA signaling is novel (Fig S4) and will require substantially more research to understand its underlying mechanisms. Being a molecular chaperon, RCA may directly interact with the JMT-protein and negatively regulate its function, something which would be possible to test once the putative NaJMT has been identified and characterized in N. attenuata.
At a functional level, it’s not clear why RCA would have evolved to play an additional role in negatively regulating JA signaling. Phytohormones and the signaling cascades they activate are known to be tightly regulated by catalytic reactions that control the pools of active hormone signals (Qin et al., 2005; Varbanova et al., 2007; Tieman et al., 2010). For example, JA is inactivated when converted to 12-OH-JA, as clearly seen in the termination of the expression of a subset of genes involved in JA-signaling. Similarly, the catabolism of JA-Ile into OH-JA-Ile or COOH-JA-Ile down-regulates JA-signaling (Miersch et al., 2008) and recently, the hydrolysis of JA-Ile by jasmonyl-L-isoleucine hydrolase 1 (JIH1) has been shown to attenuate the JA-Ile burst and allow N. attenuata plants to tailor their defense responses (Woldemariam et al., 2012). RCA should now be added to the many layers by which plants can tailor their JA-Ile signaling.
Jasmonate induced defenses impose significant costs on a plant’s growth (Redman et al., 2001); therefore, a trade-off occurs between growth and defense (Coley et al., 1985; Heil & Baldwin, 2002). Hormonal cross-talk has been proposed as a mechanism for the resource based trade-offs between growth and defense (Yang et al., 2012; Machado et al., 2013) and recently the JA-dependent reduction in photosynthetic rate have been proposed to be responsible for a plant’s decreased growth (Nabity et al., 2013). Thus, on one hand JA-signaling reduces photosynthetic capacity and on the other hand it depletes C-resources by incorporating them in defense metabolites. Moreover, the results of our extended night experiments demonstrate that JA-Ile production is also resource-dependent. In addition, regulatory elements other than JA may influence a plant’s root storage regime and re-growth capacity (Machado et al., 2013). The C-limited RCA-silenced plants were impaired in their JA-Ile bursts and JA-Ile induced defense metabolites, suggesting that RCA could be one of the factors suggested by Machado et al., (2013). When herbivore attack elicits a JA burst, N. attenuata plants could anticipate the upcoming resource constraint resulting from reduced C-assimilation through the signaling mediated by the down-regulation of RCA. Therefore, the C-limited RCA-silenced plants reorganize their resource investment strategy and redirect the attack-elicited JA flux from JA-Ile towards MeJA, thereby attenuating the expensive JA-Ile-mediated defense responses.
Resistance is thought to be costly in terms of a plant’s growth and fitness; therefore a trade-off is assumed between growth and defense (Coley et al., 1985; Heil & Baldwin, 2002). Many plants employ both resistance and tolerance strategies to cope with their herbivore communities (Leimu & Koricheva, 2006; Carmona & Fornoni, 2013). In response to herbivory, many plant species allocate C to the roots (Dyer et al., 1991; Holland et al., 1996; Schwachtje et al., 2006). However, the reallocated C -is not used for the root growth, instead it is used for post-herbivory regrowth of shoot (Schwachtje et al., 2006). This is an example of anticipatory response which occurs before the resource supply limits the activation of defense to optimize the capacity for sustained growth (Smith & Stitt, 2007). We observed that RCA-silenced plants tolerated simulated herbivory and MeJA treatments better than similarly treated WT plants. Thus, by making more MeJA, RCA-silenced plants may avoid the production of JA-Ile and their associated expensive defense responses, conserving C-resources for post-herbivory growth. This suggests that normally, RCA is down-regulated after herbivory to redirect the JA flux more towards MeJA and away from JA-Ile to facilitate the transition from the growth to defense and subsequently, from the resistance to tolerance. We therefore propose that RCA plays a direct role in attenuating JA-induced defense responses which in turn allows N. attenuata plants to anticipate the forthcoming resource constraints.
Supplementary Material
Acknowledgements
We thank Dr. K. Gase and T. Hahn for vector construction and DNA sequencing; S. Kutschbach and A. Wissgott for generating the transformed plants; the MPI greenhouse team for growing the plants; Dr. M. Kallenbach, Dr. M. Reichelt and M. Stitz for help with the LC-MS analysis, Drs. M. Woldemariam and S. Pandit for useful discussions and suggestions.
This work was supported by the Max Planck Society, HFSP (RGP0002/2012) and ERC Advanced grants, ClockworkGreen (No. 293926) to I.T.B
References
- Arai Y, Hayashi M, Nishimura M. Proteomic identification and characterization of a novel peroxisomal adenine nucleotide transporter supplying ATP for fatty acid beta-oxidation in Soybean and Arabidopsis. Plant Cell. 2008;20(12):3227–3240. doi: 10.1105/tpc.108.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(14):8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. Journal of Chemical Ecology. 1994;20(8):2139–2157. doi: 10.1007/BF02066250. [DOI] [PubMed] [Google Scholar]
- Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR, DeLucia EH. Biotic stress globally downregulates photosynthesis genes. Plant Cell and Environment. 2010;33(10):1597–1613. doi: 10.1111/j.1365-3040.2010.02167.x. [DOI] [PubMed] [Google Scholar]
- Bota J, Medrano H, Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist. 2004;162(3):671–681. doi: 10.1111/j.1469-8137.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- Carmona D, Fornoni J. Herbivores can select for mixed defensive strategies in plants. New Phytologist. 2013;197(2):576–585. doi: 10.1111/nph.12023. [DOI] [PubMed] [Google Scholar]
- Chung HS, Koo AJK, Gao XL, Jayanty S, Thines B, Jones AD, Howe GA. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiology. 2008;146(3):952–964. doi: 10.1104/pp.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. Resource availability and plant antiherbivore defense. Science. 1985;230(4728):895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Oligosaccharins, brassinolides, and jasmonates: Nontraditional regulators of plant growth, development, and gene expression. Plant Cell. 1997;9(7):1211–1223. doi: 10.1105/tpc.9.7.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE. Jasmonic acid methyl jasmonate accumulate in wounded Soybean hypocotyls and modulate wound gene-expression. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirevska-Kepova K, Holzer R, Simova-Stoilova L, Feller U. Heat stress effects on ribulose-1,5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biologia Plantarum. 2005;49(4):521–525. [Google Scholar]
- Dyer MI, Acra MA, Wang GM, Coleman DC, Freckman DW, Mcnaughton SJ, Strain BR. Source-Sink Carbon Relations in 2 Panicum-Coloratum Ecotypes in Response to Herbivory. Ecology. 1991;72(4):1472–1483. [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase-inhibitors. Plant Cell. 1992;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nature Chemical Biology. 2009;5(5):344–350. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]
- Giri AP, Wunsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiology. 2006;142(4):1621–1641. doi: 10.1104/pp.106.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K, Bednarek P, Ciolkowski I, Hamberger B, Heise A, Liedgens H, Logemann E, Nurnberger T, Schmelzer E, Somssich IE, Tan JW. Non-self recognition, transcriptional reprogramming, and secondary metabolite accumulation during plant/pathogen interactions. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14569–14576. doi: 10.1073/pnas.0831246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiology. 2001;125(2):711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZL, von Caemmerer S, Hudson GS, Price GD, Badger MR, Andrews TJ. Ribulose-1,5-bisphosphate carboxylase/oxygenase activase deficiency delays senescence of ribulose-1,5-bisphosphate carboxylase/oxygenase but progressively impairs its catalysis during tobacco leaf development. Plant Physiology. 1997;115(4):1569–1580. doi: 10.1104/pp.115.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Baldwin IT. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends in Plant Science. 2002;7(2):61–67. doi: 10.1016/s1360-1385(01)02186-0. [DOI] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants - to grow or defend. Quarterly Review of Biology. 1992;67(4):478–478. [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiology. 2001;125(2):683–700. doi: 10.1104/pp.125.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JN, Cheng WX, Crossley DA. Herbivore-induced changes in plant carbon allocation: Assessment of below-ground C fluxes using carbon-14. Oecologia. 1996;107(1):87–94. doi: 10.1007/BF00582238. [DOI] [PubMed] [Google Scholar]
- Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Current Opinion in Plant Biology. 2002;5(3):230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- Ilyin SE, Horowitz D, Belkowski SM, Xin H, Eckardt AJ, Darrow AL, Chen C, Maley D, D’Andrea M, Plata-Salaman CR, Derian CK. Integrated expressional analysis: Application to the drug discovery process. Methods. 2005;37(3):280–288. doi: 10.1016/j.ymeth.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Kallenbach M, Alagna F, Baldwin IT, Bonaventure G. Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway. Plant Physiology. 2010;152(1):96–106. doi: 10.1104/pp.109.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Wang L, Giri A, Baldwin IT. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell. 2006;18(11):3303–3320. doi: 10.1105/tpc.106.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291(5511):2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koo AJK, Cooke TF, Howe GA. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(22):9298–9303. doi: 10.1073/pnas.1103542108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12(4):177–183. [Google Scholar]
- Leimu R, Koricheva J. A meta-analysis of tradeoffs between plant tolerance and resistance to herbivores: combining the evidence from ecological and agricultural studies. Oikos. 2006;112(1):1–9. [Google Scholar]
- Liu L, White MJ, MacRae TH. Identification of ultraviolet-B responsive genes in the pea, Pisum sativum L. Plant Cell Reports. 2002;20(11):1067–1074. [Google Scholar]
- Machado R, Ferrieri A, Robert C, Glauser G, Kallenbach M, Erb M. Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytologist. 2013;200:1234–1246. doi: 10.1111/nph.12438. [DOI] [PubMed] [Google Scholar]
- Matt P, Schurr U, Klein D, Krapp A, Stitt M. Growth of tobacco in short-day conditions leads to high starch, low sugars, altered diurnal changes in the Nia transcript and low nitrate reductase activity, and inhibition of amino acid synthesis. Planta. 1998;207(1):27–41. doi: 10.1007/s004250050452. [DOI] [PubMed] [Google Scholar]
- Mckey D. Adaptive patterns in alkaloid physiology. American Naturalist. 1974;108:305–320. [Google Scholar]
- Mckey D. The distribution of secondary compounds within plants. Academic Press; New York, USA: 1979. [Google Scholar]
- Memelink J. Regulation of gene expression by jasmonate hormones. Phytochemistry. 2009;70(13-14):1560–1570. doi: 10.1016/j.phytochem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytologist. 2008;177(1):114–127. doi: 10.1111/j.1469-8137.2007.02252.x. [DOI] [PubMed] [Google Scholar]
- Mitra S, Baldwin IT. Independently silencing two photosynthetic proteins in Nicotiana attenuata has different effects on herbivore resistance. Plant Physiology. 2008;148(2):1128–1138. doi: 10.1104/pp.108.124354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. Herbivore induction of jasmonic acid and chemical defences reduce photosynthesis in Nicotiana attenuata. Journal of Experimental Botany. 2013;64(2):685–694. doi: 10.1093/jxb/ers364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ. AAA(+) superfamily ATPases: common structure-diverse function. Genes to Cells. 2001;6(7):575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Paschold A, Bonaventure G, Kant MR, Baldwin IT. Jasmonate perception regulates jasmonate biosynthesis and JA-Ile metabolism: The case of COI1 in Nicotiana attenuata. Plant and Cell Physiology. 2008;49(8):1165–1175. doi: 10.1093/pcp/pcn091. [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant Journal. 2007;51(1):79–91. doi: 10.1111/j.1365-313X.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Jolivet Y, Fontaine V, Banvoy J, Dizengremel P. Changes in Rubisco and Rubisco activase gene expression and polypeptide content in Pinus halepensis M. subjected to ozone and drought. Plant Cell and Environment. 2001;24(1):123–131. [Google Scholar]
- Portis AR. Rubisco activase - Rubisco’s catalytic chaperone. Photosynthesis Research. 2003;75(1):11–27. doi: 10.1023/A:1022458108678. [DOI] [PubMed] [Google Scholar]
- Qin GJ, Gu HY, Zhao YD, Ma ZQ, Shi GL, Yang Y, Pichersky E, Chen HD, Liu MH, Chen ZL, Qu LJ. An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell. 2005;17(10):2693–2704. doi: 10.1105/tpc.105.034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman AM, Cipollini DF, Schultz JC. Fitness costs of jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia. 2001;126(3):380–385. doi: 10.1007/s004420000522. [DOI] [PubMed] [Google Scholar]
- Rhoades DF. Evolution of plant chemical defense against herbivores. Academic Press; New York, USA: 1979. [Google Scholar]
- Schaller A, Stintzi A. Enzymes in jasmonate biosynthesis - Structure, function, regulation. Phytochemistry. 2009;70(13-14):1532–1538. doi: 10.1016/j.phytochem.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Schwachtje J, Baldwin IT. Why does herbivore attack reconfigure primary metabolism? Plant Physiology. 2008;146(3):845–851. doi: 10.1104/pp.107.112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtje J, Kutschbach S, Baldwin IT. Reverse genetics in ecological research. Plos One. 2008;3(2) doi: 10.1371/journal.pone.0001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtje J, Minchin P, Jahnke S, van Dongen J, Schittko U, Baldwin I. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan XY, Wang JX, Chua LL, Jiang DA, Peng W, Xie DX. The Role of Arabidopsis rubisco activase in jasmonate-induced leaf senescence. Plant Physiology. 2011;155(2):751–764. doi: 10.1104/pp.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbe M, Qu N, Galis I, Baldwin IT. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell. 2008;20(7):1984–2000. doi: 10.1105/tpc.108.058594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell and Environment. 2007;30(9):1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nature Protocols. 2006;1(3):1342–1345. doi: 10.1038/nprot.2006.232. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14(6):1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. Plos Biology. 2004;2(8):1074–1080. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz M, Baldwin IT, Gaquerel E. Diverting the flux of the JA pathway in Nicotiana attenuata compromises the plant’s defense metabolism and fitness in nature and glasshouse. Plos One. 2011a;6(10):e25925. doi: 10.1371/journal.pone.0025925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz M, Gase K, Baldwin IT, Gaquerel E. Ectopic expression of AtJMT in Nicotiana attenuata: Creating a metabolic sink has tissue-specific consequences for the jasmonate metabolic network and silences downstream gene expression. Plant Physiology. 2011b;157(1):341–354. doi: 10.1104/pp.111.178582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlfelder C, Lottspeich F, Mueller MJ. Purification and partial amino acid sequences of an esterase from tomato. Phytochemistry. 2002;60(3):233–240. doi: 10.1016/s0031-9422(02)00126-7. [DOI] [PubMed] [Google Scholar]
- Stuhlfelder C, Mueller MJ, Warzecha H. Cloning and expression of a tomato cDNA encoding a methyl jasmonate cleaving esterase. European Journal of Biochemistry. 2004;271(14):2976–2983. doi: 10.1111/j.1432-1033.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu GH, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the SCFCO11 complex during jasmonate signalling. Nature. 2007;448(7154):661–U662. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Tieman D, Zeigler M, Schmelz E, Taylor MG, Rushing S, Jones JB, Klee HJ. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant Journal. 2010;62(1):113–123. doi: 10.1111/j.1365-313X.2010.04128.x. [DOI] [PubMed] [Google Scholar]
- Varbanova M, Yamaguchi S, Yang Y, McKelvey K, Hanada A, Borochov R, Yu F, Jikumaru Y, Ross J, Cortes D, Ma CJ, Noel JP, Mander L, Shulaev V, Kamiya Y, Rodermel S, Weiss D, Pichersky E. Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell. 2007;19(1):32–45. doi: 10.1105/tpc.106.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dahl CC, Baldwin IT. Methyl jasmonate and cis-jasmone do not dispose of the herbivore-induced jasmonate burst in Nicotiana attenuata. Physiologia Plantarum. 2004;120(3):474–481. doi: 10.1111/j.0031-9317.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Allmann S, Wu JS, Baldwin IT. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiology. 2008;146(3):904–915. doi: 10.1104/pp.107.109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta. 2007;226(1):159–167. doi: 10.1007/s00425-007-0477-3. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates and octadecanoids: Signals in plant stress responses and development. Progress in Nucleic Acid Research and Molecular Biology. 2002;72:165–221. doi: 10.1016/s0079-6603(02)72070-9. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemariam MG, Onkokesung N, Baldwin IT, Galis I. Jasmonoyl-l-isoleucine hydrolase 1 (JIH1) regulates jasmonoyl-l-isoleucine levels and attenuates plant defenses against herbivores. Plant Journal. 2012;72(5):758–767. doi: 10.1111/j.1365-313X.2012.05117.x. [DOI] [PubMed] [Google Scholar]
- Wu JS, Wang L, Baldwin IT. Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? Planta. 2008;227(5):1161–1168. doi: 10.1007/s00425-008-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, Xiao LT, Sun TP, Li JG, Deng XW, Lee CM, Thomashow MF, Yang YN, He ZH, He SY. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(19):E1192–E1200. doi: 10.1073/pnas.1201616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl AR, Hamilton JG, Miller TJ, Crofts AR, Oxborough K, Berenbaum MR, de Lucia EH. Impact of folivory on photosynthesis is greater than the sum of its holes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):1088–1091. doi: 10.1073/pnas.022647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui DQ, Baldwin IT. Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiology. 2004;134(3):1181–1190. doi: 10.1104/pp.103.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.