Summary
How plants tailor their defense responses to attack from different insects remains largely unknown. Here we studied the role of a mitogen-activated protein kinase (MAPK), MPK4, in the resistance of a wild tobacco Nicotiana attenuata to two herbivores, the specialist Manduca sexta and the generalist Spodoptera littoralis.
Stably transformed N. attenuata plants silenced in MPK4 (irMPK4) were generated and characterized for traits important for defense against herbivores.
Only the oral secretions (OS) from M. sexta, but not the OS from S. littoralis or mechanical wounding, induced elevated levels of jasmonic acid (JA) in irMPK4 plants compared to the wild-type plants. Moreover, silencing MPK4 highly increased the resistance of N. attenuata to M. sexta in a fashion that was independent of COI1 (CORONATINE INSENSITIVE 1)-mediated JA signaling. Untargeted metabolomic screening identified several new MPK4-dependent putative defensive compounds against M. sexta. In contrast, silencing MPK4 did not affect the growth of the generalist insect S. littoralis, and we propose that this was due to the very low levels of fatty acid-amino acid conjugates (FACs) in S. littoralis OS.
Thus, MPK4 is likely to be a key signaling element that enables plants to tailor defense responses to different attackers.
Keywords: herbivore, defense, mitogen-activated protein kinase, Nicotiana attenuata, generalist insect, specialist insect
Introduction
Plants are challenged by numerous environmental stresses during all stages of their life cycles. Accordingly, they have evolved sophisticated signaling networks to cope with these challenges. These signaling networks quickly convert the extracellular stress stimuli into intracellular defense responses (Jones & Dangl, 2006; Howe & Jander, 2008; Wu & Baldwin, 2009; Wu & Baldwin, 2010). Among these signaling systems, the mitogen-activated protein kinase (MAPK) cascades play essential roles (Ichimura et al., 2000; Asai et al., 2002; Teige et al., 2004; Meszaros et al., 2006; Brader et al., 2007). Typically, a MAPK cascade consists of a three-kinase module that is conserved in all eukaryotes. MAPK, the last kinase in the cascade, is activated by the dual phosphorylation of Thr and Tyr residues in its kinase catalytic activation loop. This phosphorylation is mediated by a MAPK kinase (MAPKK or MEK), which is activated by a MAPKK kinase (MAPKKK or MEKK). Following activation, MAPKs regulate gene expression by phosphorylation of DNA-binding transcription factors or directly activate certain enzymes (Hill & Treisman, 1995; Karin & Hunter, 1995; Hazzalin & Mahadevan, 2002).
There are about 20 MAPK genes in the Arabidopsis genome (MAPK Group, 2002). However, only the functions of three MAPKs in Arabidopsis stress responses, especially pathogen-induced responses, have been intensively studied. MPK6 and MPK3 are involved in abiotic stress-elicited reactions (Ichimura et al., 2000; Ahlfors et al., 2004; Gudesblat et al., 2007; Xing et al., 2009), and are required for resistance to attack from pathogens (Desikan et al., 2001; Asai et al., 2002; Menke et al., 2004; Ren et al., 2008; Pitzschke et al., 2009). Another Arabidopsis MAPK, MPK4 was identified as a negative regulator of plant immunity to pathogens (Petersen et al., 2000). mpk4 mutants have highly elevated salicylic acid (SA) levels, which result in greatly increased levels of transcripts of PATHOGENESIS-RELATED (PR) genes. In turn, these mutants are strongly resistant to a virulent bacterial pathogen, Pseudomonas syringae pv. tomato DC3000, and a virulent isolate of the oomycete, Hyaloperonospora arabidopsidis (Petersen et al., 2000).
Herbivores pose another biotic stress to plants. Similar to resistance to pathogens, complex defense systems against herbivores have also been found in various plant species (Howe & Jander, 2008; Mithöfer & Boland, 2008; Wu & Baldwin, 2010). Following herbivore attack, plants alter phytohormone levels, including those of jasmonic acid (JA), salicylic acid (SA), and ethylene (Howe et al., 1996; Reymond & Farmer, 1998; von Dahl et al., 2007), and reconfigure their transcriptomes and proteomes (Hui et al., 2003; Reymond et al., 2004; Giri et al., 2006). These changes finally lead to enhanced levels of certain secondary metabolites, which confer resistance to herbivores directly or indirectly. The central roles of JA and its amino acid derivative, JA-Ile, in plant-herbivore interactions have been intensively studied (Howe & Jander, 2008; Wu & Baldwin, 2010). JA biosynthesis involves at least eight enzymes located in chloroplasts and peroxisomes, which produce linolenic acid and convert it to JA (Wasternack, 2007). Catalyzed by JAR (JASMONATE RESISTANT) proteins, JA is further conjugated with isoleucine to form JA-Ile, which binds to the receptor COI1 (CORONATINE INSENSITIVE1) and triggers JA-induced responses (Staswick & Tiryaki, 2004; Chini et al., 2007; Thines et al., 2007). Transgenic (or mutant) plants that are impaired in JA/JA-Ile biosynthesis or signaling have highly decreased levels of defense-related secondary metabolites and thus show reduced resistance to herbivores (Howe et al., 1996; Reymond et al., 2000; Kessler & Baldwin, 2001; Paschold et al., 2007). Many studies have identified JA-mediated herbivore defense pathways, but still little is known about JA-independent defenses (Howe & Jander, 2008).
Given the central role of JA in plant-herbivore interactions, the signaling networks that control JA biosynthesis and thus activate effective defenses are particularly important for an understanding of the mechanism by which plants regulate their defenses against herbivores. Genetic studies have indicated the involvement of MAPKs in regulating wounding- and herbivory-induced JA biosynthesis (Kandoth et al., 2007; Wu et al., 2007). In the wild tobacco Nicotiana attenuata, wounding and herbivory rapidly activate salicylic acid-induced protein kinase (SIPK; an orthologue of Arabidopsis MPK6) and wound-induced protein kinase (WIPK; an orthologue of Arabidopsis MPK3) within minutes (Wu et al., 2007). Silencing SIPK and WIPK in N. attenuata and their homologues in tomato greatly compromises wounding- and herbivory-induced JA bursts, demonstrating the critical role of MAPKs in regulating JA biosynthesis in plant defense against herbivores (Kandoth et al., 2007; Wu et al., 2007; Meldau et al., 2009).
Although increased JA levels are induced by feeding from almost all herbivore species, plants respond to herbivore feeding in an herbivore species-specific manner (Heidel & Baldwin, 2004; De Vos et al., 2005). In analogy to the well-studied pathogen-associated molecular patterns (PAMPs), herbivore-associated molecular patterns (HAMPs) have been proposed to be the main factors that plants use to distinguish different herbivores (Mithöfer & Boland, 2008). However, little is yet known about how plants perceive various HAMPs/elicitors and how these cues activate different downstream signaling systems, such as JA-dependent and –independent pathways, to deploy herbivore-specific defenses. Here we studied the function of a MAPK in N. attenuata, MPK4, in plant defense against the specialist insect M. sexta and the generalist insect Spodoptera littoralis. We show that compared with those in wild-type (WT) plants, herbivory (but not wounding) of M. sexta induces increased JA levels in MPK4-silenced plants, indicating that unlike SIPK and WIPK (Wu et al., 2007), MPK4 is a specific negative regulator of herbivory-induced JA accumulation; furthermore, MPK4 negatively regulates the defense levels of N. attenuata against the specialist, M. sexta, in a largely JA signaling-independent manner. In contrast, silencing MPK4 did not alter plant resistance levels to the generalist S. littoralis, demonstrating that MPK4 confers herbivore-specific responses in N. attenuata.
Materials and Methods
Plant growth and sample treatments
Nicotiana attenuata (Solanaceae) seeds were from a line maintained in our laboratory that was originally collected in Utah (USA) and inbred for 30 generations in the glasshouse. Seed germination and plant cultivation followed Krügel et al. (2002). Four- to 5-week-old plants were used for all experiments.
For collection of M. sexta and S. littoralis oral secretions (OSMs and OSSl), larvae were reared on N. attenuata WT plants until the third to fifth instars. OS were collected on ice as described in Halitschke et al. (2001). To analyze the FAC contents in the OS, each sample was centrifuged at 16,000 g for 10 min, the supernatants were 1/100 diluted with 15% methanol, and directly analyzed on an HPLC-MS (1200L LC-MS system, Varian) (Halitschke et al., 2001). For the simulated herbivory treatments, leaves were wounded with a pattern wheel and OS were immediately rubbed into the puncture wounds of each wounded leaf (W+OSMs or W+OSSl); for the wounding treatments, leaves were wounded with a pattern wheel, and 20 μL of water were rubbed into the puncture wounds on each wounded leaf (W+W).
Generation of transformed plants
Creation of irMPK4 plants has been described in Hettenhausen et al. (2012). Crossing irCOI1 with irMPK4 plants was done by removing anthers from flowers of irCOI1 plants before pollen maturation and pollinating the stigmas with pollen from irMPK4 plants.
Analysis of JA and JA-Ile concentrations
One milliliter of ethyl acetate spiked with 200 ng of D2-JA and 40 ng of 13C6-JA-Ile, the internal standards for JA and JA-Ile, respectively, was added to each crushed leaf sample (~ 150 mg). Samples were then ground on a FastPrep homogenizer (Thermo Electron). After being centrifuged at 13,000 g for 10 min at 4 °C, supernatants were transferred to fresh tubes and evaporated to dryness on a vacuum concentrator (Eppendorf). Each residue was resuspended in 0.5 mL of 70% methanol (v/v) and centrifuged at 13,000 g for 15 min at 4 °C to remove particles. The supernatants were analyzed on a HPLC-MS/MS (1200L LC-MS system, Varian).
Herbivore growth bioassays
Freshly hatched M. sexta larvae were placed on 30 replicated plants of each genotype (1 larva/plant). To compare herbivore growth rates on WT and irMPK4 plants, larvae were weighed on day 4, 7, 9 and 11; for the comparison of herbivore growth on WT, irMPK4, irCOI1, and irMPK4×irCOI1 plants, larval masses on day 4, 6, and 8 were recorded.
To measure S. littoralis growth, freshly hatched larvae were grown on artificial diet for 10 days and then placed on 30 replicate plants of each genotype. Larval masses were recorded 3, 6, and 9 days after transfer to the plants.
To analyze if S. littoralis was sensitive to the MPK4-dependend and JA-independent defense compounds, WT and irMPK4 plants were pretreated with W+OSMs or 20 μL of N-linolenoyl-L-Glu at 138 ng μL−1 dissolved in an aqueous solution of 0.005% (w/v) Triton X-100, one day before S. littoralis larvae were placed on these leaves. S. littoralis larvae were confined in clip cages to avoid moving to other uninduced leaves and their masses were recorded over time. The same setup was used to measure S. littoralis performance on irCOI1 and irMPK4×irCOI1 plants, which had been pretreated with W+OSMs (1 day earlier).
Analyses of trypsin proteinase inhibitor (TPI) activity, contents of nicotine, caffeoylputrescine, and diterpene glycosides
TPI activity was analyzed with a radial diffusion assay described by van Dam et al. (2001). The accumulation of the direct defenses, nicotine, caffeoylputrescine, and diterpene glycosides were analyzed in samples harvested 3 days after treatments using a HPLC method described in Keinanen et al. (2001).
Profiling of metabolites by Ultraperformance LC-ToF-MS
Leaf tissue from wild-type, irMPK4, irCOI1, and irMPK4×irCOI1 plants was harvested after 4 days of M. sexta feeding, and 5 independent biological replicates per plant genotype were used; non-treated plants served as comparisons. One hundred milligram of leaf tissue was ground with a Geno/Grinder 2000 (BTC and OPS Diagnostics) and thoroughly extracted with 1 mL of extraction buffer (40% [v/v] methanol/water and 50 mM acetate buffer, pH 4.8). Homogenized samples were centrifuged at 12,000 g for 15 min at 4 °C, the supernatants were transferred into fresh 1.5-mL microcentrifuge tubes, and the samples were centrifuged again under the same conditions. The supernatant (500 μL) was transferred into HPLC vials. Sample separation and mass spectrometry analysis followed Gaquerel et al. (2010).
Data sets were evaluated from 50 to 550 s in the mass range m/z 90 to 1400. The raw data files were converted to netCDF format using the export function of the Data Analysis version 4.0 software (Bruker Daltonics) and processed using the XCMS package (Tautenhahn et al., 2008) and the R-package CAMERA (http://www.bioconductor.org/biocLite.R) as previously described (Gaquerel et al., 2010). Peak detection was performed using the centWave method (Tautenhahn et al., 2008) and the parameter settings ppm= 20, snthresh = 10, peak width = 5 to 20 s. Retention time correctionwas achieved using the parameter settings minfrac = 1, bw = 60 s, mzwid = 0.1D, span = 1, and missing = extra = 0 (Gaquerel et al., 2010). The Metaboanalyst software (Xia et al., 2009) was used to perform multivariate analysis (PCA). The data was filtered using the coefficient of variation, and it was normalized using Pareto scaling (Xia et al., 2009; Gaquerel et al., 2010). The public metabolite databases used for analysis were Prime (http://prime.psc.riken.jp/), Metlin (http://metlin.scripps.edu/), MetDAT2 (http://www.sdwa.nus.edu.sg/METDAT2/), KEEG (http://www.genome.jp/kegg/), PubChem (http://pubchem.ncbi.nlm.nih.gov/), and Knapsack (http://kanaya.naist.jp/KNApSAcK/).
Analysis of starch levels
Starch levels were estimated using the anthrone method (Morris, 1948). Soluble sugars were removed with 80% ethanol and after extraction with perchloric acid; samples were boiled for 8 minutes with the anthrone reagent (100 mg anthrone in 100 mL 95% H2SO4) and starch concentrations were determined according to the absorbance at 540 nm.
RNA extraction and quantitative real-time PCR (qPCR)
Total RNA was extracted from ground leaf samples using TRIzol reagent (Invitrogen). For qPCR analysis, 5 replicated biological samples were used and 0.5 μg of total RNA sample was reverse-transcribed with oligo(dT) and Superscript II reverse transcriptase (Invitrogen). qPCR was performed on an ABI PRISM 7700 sequence detection system (Applied Biosystems) using qPCR Core kits (Eurogentec). A N. attenuata actin2 gene was employed as the internal standard for normalizing cDNA concentration variations. qPCR primers were (F: forward primer; R: reverse primer): for actin2, F: 5′-GGTCGTACCACCGGTATTGTG-3′; R: 5′-GTCAAGACGGAGAATGGCATG-3′, for COI1, F: 5′-CAGGGCATCTTCAGCTGGTC-3′; R: 5′-CGGGATGCTCAGCAACGA-3′, and for MPK4, F: 5′-TAGGAGCAACTCCGGTGCC; R: 5′-GCAAGGACAACATCTGAGACAGAT-3′.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) or unpaired t-test using StatView, version 5.0 (SAS Institute).
Results
Simulated M. sexta herbivory but not wounding, transiently induces elevated levels of JA in irMPK4 plants
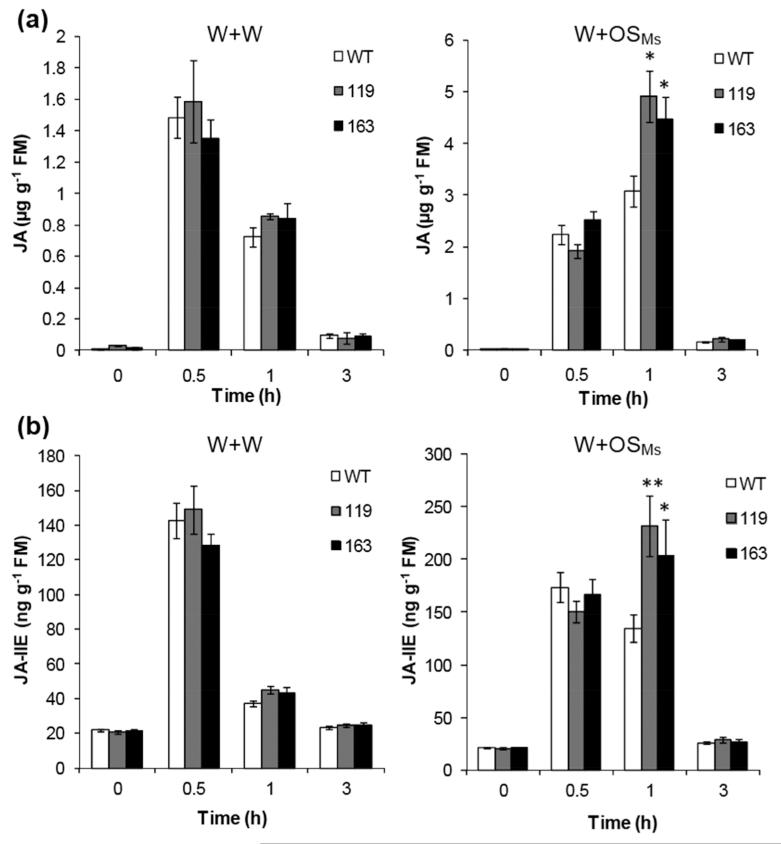
Using an RNAi (RNA interference) vector and Agrobacterium-mediated transformation, we created several independently transformed stable lines of MPK4-silenced N. attenuata (irMPK4) (Hettenhausen et al., 2012); among these, lines 119 and 163 with single T-DNA insertions were selected for this study. Given the central role of JA in plant resistance to herbivores, we first examined whether MPK4 modulates wounding- and herbivory-elicited JA levels. Leaves of rosette-staged WT and irMPK4 plants were wounded with a pattern wheel and 20 μL of M. sexta oral secretions (OSMs) were applied immediately to wounds (W+OSMs) to mimic herbivory (Halitschke et al., 2001; Halitschke et al., 2003); for comparison with mechanical wounding, 20 μL of water were applied to wounds (W+W). The JA contents in WT and irMPK4 plants after these treatments were determined by HPLC-MS/MS. No differences in JA levels were detected between WT and irMPK4 plants before and after W+W treatment (Fig. 1a). However, irMPK4 plants showed 65% increased levels of JA 1 h after W+OSMs treatment compared to the JA levels in similarly treated WT plants (Fig. 1a). Consistently, the contents of JA-Ile, the conjugate of JA and isoleucine, similarly increased: 1 h after W+ OSMs treatment, JA-Ile contents were 60% higher in irMPK4 plants, while JA-Ile levels in W+W-induced WT and irMPK4 plants were the same (Fig. 1b). The antagonistic effect of SA on JA accumulation and signaling has been well documented (Doares et al., 1995; Niki et al., 1998; Kunkel & Brooks, 2002; Spoel et al., 2003). We quantified SA concentrations in order to determine if the elevated JA contents in W+ OSMs-treated irMPK4 plants resulted from attenuated SA levels. WT and irMPK4 plants showed no difference in SA levels before and after either treatment (Fig. S1).
Fig. 1. Silencing MPK4 specifically elevates simulated M. sexta herbivory-induced JA and JA-Ile levels.
Wild-type (WT) and irMPK4 plants (line 119 and 163) were wounded with a pattern wheel and 20 μL of water (W+W) or M. sexta OS (W+OSMs) were immediately applied to wounds. Samples were harvested at indicated times, and their JA (a) and JA-Ile (b) contents (mean ± SE) were analyzed with HPLC-MS/MS. Asterisks indicate significant differences between WT and irMPK4 plants (t-test; *, P < 0.05; **, P < 0.01; N = 5).
To examine whether silencing MPK4 altered the activity of SIPK and WIPK, we measured their activity before and after W+W and W+ OSMs treatment by an in-gel kinase assay (Fig. S2). No differences of SIPK and WIPK activity between WT and irMPK4 plants were found.
We conclude that MPK4 negatively affects M. sexta herbivory-induced JA accumulation and thus comprises a part of the regulatory network that is required for the normal regulation of JA levels in N. attenuata in response to M. sexta attack.
Silencing MPK4 enhances N. attenuata’s defense against the specialist insect herbivore M. sexta
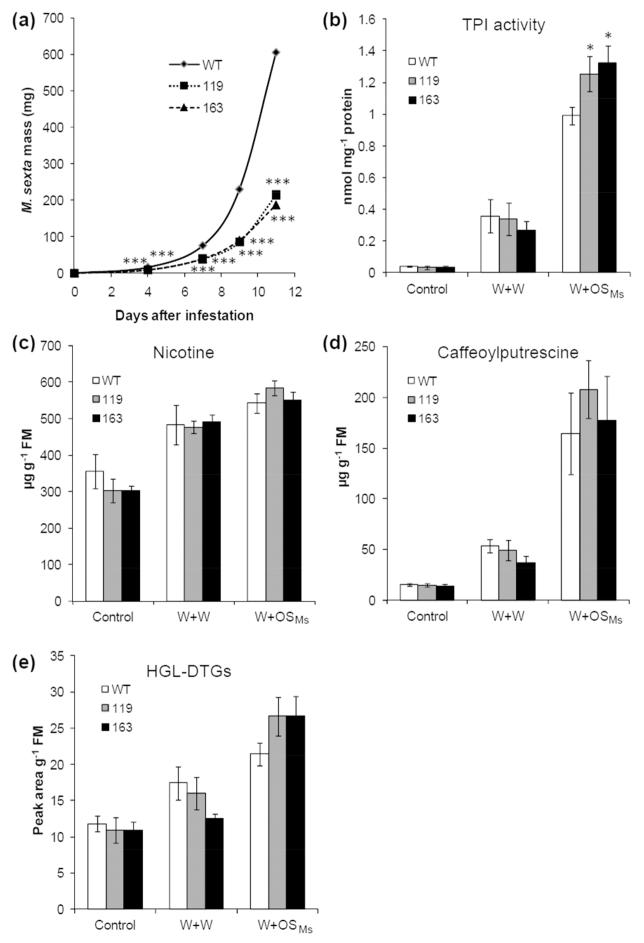
To further study the function of MPK4 in N. attenuata’s defense against herbivores, we performed bioassays on WT and irMPK4 plants to examine whether silencing MPK4 influences the growth of M. sexta. Freshly hatched M. sexta larvae were placed on WT and irMPK4 plants and larval masses were recorded over time. From as early as day 4, compared with those on WT plants, larvae on irMPK4 plants had decreased growth rates; by day 11, the average larval mass on irMPK4 was only 1/3 of that of the larvae on WT plants (Fig. 2a). Thus, MPK4 highly suppresses the resistance of N. attenuata to its specialist herbivore, M. sexta.
Fig. 2. Simulated M. sexta feeding does not induce large changes of known defensive metabolites.
(a) M. sexta growth on different plants. Wild-type (WT) and irMPK4 plants (lines 119 and 163) were infested with 30 neonate M. sexta larvae (1 larva/plant). Masses of these larvae (mean ± SE) on WT and irMPK4 plants were recorded on day 4, 7, 9, and 11. (b to e) Accumulations of defensive secondary metabolites in WT and irMPK4 plants after wounding and simulated herbivory. WT and irMPK4 plants were wounded with a pattern wheel and 20 μL of water (W+W) or M. sexta OSMs (W+OSMs) were immediately applied to the puncture wounds. The activity of TPI (b) and contents of nicotine (c), caffeoylputrescine (d), and 17-hydroxygeranyllinalool diterpene glucosides (HGL-DTGs) (e) (mean ± SE) were analyzed in samples harvested 3 days after treatment. Asterisks indicate significant differences between the masses of larvae reared on WT and irMPK4 plants [t-test; *, P < 0.05; ***, P < 0.001; N = 30 for (a) and N=5 for (b to e)].
In N. attenuata, trypsin proteinase inhibitors (TPIs) (Van Dam et al., 2001), nicotine (Steppuhn et al., 2004a), caffeoylputrescine (CP) (Kaur et al., 2010b), and 17-hydroxygeranyllinalool diterpene glucosides (HGL-DTGs) (Jassbi et al., 2008; Heiling et al., 2010) are all important direct defenses against herbivores, and the accumulations of these compounds are mainly regulated by JA signaling (Paschold et al., 2007). We sought to determine whether these metabolites were responsible for the increased resistance of irMPK4 plants. Consistent with the transiently increased levels of W+OSMs-induced JA in irMPK4 plants, irMPK4 plants had 25% higher TPI activity than did WT after W+OSMs elicitation but not after W+W treatment (Fig. 2b). WT and irMPK4 plants did not show significant differences in the contents of nicotine, CP, and HGL-DTG (Fig. 2, c to e), although irMPK4 tended to have higher HGL-DTG levels after W+OSMs treatments (Fig. 2e; t-test, P > 0.19).
Therefore, MPK4 has little influence on the levels of secondary metabolites that are known to function as direct defenses against M. sexta.
Wounding and herbivory elicit a release of green leaf volatiles (GLVs), which can attract predators of herbivores or increase herbivore loads in nature (Halitschke et al., 2008; Meldau et al., 2009; Dicke & Baldwin, 2010). In addition, GLVs also function as feeding stimulants for M. sexta larvae on N. attenuata (Halitschke et al., 2004; Meldau et al., 2009). The quantities of GLVs released from WT and irMPK4 plants after either W+W or W+OSMs were similar (Fig. S3), excluding the possibility that the reduced M. sexta larval growth resulted from impaired GLV emissions in irMPK4 plants.
MPK4 negatively affects an important JA signaling-independent defense pathway against M. sexta
In N. attenuata, the deployment of all known inducible direct and indirect defenses requires JA signaling (Paschold et al., 2007). Although irMPK4 plants have increased JA levels after OSMs elicitation, only one of the known direct defensive compounds, TPI, showed moderately elevated levels (Fig. 2b). Given the dramatically enhanced resistance levels of irMPK4 plants against M. sexta attack, we speculated that MPK4 may also regulate a novel defense pathway that is independent of JA signaling.
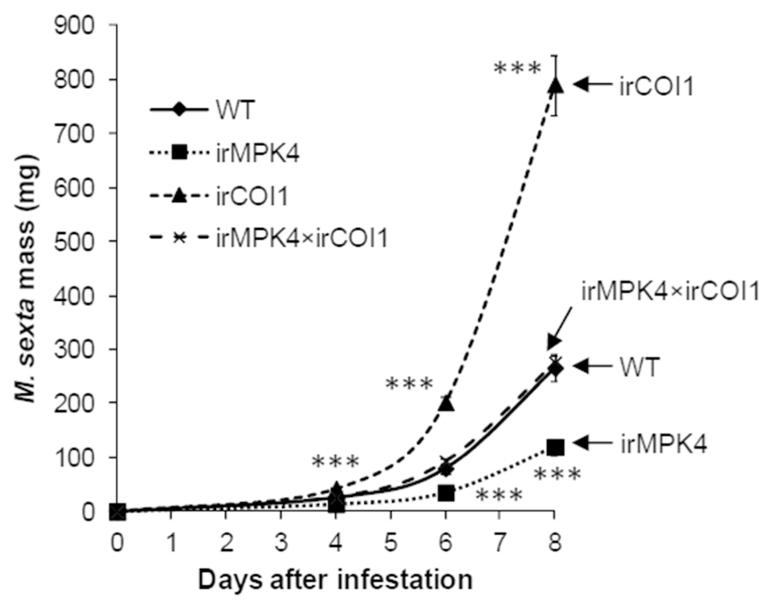
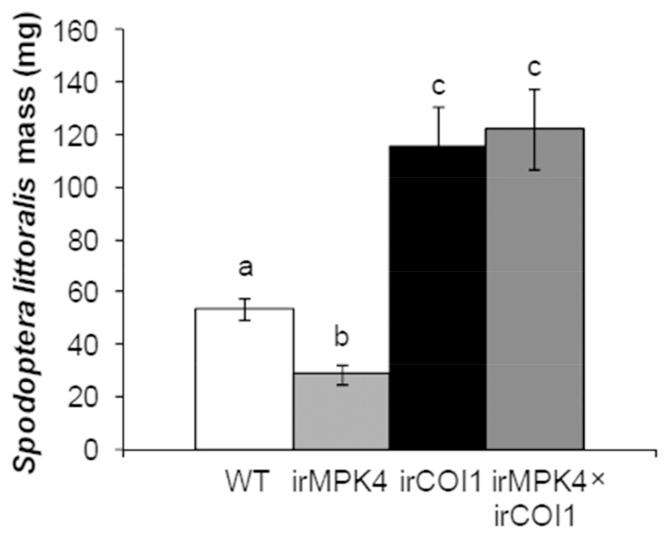
To test this hypothesis, an N. attenuata line silenced in COI1 (irCOI1 plants, deficient in JA perception) (Paschold et al., 2007) was crossed with irMPK4 (line 119) to obtain irMPK4×irCOI1 plants, which were silenced in both MPK4 and COI1 (Fig. S4a). During the rosette stage, when all experiments were performed, no obvious morphological differences were found among WT, irMPK4, irCOI1, and irMPK4×irCOI1 plants. We analyzed the secondary metabolites that function as defenses against M. sexta, and confirmed that irMPK4×irCOI1 plants had substantially diminished levels of TPI, nicotine, CP, and HGL-DGTs owing to the defect in JA signaling (Fig. S4b). M. sexta neonates were placed on WT, irCOI1, irMPK4 (line 119), and irMPK4×irCOI1 plants and herbivore growth was recorded over 8 days (Fig. 3). After 8 days, compared with those grown on WT plants (260 mg), M. sexta larvae gained ~ 200% more average mass on irCOI1 (780 mg) and 50% less average mass on irMPK4 plants (120 mg); importantly, silencing COI1 in irMPK4 plants only resulted in 1-fold increase of M. sexta masses, which were similar to the masses of the larvae on WT plants but were only one third of the caterpillar masses on irCOI1 plants (Fig. 3). Similar results were obtained in two more independently repeated bioassays.
Fig. 3. Silencing MPK4 enhances N. attenuata’s resistance to M. sexta in a largely JA signaling-independent manner.
Wild-type (WT), irMPK4 (line 119), irCOI1, and irMPK4×irCOI1 plants were infested with 30 neonate M. sexta larvae (1 larva/plant). Masses of these larvae (mean ± SE) were recorded on day 4, 6, and 8. Asterisks indicate significant differences between the masses of larvae reared on WT and irMPK4, irCOI1, or irMPK4×irCOI1 plants (t-test; ***, P < 0.001, N = 30).
Very likely, certain compounds which are regulated by MPK4 but not by JA signaling accumulate to high levels in irMPK4 plants and strongly retard the growth of M. sexta larvae.
Unbiased metabolomic analysis reveals JA signaling-dependent and –independent changes in M. sexta feeding-induced secondary metabolites
To identify the potential MPK4-regulated anti-M. sexta metabolites, the differential accumulation of metabolites in M. sexta feeding-elicited leaves from irMPK4, irCOI1, irMPK4×irCOI1, and WT plants was profiled by liquid chromatography-time-of-flight-mass spectrometry (LC-ToF-MS) analysis. Herbivory-elicited leaves were harvested after 4 days of M. sexta feeding, and polar metabolites were analyzed using a previously described method (Gaquerel et al., 2010; Gilardoni et al., 2011). Metabolites were ionized using the electrospray ionization (ESI) interface in both positive and negative ionization modes, and those metabolites that eluted from the column between 50 and 500 s and having mass-to-charge (m/z) values ranging from 90 to 1400 were selected for analysis.
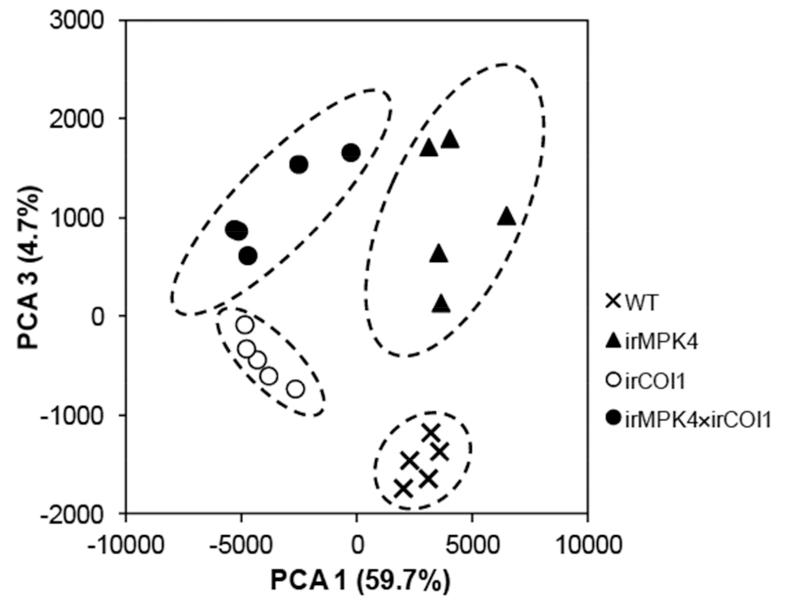
A total of 2516 and 3256 ions were detected in the negative and positive modes, respectively. To obtain an overview of the differences among genotypes, the dataset corresponding to the differentially accumulated ions was analyzed after Pareto-scaling by principal component analysis (PCA). While the ions detected in the positive mode could not separate the individual lines (Fig. S5a), they were clearly distinguished in the negative mode, indicating pronounced metabolomic changes resulting from the silencing of MPK4. The first and third principal components (PCs) together explained 64.4% of the variation within this dataset, and PC1 and 3 clearly separated the samples based on their genotypes (Fig. 4). PCA1 separated irCOI1 and irMPK4×irCOI1 from WT and irMPK4, indicating that ions within this group required JA-signaling. PCA3 separated irMPK4 and irMPK4×irCOI1 from WT and irCOI1, underscoring the ability of MPK4 to sculpt the secondary metabolites composition of leaves. Since MPK4 mediates a JA-independent defense against M. sexta, we expected that these unknown defensive compounds should have greater concentrations in irMPK4 than in WT and irCOI1 plants, and that their concentrations would not be strongly decreased in irMPK4×irCOI1 plants. To identify the ions that were specifically upregulated by silencing MPK4, individual ions, whose abundances were at least 1.5 fold (ANOVA; P < 0.05) greater in irMPK4 than in WT and in irCOI1, and were also at least 1.5 fold (ANOVA; P < 0.05) greater in irMPK4×irCOI1 than in WT and in irCOI1, were selected. Ten and five ions in negative and positive mode, respectively, accumulated to significantly (p < 0.05) higher levels in irMPK4 plants throughout all 4 comparisons (Table S1). To define the identities of these ions, a search in the public metabolite database and in home-made databases (Gaquerel et al., 2010) was performed using the m/z values. Approximately 73% of the ions did not match to any of the metabolites in the databases. Notably, the compounds which could be identified all belong to the group of phenylpropanoid metabolites, and some members of this group have been shown to confer resistance to M. sexta (Kaur et al., 2010a).
Fig. 4. Principal component analysis of the secondary metabolites detected by UPLC-ToF-MS.
Principal component analysis obtained after Pareto scaling revealed a clear differentiation of metabolite profiles in the M. sexta-elicited leaves of WT, irMPK4, irCOI1, and irMPK4×irCOI1 plants. Herbivory-elicited wild-type (WT), irMPK4, irCOI1, and irMPK4×irCOI1 leaves were harvested after 4 days of M. sexta feeding. Polar metabolites were extracted and analyzed on an UPLC-ToF-MS in negative ion detection mode as described in the Methods. Ellipses delimit the 95% statistical confidence areas for each biological group in the score plot.
In contrast to M. sexta feeding-elicited leaves, untreated WT and irMPK4 samples were not separated by the PCA, indicating very similar metabolite profiles (Fig. S5, b and c). From the analysis performed in the negative mode, no ions were found to be significantly different between untreated WT and irMPK4 plants, whereas 20 ions, all detected in the positive ionization mode, accumulated at least 1.5 fold (ANOVA; P < 0.05) greater in untreated irMPK4 than in WT, but importantly, none of these ions was identical to those identified from M. sexta-induced plants (Table S2). Thus, the anti-M. sexta JA-independent defense metabolites are probably induced by M. sexta feeding in irMPK4 plants but are not constitutively controlled by MPK4.
irMPK4 plants have elevated photosynthetic rates (Hettenhausen et al., 2012), and it is possible that these may affect the nutritional contents of irMPK4. We measured both the contents of total proteins and starch (representing the sugars) in WT and irMPK4 plants; no differences were found between the protein contents of samples harvested before and after M. sexta feeding, while in line with the elevated photosynthesis, irMPK4 contained ~ 50% more starch than did WT plants (Fig. S6). These results argue against the possibility that the decreased M. sexta growth rates on irMPK4 plants resulted from certain indirect effects of silencing MPK4, such as altered photosynthesis capacity.
MPK4 is not required for defense against the generalist herbivore S. littoralis
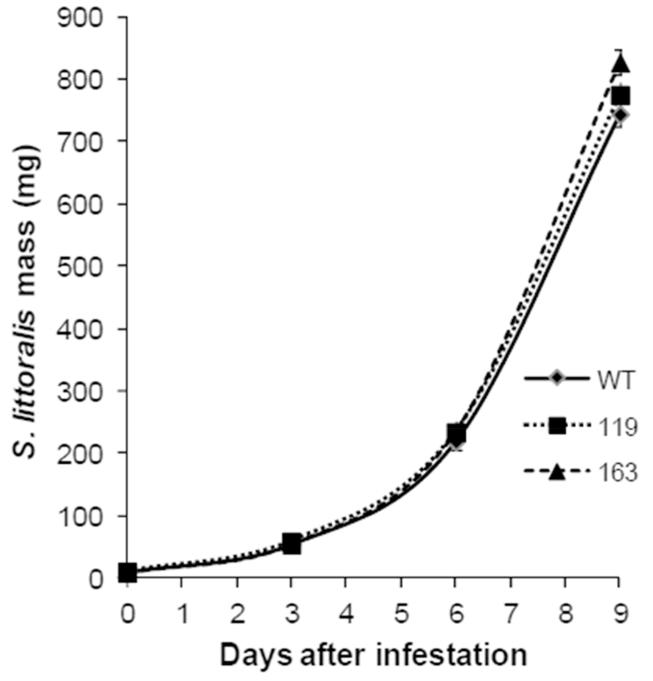
To examine whether MPK4 is also important for the defense against the generalist herbivore S. littoralis, S. littoralis larvae were allowed to feed on WT and irMPK4 plants for 9 days. In contrast to the specialist M. sexta, generalist S. littoralis did not show different growth rates on WT and irMPK4 plants (Fig. 5).
Fig. 5. S. littoralis grows similarly on wild-type and irMPK4 plants.
Wild-type (WT) and irMPK4 (lines 119 and 163) plants were infested with 30 S. littoralis larvae (1 larva/plant). Masses of these larvae (mean ± SE) were recorded on day 3, 6, and 9. No statistical differences were found between the masses of S. littoralis grew on WT and irMPK4 plants (t-test).
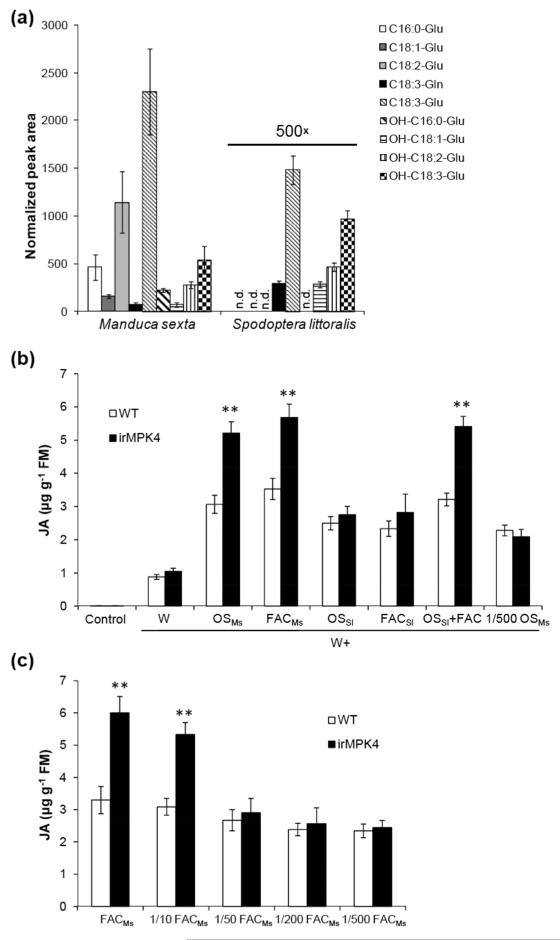
Fatty acid-amino acid conjugates (FACs) in OSMs are necessary and sufficient to trigger M. sexta feeding-specific responses in N. attenuata, including the rapid activation of SIPK and WIPK and the initiation of JA and JA-Ile biosynthesis (Halitschke et al., 2001; Halitschke et al., 2003; Wu et al., 2007). Given the important role of FACs in activating herbivore defense-related responses, including the activation of SIPK and WIPK signaling in N. attenuata, the FAC contents in OSSl were analyzed. Compared with those in OSMs, substantially lower levels (~ 500 times lower) of all FACs were found in OSSl (Fig. 6a). We next explored whether the concentrations of FACs were important for W+OS-induced JA levels in irMPK4 plants. Applying OSMs (W+OSMs) or FACs in a concentration similar to those in OSMs (W+FACMs) to wounds both elicited about 60% more JA in irMPK4 than in WT plants; in contrast, W+OSSl did not induce different JA levels in WT and irMPK4 plants, although FACs in OSSl were still sufficient to elicit high levels of JA (~ 2.5 μg g−1 FM), which were more than 1.6 fold greater than those induced by W+W (0.95 μg g−1 FM) (Fig. 6b). Treating irMPK4 plants with OSSl, which had been supplemented with FAC-A (N-linolenoyl-L-Glu) (Halitschke et al., 2001) to mimic OSMs (W+OSSl+FAC), elevated the JA levels to those in W+OSMs-elicited plants; furthermore, compared with OSMs, 500-fold diluted OSMs showed reduced ability to elicit JA accumulation in irMPK4 plants (Fig. 6b). A FAC titration assay was also performed to examine the effect of FAC concentrations on the elicitation of JA accumulation in WT and irMPK4 plants, and it was found that only when FAC-A concentration was at least 13.8 ng μL−1 (similar to that in 10-fold diluted OSMs), JA levels in irMPK4 were significantly greater than in WT plants (Fig. 6c). FACs in OSSl had very low concentration and therefore S. littoralis feeding could not induce greater levels of JA in irMPK4 than in WT plants.
Fig. 6. FAC in low concentrations does not induce different JA levels in WT and irMPK4 plants.
(a) Comparison of the FAC contents in the oral secretions of M. sexta and S. littoralis. (b) JA induced by wounding and simulated herbivory. Wild-type (WT) and irMPK4 (line 119) were wounded with a pattern wheel and 20 μL of water (W+W), M. sexta OS (W+OSMs), S. littoralis OS (W+OSSl), FAC-A in a concentration similar to that in S. littoralis OS (W+FACSl) or M. sexta OS (W+FACMs), S. littoralis OS supplied with FAC-A (OSSl+FAC), or 1 to 500 diluted M. sexta OS, were immediately applied to wounds and JA contents were measured in samples collected 1 h after treatments. Non-treated plants served as controls. (c) FAC titration assay. Plants were wounded with a pattern wheel and 20 μL of FAC-A in a serial dilution were applied to wounds, and JA contents were measured in samples collected 1 h after treatments (FACMs represents FAC-A in a concentration similar to that in OSMs, 138 ng μL−1). Asterisks indicate significant differences between WT and irMPK4 plants (t-test; **, P < 0.01; N=5).
The similar levels of JA in S. littoralis-induced WT and irMPK4 plants were consistent with the equal growth rates of S. littoralis on WT and irMPK4 plants. The possibility that the contents of FACs in OSSl were too low to activate the JA-independent defense pathway in which MPK4 plays a negative role, or that S. littoralis can tolerate this JA-independent defense could not be ruled out.
The MPK4-regulated JA signaling-independent defense pathway in irMPK4 does not inhibit the growth of S. littoralis
Our genetic analysis indicated that the highly increased resistance to M. sexta in irMPK4 plants was largely contributed by certain defensive compounds regulated by a novel JA-independent defense (hereafter JID) pathway (Fig. 3), but silencing MPK4 did not change the resistance levels of N. attenuata against S. littoralis. We speculated that N. attenuata evolved the JID pathway (which is suppressed by MPK4) to counteract the specialist M. sexta but not the generalist S. littoralis; thus, it is likely that these compounds have no effect on S. littoralis growth. To examine this possibility, we treated WT and irMPK4 with W+OSMs to ensure the accumulation of the JA-independent metabolites. One day after W+OSMs treatment, S. littoralis larvae were placed on these plants and their masses were recorded. By day 4, S. littoralis grown on WT plants had an average mass of 54 mg, but those on irMPK4 plants reached only about 28 mg (Fig. 7). Since W+OSMs treatment induces JA-dependent (such as TPI) and –independent defenses, caterpillar growth was monitored in parallel on irCOI1 and irMPK4×irCOI1, which were also treated with W+OSMs 1 day prior to the bioassay: S. littoralis average masses increased equally to ~ 120 mg on both irCOI1 and irMPK4×irCOI1 (Fig. 7). Similar results were obtained from an independently repeated experiment, in which the S. littoralis bioassay was performed on plants that were pre-treated with W+FAC-A (138 ng μL−1, similar to those in OSMs) (Fig. S7a). Given that after W+OSMs treatment, only TPI had elevated levels in irMPK4 plants, we examined S. littoralis growth on irTPI, which were silenced in TPI transcripts (Steppuhn et al., 2004b), and on irMPK4×irTPI plants, which were pretreated with W+OSMs. The results confirmed that the increased TPI activity in irMPK4 plants after W+FAC-A treatment was responsible for the decreased growth of S. littoralis (Fig. S7b).
Fig. 7. The JA-independent defense pathway does not inhibit the growth of S. littoralis.
Wild-type (WT), irMPK4 (line 119), irCOI1, and irMPK4×irCOI1 plants were wounded with a pattern wheel, and 20 μL of M. sexta OS were applied to wounds immediately. After 1 day, each plant type was infested with 30 S. littoralis larvae (1 larva/plant). Masses of these larvae (mean ± SE) were recorded after 3 days of feeding. Different small letters represent statistical differences (ANOVA, P < 0.05, N = 30).
Thus, S. littoralis is tolerant of the anti-M. sexta compounds that are regulated by the JA-independent pathway in irMPK4 plants, but its growth is negatively affected by defenses elicited by JA signaling.
Discussion
Deactivation of a physiological process is equally as important as its activation. However, much less is known about proteins that play negative regulatory roles in plant physiology than those playing positive roles. Using a reverse genetic approach, we examined the function of a MAPK, MPK4, in plant resistance to insect attack. Unlike SIPK and WIPK, two MAPKs that play positive roles in mediating JA levels, MPK4 negatively affected JA accumulation elicited by M. sexta feeding (but was not involved in S. littoralis-induced JA accumulation). Importantly, MPK4 also suppressed an essential anti-M. sexta pathway that was independent of JA signaling. In contrast, the MPK4-suppressed defense pathways were not required for the N. attenuata-S. littoralis interaction. In addition to being a novel negative MAPK regulator of FAC-induced JA, we further demonstrate that MPK4 likely represents a node in the signaling network that confers herbivore species-specific responses.
Concentrations of FACs are important in activating MPK4-dependent JA accumulations
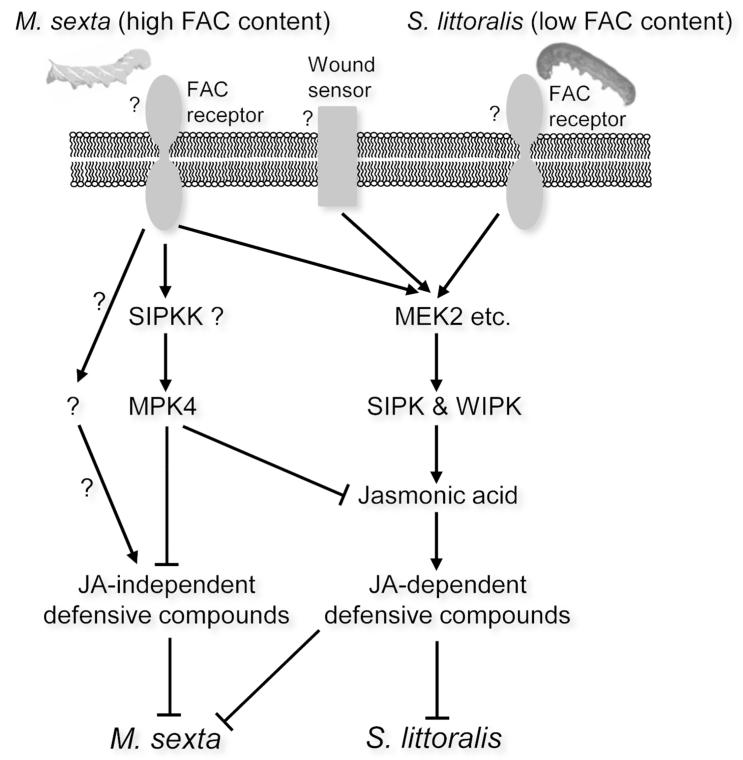
Although the mechanisms of perception remain unclear, some plants use FACs as HAMPs and deploy specific defense reactions (Mithöfer & Boland, 2008; Bonaventure et al., 2011). OSMs contain FACs at milli-molar concentrations (Halitschke et al., 2001), however, Spodoptera spp. appear to have much less FACs in their OS, as FACs in OSSl are about 500 times lower in concentrations than those in OSMs, and similarly low contents of FACs were also found in S. exigua (Diezel et al., 2009). Consistent with the finding that FACs in low concentration can still effectively activate SIPK and WIPK (Wu et al., 2007), W+OSSl induced greater JA levels than did W+W in N. attenuata. However, using OSMs, OSSl, supplementation of FAC-A to OSSl, and FAC-A in different concentrations, we found that FAC concentrations play a critical role in eliciting MPK4-dependent JA accumulation. Furthermore, our titration assay using FAC-A at different concentrations confirmed that relatively high concentrations of FACs were required to induce high JA levels in irMPK4 plants (Fig. 6C). We propose that it is likely that similar to SIPK and WIPK, MPK4 is also located downstream of the putative FAC receptor, and N. attenuata uses FAC concentrations to recognize different insect herbivores (such as the specialist M. sexta and generalist S. littoralis) and activates herbivore-specific reactions, including the MPK4-mediated responses (Fig. 8). It is possible that the different concentrations of FACs in the OS of the specialist M. sexta and the generalist S. littoralis not only account for the different levels of herbivory-induced JA and thus the JA-dependent defenses, but also the JIDs. This possibility should be examined after the identities of the JA-independent metabolites are determined.
Fig. 8. A hypothetical working model summarizing M. sexta- and S. littoralis-induced MAPK signaling in N. attenuata.
The FACs in M. sexta oral secretions (OS) are perceived by a putative FAC receptor in N. attenuata and thereby activate at least MEK2 (Heinrich et al., 2011) and its downstream MAPKs, SIPK and WIPK, which positively regulate the accumulation of JA. Highly increased JA levels induce the biosynthesis of defensive compounds, such as TPI, HGL-DTGs, and caffeoylputrescine. Perception of FACs in M. sexta OS might also activate MPK4 [probably through activation of SIPKK (Gomi et al., 2005)], which suppresses JA levels and more importantly, the accumulation of unknown anti-M. sexta compounds in a JA signaling-independent manner. It is unclear which signaling pathway positively regulates the production of these unknown compounds. Compared with those in M. sexta OS, FACs in S. littoralis OS occur at much lower concentrations and thus do not activate the MPK4 suppressor pathway, but are still sufficiently abundant to activate an amplification of SIPK and WIPK activity compared to mechanical wounding and induce JA-dependent defenses.
FACs are thought to function in nitrogen metabolism of certain insects (Yoshinaga et al., 2008) and they are likely essential for insect physiology, as they have been found not only in many caterpillars but also in crickets (Teleogryllus taiwanemma) and fruit flies (Drosophila melanogaster) (Yoshinaga et al., 2007). In the evolutionary arms races between N. attenuata and generalist and specialist insects, FACs in the OS of insects likely play a critical role in plant-insect interactions: plants have evolved recognition systems to deploy herbivore-specific defenses to achieve optimal growth and defense output and/or insects may also have evolved to alter the components of their OS, e.g. FAC contents, to minimize the risks of triggering strong defenses from their hosts.
JA biosynthesis takes place in the chloroplast and peroxisome. SIPK, WIPK, and MPK4 are mainly located in cytoplasm and nucleus (Ahlfors et al., 2004; Hettenhausen et al., 2012) and there is no evidence that they are also located in or can translocate into chloroplasts and/or peroxisomes. The enzymatic reactions in JA biosynthesis have been intensively studied (Delker et al., 2006). However, little is known about the mechanisms underlying the regulation of JA biosynthesis – how plants rapidly activate JA biosynthesis. To further study the mechanisms by which these MAPKs influence JA biosynthesis, new methods are required to determine the in vivo activity of the biosynthetic enzymes (at least 8 enzymes) and to quantify the intermediate products (precursors) of JA, all of which have high turn-over rates. The catabolism of JA remains unclear. The possibility that irMPK4 plants have decreased catabolic rates of JA cannot be ruled out.
MPK4 suppresses a novel JA-independent defense pathway against the specialist insect herbivore, M. sexta, but is not important in the resistance to the generalist S. littoralis
Several studies have shown that wounding not only activates the JA pathway, but also the JA-independent responses. For example, in Arabidopsis JA and wounding induce overlapping but yet different sets of genes, and after wounding several genes are still induced in coi1 mutants (Titarenko et al., 1997); many wounding-induced genes are also highly elevated in Arabidopsis jar1 mutants, which are deficient in JA-Ile (Suza & Staswick, 2008). It is possible that wounding- and herbivory-induced JA-independent pathways are mediated by other phytohormones, such as ethylene, salicylic acid, and abscisic acid signaling (Erb et al., 2012). Similarly, JA-independent defenses are also involved in Arabidopsis-Botrytis cinerea (a necrotrophic fungus) interactions (Rowe et al., 2010).
In bioassays, we found that irMPK4 plants have high levels of resistance to M. sexta. Using irMPK4×irCOI1 plants, which are deficient in both MPK4 and COI1, we examined the contribution of JA signaling. Strikingly, despite highly compromised levels of all known anti-herbivore compounds, irMPK4×irCOI1 plants still largely retained their elevated resistance levels compared to irCOI1. Clearly, MPK4 suppresses certain defensive compounds in a JA signaling-independent manner (Fig. 3). Given that silencing COI1 in irMPK4 highly compromised the accumulation of CP, HGL-DGTs, and TPIs (Fig. S4b), this JID pathway in irMPK4 plants is remarkably effective against M. sexta. In contrast, S. littoralis grew similarly on WT and irMPK4 plants, demonstrating that MPK4 is not important in the interaction between N. attenuata and S. littoralis larvae.
We found that only high levels of FACs (such as those in OSMS) elicited greater levels of JA in irMPK4 than in WT plants (Fig. 6). Most likely due to the low FAC concentrations in OSSl, S. littoralis feeding did not elicit greater levels of JA in irMPK4 than in WT N. attenuata, and thus MPK4 is not important for the JA-dependent defenses in S. littoralis-N. attenuata interactions. Given the low FAC contents in OSSl, it is possible that S. littoralis feeding does not elicit the JID pathway, and this resulted in the equal growth of S. littoralis on irMPK4 and WT plants; another possibility is that S. littoralis feeding activates the accumulation of JA-independent defensive metabolites, but S. littoralis tolerates these metabolites. By applying OSMs to ensure the production of JA-independent metabolites, we provide in planta evidence that despite their strong inhibitory effects on M. sexta growth, these compounds do not interfere with the growth of S. littoralis, indicating the tolerance of S. littoralis larvae. Using a large-scale metabolomic analysis, we identified 15 putative metabolites that were upregulated after M. sexta feeding in MPK4-silenced plants compared to those in WT plants, and it is likely that some of these metabolites play important roles in the interaction between M. sexta and N. attenuata. The identity of most of the corresponding metabolites could not be unambiguously determined and the characterization of these compounds will be the focus of future work. Comparisons between the metabolic profiles of untreated and M. sexta-induced samples indicated that several JA-independent metabolites are induced by M. sexta feeding in irMPK4 plants (Fig. 4 and Table S1). It is also interesting to study whether low concentrations of FACs (such as those in OSSl) can also elicit the accumulation of these JA-independent metabolites in irMPK4 plants. Since mass spectrometry-based metabolic screening only identifies compounds that can be ionized, it is possible that there are certain other undetected MPK4-regulated JA-independent metabolites. In tomato, threonine deaminase (TD) plays a defense role against M. sexta by degrading the essential amino acids arginine and threonine in M. sexta midgut (Chen et al., 2005). Whether proteins are a part of the JIDs remains unknown.
In N. tabacum, wounding transiently enhances the activity of MPK4 (Gomi et al., 2005). Thus we speculate that M. sexta herbivory (and FACs in high concentrations) might also activate MPK4, and this might lead to the suppression of JA biosynthesis and the JIDs (Fig. 8). Additionally, it is possible that another pathway, which is also activated by perception of FACs in M. sexta OS, positively regulates the concentrations of these JA-independent defensive compounds (Fig. 8). After M. sexta feeding, FACs activate both a positive and a negative (the MPK4) pathway in a concerted (e.g., different timings and scales) manner and thereby N. attenuata responds to M. sexta feeding with the accumulation of a particular blend of defensive metabolites, including the JA-independent ones (Fig. 8). However, the possibility that MPK4 is deactivated (dephosphorylated) by M. sexta feeding or FAC treatment cannot be ruled out. We hypothesize that when S. littoralis attacks N. attenuata, the FACs in OSSl are likely too low to activate this positive regulatory pathway (and the suppressor MPK4) to increase the levels of JIDs. Identification of the JA-independent metabolites is necessary to rigorously test these hypotheses and study how MPK4 suppresses their accumulation and which signaling pathway in N. attenuata positively regulates these metabolites, and if the generalist S. littoralis feeding or FACs in low concentrations induce the JIDs.
Unlike SIPK and WIPK, which are important in wounding- and generalist and specialist herbivore-induced responses, MPK4 is only required in the interaction between N. attenuata and M. sexta, but not S. littoralis; furthermore, our bioassays using generalist and specialist insects and genetically modified plants, also revealed herbivore-specific effectiveness of different secondary metabolites. These findings are consistent with the expectations of an evolutionary arms-race between plants and herbivores (Becerra et al., 2009; Ali & Agrawal, 2012). Our previous study has indicated that MPK4 plays a key role in controlling various ecologically important traits. irMPK4 plants are very sensitive to drought stress and deficient in stomatal closure responses due to their insensitivity to abscisic acid; furthermore, they are also highly compromised in guard cell-based resistance to surface-deposited bacterial pathogens (Hettenhausen et al., 2012). Here we show that MPK4 is likely to be a part of a signaling network that is important for N. attenuata to recognize different types of predators: it negatively affects plant defense against the specialist herbivore, M. sexta, but not against S. littoralis. Thus, in N. attenuata MPK4 has both positive and negative functions in plant interactions with various environmental factors. Large scale transcriptome analysis and phosphorylation target screening will shed further light on the molecular basis of MPK4 function, such as the activation of transcription factors that are downstream of MPK4. Identification of the specific anti-M. sexta compounds (the JIDs) in irMPK4, which are regulated by the JA-independent pathway, and elucidation of their biosynthesis pathways will enrich our understanding of this new form of herbivore defense. It is also important to discover the positive signaling components that influence their biosynthesis, which could be other MAPKs.
Supplementary Material
Acknowledgments
We thank Dr. Mario Kallenbach for synthesizing FACs, Dr. Klaus Gase, Susan Kutschbach and Wibke Kröber for technical assistance, Dr. Tamara Krügel, Andreas Schünzel, and Andreas Weber for plant cultivation, an ERC advanced grant, ClockworkGreen (No. 293926) to I.T.B. and the Max Plank Society for financial support.
Footnotes
Fig. S1 Silencing MPK4 does not change SA accumulation after elicitations in N. attenuata.
Fig. S2 irMPK4 plants have normal induced levels of SIPK and WIPK activity after M. sexta herbivory.
Fig. S3 Wounding- and herbivory-induced plant green leaf volatiles in wild-type and irMPK4 plants.
Fig. S4 Silencing COlI in irMPK4 plants abolishes the accumulation of JA-inducible defensive metabolites.
Fig. S5 Principal component analysis after Pareto scaling of the secondary metabolites detected by UPLC-ToF-MS.
Fig. S6 Total protein and starch contents in wild-type and MPK4-silenced plants.
Fig. S7 The JA-independent defense pathway does not inhibit the growth of S. littoralis.
Table S1 Ions differentially detected in wild-type (WT), irMPK4, irCOI1, and irMPK4×irCOI1 plants.
Table S2 Ions differentially detected in untreated wild-type (WT) and irMPK4 plants.
References
- Ahlfors R, Macioszek V, Rudd J, Brosche M, Schlichting R, Scheel D, Kangasjarvi J. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004;40(4):512–522. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- Ali JG, Agrawal AA. Specialist versus generalist insect herbivores and plant defense. Trends in Plant Science. 2012;17(5):293–302. doi: 10.1016/j.tplants.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415(6875):977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Becerra JX, Noge K, Venable DL. Macroevolutionary chemical escalation in an ancient plant-herbivore arms race. Proc Natl Acad Sci U S A. 2009;106(43):18062–18066. doi: 10.1073/pnas.0904456106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Vandoorn A, Baldwin IT. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011;16(6):294–299. doi: 10.1016/j.tplants.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Brader G, Djamei A, Teige M, Palva ET, Hirt H. The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis. Molecular Plant-Microbe Interactions. 2007;20(5):589–596. doi: 10.1094/MPMI-20-5-0589. [DOI] [PubMed] [Google Scholar]
- Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc Natl Acad Sci U S A. 2005;102(52):19237–19242. doi: 10.1073/pnas.0509026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–673. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Metraux JP, Van Loon LC, Dicke M, Pieterse CM. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact. 2005;18(9):923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- Delker C, Stenzel I, Hause B, Miersch O, Feussner I, Wasternack C. Jasmonate biosynthesis in Arabidopsis thaliana – Enzymes, products, regulation. Plant Biology. 2006;8(3):297–306. doi: 10.1055/s-2006-923935. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiology. 2001;126(4):1579–1587. doi: 10.1104/pp.126.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends in plant science. 2010;15(3):167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009;150(3):1576–1586. doi: 10.1104/pp.109.139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995;108(4):1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17(5):250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaquerel E, Heiling S, Schoettner M, Zurek G, Baldwin IT. Development and validation of a liquid chromatography-electrospray ionization-time-of-flight mass spectrometry method for induced changes in Nicotiana attenuata leaves during simulated herbivory. J Agric Food Chem. 2010;58(17):9418–9427. doi: 10.1021/jf1017737. [DOI] [PubMed] [Google Scholar]
- Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G. LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. The Plant cell. 2011;23(9):3512–3532. doi: 10.1105/tpc.111.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri AP, Wunsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiology. 2006;142(4):1621–1641. doi: 10.1104/pp.106.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Ogawa D, Katou S, Kamada H, Nakajima N, Saji H, Soyano T, Sasabe M, Machida Y, Mitsuhara I, Ohashi Y, Seo S. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant and Cell Physiology. 2005;46(12):1902–1914. doi: 10.1093/pcp/pci211. [DOI] [PubMed] [Google Scholar]
- Group M. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7(7):301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Gudesblat GE, Iusem ND, Morris PC. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytologist. 2007;173(4):713–721. doi: 10.1111/j.1469-8137.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui DQ, Schmidt DD, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiology. 2003;131(4):1894–1902. doi: 10.1104/pp.102.018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125(2):711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. Shared signals – ‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecology Letters. 2008;11(1):24–34. doi: 10.1111/j.1461-0248.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Ziegler J, Keinanen M, Baldwin IT. Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. Plant Journal. 2004;40(1):35–46. doi: 10.1111/j.1365-313X.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3(1):30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- Heidel AJ, Baldwin IT. Microarray analysis of salicylic acid- and jasmonic acid-signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant Cell Environ. 2004;27(11):1362–1373. [Google Scholar]
- Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, Jassbi AR, Baldwin IT. Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-Hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell. 2010;22(1):273–292. doi: 10.1105/tpc.109.071449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, Baldwin IT, Wu JQ. Two mitogen-activated protein kinase kinases, MKK1 and MEK2, are involved in wounding- and specialist lepidopteran herbivore Manduca sexta-induced responses in Nicotiana attenuata. Journal of Experimental Botany. 2011;62(12):4355–4365. doi: 10.1093/jxb/err162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettenhausen C, Baldwin I, Wu J. Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv. tomato DC3000. Plant Physiol. 2012;158:759–776. doi: 10.1104/pp.111.190074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80(2):199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8(11):2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DQ, Iqbal J, Lehmann K, Gase K, Saluz HP, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiology. 2003;131(4):1877–1893. doi: 10.1104/pp.102.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24(5):655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT. Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiology. 2008;146(3):974–986. doi: 10.1104/pp.107.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci U S A. 2007;104(29):12205–12210. doi: 10.1073/pnas.0700344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5(7):747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- Kaur H, Heinzel N, Schottner M, Baldwin IT, Galis I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol. 2010a;152(3):1731–1747. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Heinzel N, Schottner M, Baldwin IT, Galis I. R2R3-NaMYB8 Regulates the Accumulation of Phenylpropanoid-Polyamine Conjugates, Which Are Essential for Local and Systemic Defense against Insect Herbivores in Nicotiana attenuata. Plant Physiology. 2010b;152(3):1731–1747. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinanen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. Journal of Agricultural and Food Chemistry. 2001;49(8):3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291(5511):2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12(4):177–183. [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5(4):325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Meldau S, Wu JQ, Baldwin IT. Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytologist. 2009;181(1):161–173. doi: 10.1111/j.1469-8137.2008.02645.x. [DOI] [PubMed] [Google Scholar]
- Menke FL, van Pelt JA, Pieterse CM, Klessig DF. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell. 2004;16(4):897–907. doi: 10.1105/tpc.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros T, Helfer A, Hatzimasoura E, Magyar Z, Serazetdinova L, Rios G, Bardoczy V, Teige M, Koncz C, Peck S, Bogre L. The Arabidopsis MAP kinase kinase MKK1 participates in defence responses to the bacterial elicitor flagellin. Plant J. 2006;48(4):485–498. doi: 10.1111/j.1365-313X.2006.02888.x. [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146(3):825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL. Quantitative determination of carbohydrates with dreywoods anthrone reagent. Science. 1948;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant and Cell Physiology. 1998;39(5):500–507. [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant Journal. 2007;51(1):79–91. doi: 10.1111/j.1365-313X.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103(7):1111–1120. doi: 10.1016/s0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Schikora A, Hirt H. MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol. 2009;12(4):421–426. doi: 10.1016/j.pbi.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci U S A. 2008;105(14):5638–5643. doi: 10.1073/pnas.0711301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell. 2004;16(11):3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Current Opinion in Plant Biology. 1998;1(5):404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12(5):707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe HC, Walley JW, Corwin J, Chan EK, Dehesh K, Kliebenstein DJ. Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. Plos Pathogens. 2010;6(4):e1000861. doi: 10.1371/journal.ppat.1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CM. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15(3):760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16(8):2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. Plos Biology. 2004a;2(8):1074–1080. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. PLoS Biol. 2004b;2(8):E217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suza WP, Staswick PE. The role of JAR1 in Jasmonoyl-L:-isoleucine production during Arabidopsis wound response. Planta. 2008;227(6):1221–1232. doi: 10.1007/s00425-008-0694-4. [DOI] [PubMed] [Google Scholar]
- Tautenhahn R, Bottcher C, Neumann S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinf. 2008;9:504. doi: 10.1186/1471-2105-9-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15(1):141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Titarenko E, Rojo E, Leon J, Sanchez-Serrano JJ. Jasmonic acid-dependent and –independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol. 1997;115(2):817–826. doi: 10.1104/pp.115.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam NM, Horn M, Mares M, Baldwin IT. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. Journal of Chemical Ecology. 2001;27(3):547–568. doi: 10.1023/a:1010341022761. [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Winz RA, Halitschke R, Kuhnemann F, Gase K, Baldwin IT. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant Journal. 2007;51(2):293–307. doi: 10.1111/j.1365-313X.2007.03142.x. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. Herbivory-induced signalling in plants: perception and action. Plant Cell Environ. 2009;32(9):1161–1174. doi: 10.1111/j.1365-3040.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell. 2007;19:1096–1122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37(Web Server issue):W652–660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jia W, Zhang J. AtMKK1 and AtMPK6 are involved in abscisic acid and sugar signaling in Arabidopsis seed germination. Plant Mol Biol. 2009;70(6):725–736. doi: 10.1007/s11103-009-9503-0. [DOI] [PubMed] [Google Scholar]
- Yoshinaga N, Aboshi T, Abe H, Nishida R, Alborn HT, Tumlinson JH, Mori N. Active role of fatty acid amino acid conjugates in nitrogen metabolism in Spodoptera litura larvae. Proc Natl Acad Sci U S A. 2008;105(46):18058–18063. doi: 10.1073/pnas.0809623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga N, Aboshi T, Ishikawa C, Fukui M, Shimoda M, Nishida R, Lait CG, Tumlinson JH, Mori N. Fatty acid amides, previously identified in caterpillars, found in the cricket Teleogryllus taiwanemma and fruit fly Drosophila melanogaster larvae. J Chem Ecol. 2007;33(7):1376–1381. doi: 10.1007/s10886-007-9321-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.